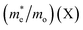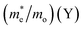Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structural and compositional properties of 2D CH3NH3PbI3 hybrid halide perovskite: a DFT study†
Sandip R. Kumavata,
Geeta Sachdeva b,
Yogesh Sonvane
b,
Yogesh Sonvane *a and
Sanjeev K. Gupta
*a and
Sanjeev K. Gupta c
c
aAdvanced Materials Lab, Department of Physics, Sardar Vallabhbhai National Institute of Technology, Surat 395007, India. E-mail: yas@phy.svnit.ac.in
bDepartment of Physics, Michigan Technological University, Houghton, Michigan 49931, USA
cComputational Materials and Nanoscience Group, Department of Physics, St. Xavier’s College, Ahmedabad 380009, India
First published on 13th September 2022
Abstract
Two-dimensional (2D) hybrid halide perovskites have been scrutinized as candidate materials for solar cells because of their tunable structural and compositional properties. Results based on density functional theory demonstrate its thickness-dependent stability. We have observed that the bandgap decreases from the mono- to quad-layer because of the transformation from 2D towards 3D. Due to the transformation, the carrier mobility is lowered with the corresponding smaller effective mass. On the other hand, the multilayer structures have good optical properties with an absorption coefficient of about 105 cm−1. The calculated absorption spectra lie between 248 nm and 496 nm, leading to optical activity of the 2D multilayer CH3NH3PbI3 systems in the visible and ultraviolet regions. The strength of the optical absorption increases with an increase in thickness. Overall results from this theoretical study suggest that this 2D multilayer CH3NH3PbI3 is a good candidate for photovoltaic and optoelectronic device applications.
1 Introduction
All over the world, population growth and human development has led to industry using much energy, which increases environmental pollution. To overcome this situation many researchers and scientists are working on finding alternative effective, clean and sustainable sources of energy. In the past two decades, researcher have been working on solar energy, because it is a clean and cost-free source of energy. The task is how to efficiently harvest the solar energy. Many research groups are working on finding new technologies and materials for harvesting solar energy more efficiently.In recent years, low cost and high performance hybrid perovskites have attracted much attention from the scientific community.1,2 The flexible structural and compositional properties of hybrid perovskite materials make them preferable candidates for solar cell applications,3,4 light-emitting diodes,5,6 field-effect transistors,7 lasers, non-volatile memory devices8–10 and photodetectors,11 etc. The use of hybrid halide perovskites with the structural formula ABX3, where A is an organic molecule like CH3NH3, NH2CH etc., B is a cation such as Ge, Pb, Sn etc. and X represents anions such as Cl, Br, I, etc. has been explored. In 2009, hybrid halide perovskites as light absorbers were reported for the first time, with a power conversion efficiency (PCE) of 3.8%,12 which was useful to increase research in the photovoltaics field13,18 due to thhe tunable bandgap, charge carrier transport properties, and intense photoluminescence.14–16,19–24 Within a few years, the PCE of hybrid halide perovskites reached 22%, in comparison with traditional silicon-based solar cells.14,25–28
Despite good efficiency, three-dimensional hybrid perovskite shows low stability and device degradation when interacting with oxygen and moisture.29–31 The use of 2D forms of these structures is an efficient way to increase the stability, transport charge mobility, and flexibility.32–35 Therefore, it is important to study perovskite formation to understand the interaction between the inorganic PbI3 and organic CH3NH3 compound.
In experimental studies, Dou et al. were first to describe the single layer and multilayer thick single crystal of 2D (C4H9NH3)2PbBr4 perovskites, with a conformation of 2D perovskites separated by layers of PbBr4−. They showed the electronic properties of the 2D materials were different from the 3D bulk structure.36 As the absorber, 2D perovskites (BA)2PbI4 fabricated by Tsai et al. reached an efficiency of up to 12%, with greater stability and high efficiency.37 Also, the light–matter interactions, photoluminescence and photoresponse of 2D perovskites have been reported, and these 2D perovskites can be suitable for optoelectronic device applications.38,39 Experimentally Liu et al. have reported the 2D (MA)2PbI4, thin film as efficient in solar absorber applications and the electronic and optical properties.40 The halide perovskite’s structural and compositional properties modulation has been achieved in 0D, 1D, 2D, and 3D.36 It has been found that the 2D layered BA2GeI4 and BA2SnI4 are potential candidates for light-emitting devices.41,42 Also, Zhang et al.43 found that the bandgap, Rashba spin splitting, and effective mass vary with the thickness of 2D perovskites (Cs2PbI4, (MA)2PbI4) suggesting the 2D materials as good candidates for optoelectronic devices.
