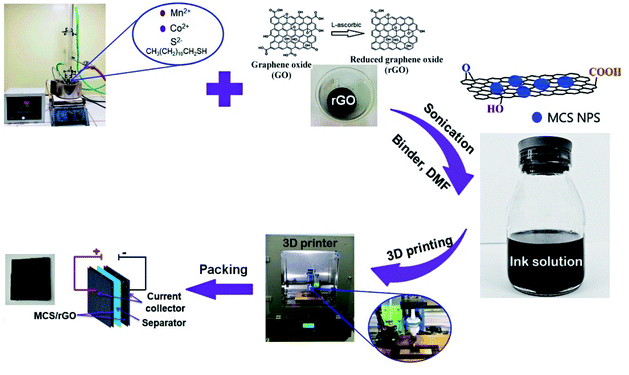
Open Access Article
Open Access ArticleHigh electrochemical performance of ink solution based on manganese cobalt sulfide/reduced graphene oxide nano-composites for supercapacitor electrode materials†
Le Thi Thanh Tam a,
Doan Thanh Tung
a,
Doan Thanh Tung *ab,
Ha Minh Nguyetab,
Nguyen Thi Ngoc Linhc,
Ngo Thanh Dung
*ab,
Ha Minh Nguyetab,
Nguyen Thi Ngoc Linhc,
Ngo Thanh Dung a,
Nguyen Van Quynhd,
Nguyen Van Dangc,
Dimitra Vernardoue,
Top Khac Le
a,
Nguyen Van Quynhd,
Nguyen Van Dangc,
Dimitra Vernardoue,
Top Khac Le fg,
Le Anh Tuan
fg,
Le Anh Tuan h,
Phan Ngoc Minh*b and
Le Trong Lu
h,
Phan Ngoc Minh*b and
Le Trong Lu *ab
*ab
aInstitute for Tropical Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Hanoi 1000, Vietnam. E-mail: dtungtnt167@gmail.com; ltlu@itt.vast.vn
bGraduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Hanoi 1000, Vietnam. E-mail: pnminh@vast.vn
cThai Nguyen University of Sciences, Tan Thinh Ward, Thai Nguyen City 25000, Thai Nguyen, Vietnam
dUniversity of Science and Technology of Hanoi, Vietnam Academy of Science and Technology, Hanoi 1000, Vietnam
eDepartment of Electrical and Computer Engineering, School of Engineering, Hellenic Mediterranean University, 71410 Heraklion, Greece
fFaculty of Materials Science and Technology, University of Science, Ho Chi Minh City, 700000, Viet Nam
gVietnam National University, Ho Chi Minh City, 700000, Viet Nam
hPhenikaa University, Nguyen Thanh Binh Street, Yen Nghia Ward, Ha Dong District, Hanoi, 12116, Vietnam
First published on 13th July 2022
Abstract
Large scale supercapacitor electrodes were prepared by 3D-printing directly on a graphite paper substrate from ink solution containing manganese cobalt sulfide/reduced graphene oxide (MCS/rGO) nanocomposites. The MCS/rGO composite solution was synthesized through the dispersion of MCS NPs and rGO in dimethylformamide (DMF) solvent at room temperature. Their morphology and composition were investigated by scanning electron microscopy (SEM), transmission electron microscopy (TEM), and energy dispersive X-ray diffraction (EDS). The role of rGO on decreasing charge transfer resistance and enhancing ion exchange was discussed. The MCS/rGO electrode exhibits an excellent specific capacitance of 3812.5 F g−1 at 2 A g−1 and it maintains 1780.8 F g−1 at a high current density of 50 A g−1. The cycling stability of the electrodes reveals capacitance retention of over 92% after 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 50 A g−1.
000 cycles at 50 A g−1.