Up to now, many studies have been done on 2D hybrid perovskites. But how the effective transport and optical properties of the 2D hybrid perovskite CH3NH3PbI3 vary with thickness has rarely been studied. In this study, the transport charge mobility and optical absorption of multilayered CH3NH3PbI3 are systematically investigated at the density functional theory level. First, structural properties of monolayer and multilayer CH3NH3PbI3 are discussed. Then, with the electronic and charge carrier properties, the absorption properties of the multilayer hybrid are uncovered.
2 Methodology
Density functional theory (DFT) was employed with VASP implemented with the PAW method.44–46 The exchange–correlation functional (GGA-PBE) was used.47,48 It is well known that GGA-PBE cannot describe the weak interaction between CH3NH3 and PbI3 molecules. Therefore we applied Grimme DFT-D2,48 nonlocal density functional vdW (DFT-D2) implemented in VASP in the study. We also included SOC and HSE49 to get clear images of the electronic and transport properties of the 2D multilayer CH3NH3PbI3. In our previous work on the 2D single layer CH3NH3PbI3 perovskite, we have observed the underestimation and overestimation of the bandgaps using SOC and HSE calculations as shown in Fig S2 and S3.†49,52 The kinetic energy cut-off was set to 500 eV. To avoid successive interaction, a vacuum space of 20 Å was applied along the Z-direction. For the bilayer, trilayer and quadlayer, the distance between two successive layers was set as 3 Å, to check the interaction between layers and the effect of N layers on the cell phase. As we increase the number of layers, the Z direction increases up to ∼20 Å, ∼30 Å, and ∼45 Å for the bilayer, trilayer and quadlayer, respectively.Structural optimization was performed using a (5 × 5 × 1) grid, and a (15 × 15 × 1) grid was used for the electronic properties. All atomic structures were optimized up to a force of 0.001 eV Å−1 with energy convergence about 10−4 eV for multilayer CH3NH3PbI3. The lattices agree well with experimental and theoretical studies.50,53
The formula for the perovskite crystal structure, with effective ionic radii for the ions at the A, B, and X sites, is shown in eqn (1).54
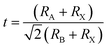 | (1) |
The dielectric function optical absorption coefficient can be calculated using the following equation.53,55,58,59
 | (2) |
 | (3) |
3 Results and discussion
3.1 Structural properties
The equilibrium configurations of multilayer CH3NH3PbI3 are shown in Fig. 1(a–d), while the structural parameters are listed in Table 1. The calculated in-plane lattice constants are a = 6.33 Å and b = 6.21 Å.50–53,60 The calculated bond length PbI is in the range of 3.09–3.27 Å for multilayer CH3NH3PbI3. | ||
| Fig. 1 Atomic structures of multilayer hybrid halide perovskites. Pb, I, C, N, and H are represented in blue, orange, black, pink and sky blue, respectively. | ||
| Structural parameter (Å) | A | B | d(PbI3–CH3NH3) | d(C–C) | d(N–N) | d(Pb–Pb) | d(I–I) | Formation energy (eV) |
|---|---|---|---|---|---|---|---|---|
| Monolayer | 6.33 | 6.21 | 4.64 | 6.33 | 6.33 | 6.33 | 4.29 | −70.8 |
| Bilayer | 6.34 | 6.21 | 4.50 | 4.12 | 5.37 | 10.23 | 3.91 | −141.3 |
| Trilayer | 6.34 | 6.21 | 4.44 | 4.22 | 5.23 | 10.22 | 3.89 | −211.9 |
| Quadlayer | 6.34 | 6.21 | 4.43 | 4.18 | 5.36 | 10.19 | 3.92 | −282.4 |
| Bulk | 6.31, 6.33 | 6.31 |
The calculated formation energy is [Eform = (Elayer − nEPb − nEI − nECH3NH3)] where Eform is formation energy, Elayer is total energy of the layer, EPb is total energy of the Pb atoms, EI is the total energy of the iodine atoms, ECH3NH3 is the total energy of the CH3NH3 molecules. The formation energies of the multilayer perovskites are negative and increase with the corresponding increase in number of layers of CH3NH3PbI3, as shown in Fig. 2. The tolerance factor calculated using eqn (1) is 0.91 which is suitable for the hybrid halide perovskite structure.38–40
3.2 Electronic properties
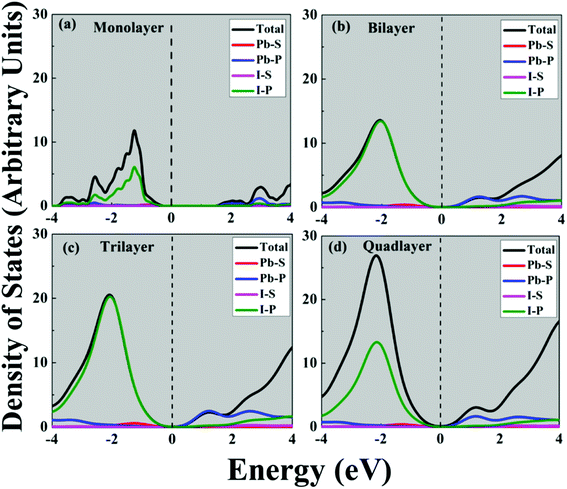
The calculated band gap for the perovskite CH3NH3PbI3 monolayer is 1.63 eV; in the bilayer case it is 1.81 eV; for the trilayer 1.68 eV; and for the quadlayer, it is 1.55 eV as shown in Table 2 and Fig. 3. Similar to the bulk structure, multilayer CH3NH3PbI3 systems also have direct band gaps. The variation in the direct band gap occurred when we shifted from the monolayer to bilayer to trilayer to quadlayer due to an increase in localized states in the CBM and VBM near the Fermi level. Which appears as denser bands for the VBM compared to the CBM. In the case of the bilayer, the energy of the bottom of the near Fermi conduction band decreases and the energy of the top of the valence band increases, as shown in Fig. S5.† Which affects the band gap of the bilayer, which is higher than that of monolayer. Further we observed that when we shifted from bilayer to trilayer to quadlayer, the near Fermi CBM and VBM band energies decrease as we increase the numbers of layers, as shown in Fig. S5.† Further, we find that an indirect band gap occurs along the X to R point. The bandgap values are comparable with the corresponding bulk value of 1.50 eV.61 Moreover, Fig. 4 and Fig. S1† show the total density of states (TDOS) and that the edge of the valence band is mostly contributed by the Pb-6s and I-5p orbitals whereas the edge of the conduction band is dominated by the Pb-6p orbital.| Bandgap PBE (eV) | Bandgap HSE (eV) | |||
|---|---|---|---|---|
| R–R | X–R | R–R | X–R | |
| Monolayer | 1.63 | 2.33 | 2.62 | 3.41 |
| Bilayer | 1.81 | 2.05 | 2.50 | 2.90 |
| Trilayer | 1.68 | 1.87 | ||
| Quadlayer | 1.55 | 1.76 | ||
3.3 Strain engineering
Further we have explored the strain dependent electronic properties of the multilayer CH3NH3PbI3 hybrid halide perovskites as shown in Table 3. Here we have applied both tensile and compressive strains up to 2%. We have observed that upon application of both types of strain the band gap of the system increases. For the monolayer it increases up to 2.01 eV for tensile strain, while in the cases of the bi-, tri- and quadlayer it increases up to 1.83 eV, 1.69 eV, 1.59 eV respectively. Near the Fermi level in the CBM most of the band line is occupied by non-antibonding states of the Pb-p and I-p orbitals and in the VBM most of the band lines are occupied by antibonding states of the Pb-s and I-p orbitals. As the number of layers increases from mono to bi to tri to quad, the corresponding band gap decreases as shown in Fig. S6, S7, S8 and S9† for the mono-, bi-, tri- and quadlayer, respectively. Similarly the variation in band gap occurs due to an increase in the localized state near the Fermi level. Fig. S4† shows the variation in formation energy and total energy with applied tensile and compressive strains for mono-, bi-, tri- and quadlayer CH3NH3PbI3. As we can observe, with tensile and compressive strain, the total energy of the system decreases with respect to the unstrained systems. From Fig. S4† we can observe the formation energy increase as we go from the monolayer to quadlayer.| Strain | Band gap (eV) | ||||
|---|---|---|---|---|---|
| −2% | −1% | 0% | +1% | +2% | |
| Monolayer | 2.01 | 1.98 | 1.63 | 1.98 | 1.97 |
| Bilayer | 1.78 | 1.82 | 1.81 | 1.83 | 1.83 |
| Trilayer | 1.65 | 1.66 | 1.68 | 1.69 | 1.67 |
| Quadlayer | 1.59 | 1.61 | 1.55 | 1.59 | 1.587 |
3.4 Carrier mobility
To evaluate the charge carrier efficiency in multilayer CH3NH3PbI3, we determined the carrier mobility via calculations of the deformation potential by applying compressive and tensile strain up to 2%. as displayed in Fig. S4† which shows formation energy under mechanical strain, and Fig. S10†shows the variation in CBM and VBM under both tensile and compressive strain. The directional dependent effective mass, as shown in Table 4, for the in-plane CBM electrons and VBM holes can be calculated using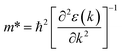 where ε(k) is the wave vector k’s eigenvalue along the transport direction. This is an effective way to moderate transport properties. The charge carrier has some limitations in the bulk hybrid halide perovskite.
where ε(k) is the wave vector k’s eigenvalue along the transport direction. This is an effective way to moderate transport properties. The charge carrier has some limitations in the bulk hybrid halide perovskite.
Then, we compared the single layer (2D) transport properties40,54 of CH3NH3PbI3 with multilayer 2D CH3NH3PbI3 perovskite. In the comparison of the 2D single layer system with the 2D multilayer system, the average directional dependent effective mass of the electrons is decreased, from 3.92 to 0.72, while in the case of holes, the effective mass decreases from 1.51 to 0.79 as shown in Table 4. It is observed that the carrier mobility of the perovskite materials encompass a valuable range. The anisotropic nature of the carrier mobility is due to the difference in fabrication techniques and materials’ morphologies.63 2D CH3NH3PbI3 has flexible structural properties, which affect the transformation from monolayer to bilayer and carrier mobilities are decreased. In the case of 2D multilayer hybrid halide perovskites, from bilayer to quadlayer, carrier mobilities increase from 3.00 cm2 V−1 s−1 to 24.00 cm2 V−1 s−1, which is consistent with previously reported 3D bulk halide perovskites, where 0.4 cm2 V−1 s−1 to 25 cm2 V−1 s−1 was found.19,63 We observed that as the number of layers increases from the monolayer to the quadlayer, the effective masses and carrier mobilities of the electrons and holes resemble the bulk nature of the CH3NH3PbI3 hybrid perovskites.19,41,51,52,57,62,63
3.5 Optical properties
The optical properties of multilayer CH3NH3PbI3 are shown in Fig. 5(a) and (b). We find that broadening of the peaks occurs with the increase in thickness for both ε1(ω) and ε2(ω). The calculated real ε1(ω) static dielectric constant of CH3NH3PbI3 increases from 2.43 to 3.40, monolayer to quadlayer, respectively. In the imaginary part of the dielectric function ε2(ω) the wide peak orientation is due to transition of Pb s to I p orbitals. Likewise, the absorption coefficient slightly increases from 14.1 × 105 cm−1 for the monolayer to 16.0 × 105 cm−1 for the quadlayer. Note that the calculated spectra lie in the range of 248 nm to 496 nm for multilayer CH3NH3PbI3. The calculated results are consistent with previously reported experimental results, containing CH3NH3PbI3 thin films with high PbI2 concentration.8,17 As well as the absorption coefficient is significantly more as compared with the bulk CH3NH3PbI3 (2.5 × 104 cm−1 to 8.7 × 104 cm−1).8,52,56,58,59 The high energy peaks at ∼4 eV occur due to the presence of a large number of interband transitions of the Pb s VBM to I p CBM at the R point. | ||
| Fig. 5 Optical properties of the 2D multilayer CH3NH3PbI3 perovskites: (a) ε1(ω), (b) ε2(ω), (c) absorption coefficient and (d) reflectivity. | ||
In the optical reflectivity of the multilayer, shown in Fig. 5(d), the reflectivity spectra of multilayer CH3NH3PbI3 significantly enhance with an increase in photon energy. This reflects that the 1st peak is in the visible region and the 2nd highest peak is in the ultraviolet region of the spectrum. We observed that the maximum light is in the UV region, compared with the visible region. Therefore, the light absorption by the CH3NH3PbI3 surface has been greatly enhanced in the visible (2 eV to 3.26 eV) as well as UV regions.
4 Conclusions
The calculated structural, electronic, and optical properties of the multilayer CH3NH3PbI3 hybrid halide perovskite system are investigated comprehensively with varying thickness using the DFT method. The formation energies, band gaps, and effective masses vary with the corresponding mono-, bi-, tri-, and quadlayer. We also observed that from mono- to quadlayer the bandgap decreases because of the transformation from 2D towards 3D. Due to strong antibonding states in the iodine and lead, high carrier mobilities are found with corresponding small effective masses. Besides, the multilayer has good optical properties with an absorption coefficient of about 105 cm−1. The calculated absorption spectra lie between 248 nm and 496 nm, which leads to optical activity of the 2D multilayer CH3NH3PbI3 in the visible and ultraviolet regions. We believe that the reasonable absorption in the visible region with high carrier mobilities could make multilayer CH3NH3PbI3 a candidate material for efficient photovoltaic devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. R. K. and Y. A. S. are thankful to SERB, government of India, for a grant (number EEQ/2016/000217). We also thank (C-DAC) Pune for advanced computational facilities. We also gratefully acknowledge access to a high performance computing cluster (HPC) facility at Michigan Technological University (MTU).References
- S. Ahmad, P. K. Kanaujia, H. J. Beeson, A. Beeson, F. Deschler, D. Credgington, U. Steiner, G. V. Prakash and J. J. Baumberg, Strong photocurrent from two-dimensional excitons in solution-processed stacked perovskite semiconductor sheets, ACS Appl. Mater. Interfaces, 2015, 7, 25227–25236 CrossRef CAS PubMed.
- M. Alidaei, M. Izadifard, M. E. Ghazi and V. Ahmadi, Efficiency enhancement of perovskite solar cells using structural and morphological improvement of CH3NH3PbI3 absorber layers, Mater. Res. Express, 2018, 5, 016412 CrossRef.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites, Science, 2012, 338, 643–647 CrossRef CAS PubMed.
- M. Jiang, N. Deng, L. Wang, H. Xie and Y. Qiu, The structural, electronic, and optical properties of organic–inorganic mixed halide perovskites CH3NH3Pb (I1−yXy)3 (X = Cl, Br), Chin. Phys. B, 2018, 27, 067102 CrossRef.
- M. E. Kamminga, H.-H. Fang, M. A. Loi, G. H. Ten Brink, G. R. Blake, T. T. Palstra and J. E. Ten Elshof, Micropatterned 2D hybrid perovskite thin films with enhanced photoluminescence lifetimes, ACS Appl. Mater. Interfaces, 2018, 10, 12878–12885 CrossRef CAS.
- R. K. Misra, B. E. Cohen, L. Iagher and L. Etgar, Low-dimensional organic–inorganic halide perovskite: structure, properties, and applications, ChemSusChem, 2017, 10, 3712–3721 CrossRef CAS PubMed.
- X. Y. Chin, D. Cortecchia, J. Yin, A. Bruno and C. Soci, Lead iodide perovskite light-emitting field-effect transistor, Nat. Commun., 2015, 6, 1–9 CrossRef.
- Z. Wang, J. Liu, Z.-Q. Xu, Y. Xue, L. Jiang, J. Song, F. Huang, Y. Wang, Y. L. Zhong and Y. Zhang, Wavelength-tunable waveguides based on polycrystalline organic–inorganic perovskite microwires, Nanoscale, 2016, 8, 6258–6264 RSC.
- E. J. Yoo, M. Lyu, J. H. Yun, C. J. Kang, Y. J. Choi and L. Wang, Resistive switching behavior in organic–inorganic hybrid CH3NH3PbI3−xClx perovskite for resistive random access memory devices, Adv. Mater., 2015, 27, 6170–6175 CrossRef CAS PubMed.
- G. Xing, N. Mathews, S. S. Lim, N. Yantara, X. Liu, D. Sabba, M. Grätzel, S. Mhaisalkar and T. C. Sum, Low-temperature solution-processed wavelength-tunable perovskites for lasing, Nat. Mater., 2014, 13, 476–480 CrossRef CAS PubMed.
- Y. Fang, Q. Dong, Y. Shao, Y. Yuan and J. Huang, Highly narrowband perovskite single-crystal photodetectors enabled by surface-charge recombination, Nat. Photonics, 2015, 9, 679–686 CrossRef CAS.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal halide perovskites as visible-light sensitizers for photovoltaic cells, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS.
- C. Quarti, G. Grancini, E. Mosconi, P. Bruno, J. M. Ball, M. M. Lee, H. J. Snaith, A. Petrozza and F. De Angelis, The Raman spectrum of the CH3NH3PbI3 hybrid perovskite: interplay of theory and experiment, J. Phys. Chem. Lett., 2014, 5, 279–284 CrossRef CAS.
- Y.-Q. Zhao, Q.-R. Ma, B. Liu, Z.-L. Yu, J. Yang and M.-Q. Cai, Layer-dependent transport and optoelectronic property in two-dimensional perovskite: (PEA)2PbI4, Nanoscale, 2018, 10, 8677–8688 RSC.
- J.-H. Lei, Y.-Q. Zhao, Q. Tang, J.-G. Lin and M.-Q. Cai, High transport and excellent optical property of a two-dimensional single-layered hybrid perovskite (C4H9NH3)2PbBr4: a theoretical study, Phys. Chem. Chem. Phys., 2018, 20, 13241–13248 RSC.
- S. Yang, W. Fu, Z. Zhang, H. Chen and C.-Z. Li, Recent advances in perovskite solar cells: efficiency, stability and lead-free perovskite, J. Mater. Chem. A, 2017, 5, 11462–11482 RSC.
- A. W. Sanches, M. A. da Silva, N. J. Cordeiro, A. Urbano and S. A. Lourenço, Effect of intermediate phases on the optical properties of PbI2-rich CH3NH3PbI3 organic–inorganic hybrid perovskite, Phys. Chem. Chem. Phys., 2019, 21, 5253–5261 RSC.
- J. Feng and B. Xiao, Crystal structures, optical properties, and effective mass tensors of CH3NH3PbX3 (X = I and Br) phases predicted from HSE06, J. Phys. Chem. Lett., 2014, 5, 1278–1282 CrossRef CAS.
- C. Motta, F. El-Mellouhi and S. Sanvito, Charge carrier mobility in hybrid halide perovskites, Sci. Rep., 2015, 5, 1–8 Search PubMed.
- G. Giorgi, J.-I. Fujisawa, H. Segawa and K. Yamashita, Small photocarrier effective masses featuring ambipolar transport in methylammonium lead iodide perovskite: a density functional analysis, J. Phys. Chem. Lett., 2013, 4, 4213–4216 CrossRef CAS PubMed.
- G. Kakavelakis, E. Kymakis and K. Petridis, 2D materials beyond graphene for metal halide perovskite solar cells, Adv. Mater. Interfaces, 2018, 5, 1800339 CrossRef.
- P. Li, B. Shivananju, Y. Zhang, S. Li and Q. Bao, High performance photodetector based on 2D CH3NH3PbI3 perovskite nanosheets, J. Phys. D: Appl. Phys., 2017, 50, 094002 CrossRef.
- F. Biccari, F. Gabelloni, E. Burzi, M. Gurioli, S. Pescetelli, A. Agresti, A. E. Del Rio Castillo, A. Ansaldo, E. Kymakis and F. Bonaccorso, Graphene-based electron transport layers in perovskite solar cells: a step-up for an efficient carrier collection, Adv. Energy Mater., 2017, 7, 1701349 CrossRef.
- J. Yan, W. Qiu, G. Wu, P. Heremans and H. Chen, Recent progress in 2D/quasi-2D layered metal halide perovskites for solar cells, J. Mater. Chem. A, 2018, 6, 11063–11077 RSC.
- D. Bi, C. Yi, J. Luo, J.-D. Décoppet, F. Zhang, S. M. Zakeeruddin, X. Li, A. Hagfeldt and M. Grätzel, Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%, Nat. Energy, 2016, 1, 1–5 Search PubMed.
- Y. Lee, S. Paek, K. T. Cho, E. Oveisi, P. Gao, S. Lee, J.-S. Park, Y. Zhang, R. Humphry-Baker and A. M. Asiri, Enhanced charge collection with passivation of the tin oxide layer in planar perovskite solar cells, J. Mater. Chem. A, 2017, 5, 12729–12734 RSC.
- B. Liu, Y.-Q. Zhao, Z.-L. Yu, L.-Z. Wang and M.-Q. Cai, Tuning the Schottky rectification in graphene-hexagonal boron nitride-molybdenum disulfide heterostructure, J. Colloid Interface Sci., 2018, 513, 677–683 CrossRef CAS PubMed.
- M. A. Green, Y. Hishikawa, E. D. Dunlop, D. H. Levi, J. Hohl-Ebinger and A. W. Ho-Baillie, Solar cell efficiency tables (version 52), Prog. Photovolt., 2018, 26, 427–436 CrossRef.
- C. Liu, W. Ding, X. Zhou, J. Gao, C. Cheng, X. Zhao and B. Xu, Efficient and stable perovskite solar cells prepared in ambient air based on surface-modified perovskite layer, J. Phys. Chem. C, 2017, 121, 6546–6553 CrossRef CAS.
- G. Lozano, The role of metal halide perovskites in next-generation lighting devices, J. Phys. Chem. Lett., 2018, 9, 3987–3997 CrossRef CAS PubMed.
- G. Mathiazhagan, L. Wagner, S. Bogati, K. Y. Ünal, D. Bogachuk, T. Kroyer, S. Mastroianni and A. Hinsch, Double-mesoscopic hole-transport-material-free perovskite solar cells: overcoming charge-transport limitation by sputtered ultrathin Al2O3 isolating layer, ACS Appl. Nano Mater., 2020, 3, 2463–2471 CrossRef CAS PubMed.
- R. Ansari, S. Malakpour and M. Faghihnasiri, Effects of in-plane electric field and temperature change on Young’s modulus of hexagonal boron nitride nanosheets with different chiralities, Superlattices Microstruct., 2014, 68, 16–26 CrossRef CAS.
- R. Ansari, S. Malakpour, M. Faghihnasiri and S. Sahmani, An ab initio investigation into the elastic, structural and electronic properties of MoS2 nanotubes, Superlattices Microstruct., 2015, 82, 188–200 CrossRef CAS.
- T. Hattori, T. Taira, M. Era, T. Tsutsui and S. Saito, Highly efficient electroluminescence from a heterostructure device combined with emissive layered-perovskite and an electron-transporting organic compound, Chem. Phys. Lett., 1996, 254, 103–108 CrossRef CAS.
- Z. Wang, A. M. Ganose, C. Niu and D. O. Scanlon, First-principles insights into tin-based two-dimensional hybrid halide perovskites for photovoltaics, J. Mater. Chem. A, 2018, 6, 5652–5660 RSC.
- L. Dou, A. B. Wong, Y. Yu, M. Lai, N. Kornienko, S. W. Eaton, A. Fu, C. G. Bischak, J. Ma and T. Ding, Atomically thin two-dimensional organic–inorganic hybrid perovskites, Science, 2015, 349, 1518–1521 CrossRef CAS.
- H. Tsai, W. Nie, J.-C. Blancon, C. C. Stoumpos, R. Asadpour, B. Harutyunyan, A. J. Neukirch, R. Verduzco, J. J. Crochet and S. Tretiak, High-efficiency two-dimensional Ruddlesden–Popper perovskite solar cells, Nature, 2016, 536, 312–316 CrossRef CAS PubMed.
- Z. Tan, Y. Wu, H. Hong, J. Yin, J. Zhang, L. Lin, M. Wang, X. Sun, L. Sun and Y. Huang, Two-dimensional (C4H9NH3)2PbBr4 perovskite crystals for high-performance photodetector, J. Am. Chem. Soc., 2016, 138, 16612–16615 CrossRef CAS.
- J. Zhou, Y. Chu and J. Huang, Photodetectors based on two-dimensional layer-structured hybrid lead iodide perovskite semiconductors, ACS Appl. Mater. Interfaces, 2016, 8, 25660–25666 CrossRef CAS PubMed.
- D. Wang, B. Wen, Y.-N. Zhu, C.-J. Tong, Z.-K. Tang and L.-M. Liu, First-principles study of novel two-dimensional (C4H9NH3)2PbX4 perovskites for solar cell absorbers, J. Phys. Chem. Lett., 2017, 8, 876–883 CrossRef CAS.
- L. Ma, J. Dai and X. C. Zeng, Two-Dimensional Single-Layer Organic–Inorganic Hybrid Perovskite Semiconductors, Adv. Energy Mater., 2017, 7, 1601731 CrossRef.
- M. G. Ju, J. Dai, L. Ma and X. C. Zeng, Perovskite chalcogenides with optimal bandgap and desired optical absorption for photovoltaic devices, Adv. Energy Mater., 2017, 7, 1700216 CrossRef.
- L. Zhang and W. Liang, How the structures and properties of two-dimensional layered perovskites MAPbI3 and CsPbI3 vary with the number of layers, J. Phys. Chem. Lett., 2017, 8, 1517–1523 CrossRef CAS PubMed.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef PubMed.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS.
- G. Kresse and D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- S. Grimme, Semiempirical GGA-type density functional constructed with a long-range dispersion correction, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, Hybrid functionals based on a screened Coulomb potential, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- M. Faghihnasiri, M. Izadifard and M. E. Ghazi, DFT study of electronic structure and optical properties of layered two dimensional CH3NH3PbX3 (X = Cl, Br, I), Energy Sources, Part A, 2019, 41, 2734–2745 CrossRef CAS.
- W.-J. Yin, J.-H. Yang, J. Kang, Y. Yan and S.-H. Wei, Halide perovskite materials for solar cells: a theoretical review, J. Mater. Chem. A, 2015, 3, 8926–8942 RSC.
- S. R. Kumavat, Y. Sonvane, D. Singh and S. K. Gupta, Two-dimensional CH3NH3PbI3 with high efficiency and superior carrier mobility: a theoretical study, J. Phys. Chem. C, 2019, 123, 5231–5239 CrossRef CAS.
- Y. Yamada, T. Nakamura, M. Endo, A. Wakamiya and Y. Kanemitsu, Photocarrier recombination dynamics in perovskite CH3NH3PbI3 for solar cell applications, J. Am. Chem. Soc., 2014, 136, 11610–11613 CrossRef CAS.
- W. Travis, E. Glover, H. Bronstein, D. Scanlon and R. Palgrave, On the application of the tolerance factor to inorganic and hybrid halide perovskites: a revised system, Chem. Sci., 2016, 7, 4548–4556 RSC.
- M. Dressel and M. Scheffler, Verifying the Drude response, Ann. Phys., 2006, 15, 535–544 CrossRef CAS.
- Y. Yamada, T. Yamada, L. Q. Phuong, N. Maruyama, H. Nishimura, A. Wakamiya, Y. Murata and Y. Kanemitsu, Dynamic optical properties of CH3NH3PbI3 single crystals as revealed by one-and two-photon excited photoluminescence measurements, J. Am. Chem. Soc., 2015, 137, 10456–10459 CrossRef CAS.
- G. Wang, R. Pandey and S. P. Karna, Carbon phosphide monolayers with superior carrier mobility, Nanoscale, 2016, 8, 8819–8825 RSC.
- A. Kumar, G. Sachdeva, R. Pandey and S. P. Karna, Optical absorbance in multilayer two-dimensional materials: graphene and antimonene, Appl. Phys. Lett., 2020, 116, 263102 CrossRef CAS.
- B. Veal and A. Paulikas, Optical properties of molybdenum. I. Experiment and Kramers–Kronig analysis, Phys. Rev. B: Solid State, 1974, 10, 1280 CrossRef CAS.
- G. Grancini, C. Roldán-Carmona, I. Zimmermann, E. Mosconi, X. Lee, D. Martineau, S. Narbey, F. Oswald, F. De Angelis and M. Graetzel, One-year stable perovskite solar cells by 2D/3D interface engineering, Nat. Commun., 2017, 8, 1–8 CrossRef PubMed.
- H.-S. Kim, C.-R. Lee, J.-H. Im, K.-B. Lee, T. Moehl, A. Marchioro, S.-J. Moon, R. Humphry-Baker, J.-H. Yum and J. E. Moser, Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%, Sci. Rep., 2012, 2, 1–7 Search PubMed.
- D. Demchenko, N. Izyumskaya, M. Feneberg, V. Avrutin, Ü. Özgür, R. Goldhahn and H. Morkoç, Optical properties of the organic–inorganic hybrid perovskite CH3NH3PbI3: Theory and experiment, Phys. Rev. B, 2016, 94, 075206 CrossRef.
- L. M. Herz, Charge-carrier mobilities in metal halide perovskites: fundamental mechanisms and limits, ACS Energy Lett., 2017, 2, 1539–1548 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Total density of states for mono-, bi-, tri-, quadlayers of 2D CH3NH3PbI3 (Fig. S1). SOC and HSE band structures (Fig. S2 and S3). Effect of applied tensile and compressive strain on formation energy (Fig. S4) and on band gap (CBM and VBM) as we increase the number of layers from mono to quad (Fig. S5–S9). Fig. S10 presents variation in CBM and VBM under mechanical strain. Fig. S11 presents optical behavior of the multilayer CH3NH3PbI3 system. See https://doi.org/10.1039/d2ra02874c |
| This journal is © The Royal Society of Chemistry 2022 |