1. Introduction
Recently, electrochemical energy storage and conversion devices such as batteries, fuel cells, and supercapacitors have been widely studied for portable electronic devices, electric vehicles, storage and backup equipment, and emergency power systems.1–3 Among various energy devices, supercapacitors (SCs) are of particular interest due to their superior properties such as super charging/discharging ability and high-power density.4–6 Nevertheless, SC systems still exhibit drawbacks such as low specific energy density and high cost, leading to large size and difficult practical application.7,8 Therefore, scientists often combine a pseudocapacitive mechanism with a double-layer capacitive mechanism to improve the quality of supercapacitors.9–12Owing to their high electrical conductivity, large theoretical specific capacitance, and low cost, transition metal sulfides such as CoS, MnS, NiS, MoS, CuCo2S4 and MnCo2S4 have drawn growing attention as intriguing electrode materials for SCs.5,13–16 Among them, binary metal sulfides have attracted more interest due to their multiple faradaic redox reactions.3 Furthermore, these sulfide compounds present higher electrical conductivity than their monometal sulfide or oxide counterparts because the introduction of sulfur reduces the band-gap and facilitates electron transport.17
Chen et al. prepared three types of hollow tubular MxCo3−xS4 structures (M = Mn, Ni, and Zn) for supercapacitor, where MnCo2S4 exhibits the best electrochemical performance with the specific capacitance of 1094 F g−1 at 10 A g−1.18 The fabrication of MCS material with various morphologies using hydrothermal, solvothermal, and electrochemical deposition has also been reported with specific capacitance up to 2400 F g−1.19–21 One can realize that it has not been studied much as a promising electrode for SCs, in spite of the fact that cobalt provides a larger oxidation potential and manganese delivers more electrons favoring the rate capacity.22 To date, the development of MCS electrode material is restricted because of their low rate performance at high current densities and short cycling stability.23
In order to overcome the limitations mentioned above about MCSs, one of the favorable approaches is the combination of MCS with nanocarbon-based material. Among carbon-based materials, reduced graphene oxide (rGO) has been widely applied as an efficient electrode material for SCs because of its great chemical stability, excellent electrical conductivity, and higher specific surface area.3,24–26 As a consequence, the electrochemical performance of cathodes is expected to be improved due to the presence rGO.27–31 In the MCS/rGO structure, MCS acts as a supporting agent to prevent the re-stacking of rGO sheets, while rGO acts as an interconnected porous matrix to support the anchoring of pseudo-charge ternary metal sulfides and provide excellent electron transport channels, resulting in improved capacitance, cycling stability, and rate capability. Currently, MCS/rGO nanocomposites can be fabricated by growing MCS materials directly on rGO surface or by the simple mixing of rGO with the as-prepared MCS materials.28,29 However, in order to maximize the electrochemical performance of MCS materials, precisely controlled shape, size, composition and high phase purity are required. Therefore, it is better to prepare monodisperse MCS nanomaterials first and then assemble them onto rGO sheets, giving MCS/rGO composites with MCS size, shape, and composition well-defined.30
In our previous works,32–35 high quality porous CuCo2S4–rGO electrode material for SCs was successfully fabricated by 3D printing technique. CuCo2S4 NPs were in situ grown on the surface of GO sheets under hydrothermal condition. The CuCo2S4–GO nanocomposite was then purified and redispersed in phenol to form printing ink solution. However, phenol is not good solvent for the dispersion of graphene materials due to their natural hydrophilic surface, which leads to irreversible agglomeration or restack of graphene sheets.36,37 In addition, hydrothermal method requires autoclave leading to difficulties in scale-up. Thus, a judicious selection of a proper organic solvent with a strong interaction on the graphene sheets will possibly increase the dispersion and colloidal stability of the material in solution. In this work, dimethylform-amide (DMF) solvent was used to disperse materials in the form of rGO/MCS mixture at room temperature. Different electrode materials were fabricated by 3D-printer and their electrochemical characteristics were analyzed. The role of DMF solution of polyvinylpyrrolidone (PVP) to form MCS NPs/rGO sheets composites for SCs was clarified.
2. Experimental
2.1. Chemicals
The chemicals, manganese(II) chloride tetrahydrate 99%, cobalt(II) chloride hexahydrate 99%, 1-dodecanethiol 98% (DDT), 1-octadecene (ODE), dimethylform-amide 99% (DMF), graphite flakes, KMnO4 99.5%, H2SO4 98%, H2O2 30%, HCl, ascorbic acid 99%, polyvinylpyrrolidone (PVP), ethanol, and methanol were purchased from Sigma-Aldrich and directly used as received without any further purification. The graphite papers were supplied by Xiamen TOB new energy technology Co., Ltd, China.2.2. Fabrication of MCS–rGO ink solution
The whole process for the preparation of MCS/rGO electrode is shown in Fig. 1. The GO sheets were prepared by modified Hummer's method.31,32 Typically, 1.0 g of nature graphite flakes and 1.0 g of KMnO4 were mixed in 35 mL of H2SO4 solution under overnight stirring. The reaction mixture was diluted in 300 mL of deionized water and 25 mL of H2O2. The solution was subsequently washed in 10% HCl under ultrasonic conditions. The GO solution was finally reduced by ascorbic acid to form rGO.MCS NPs were synthesized by a thermal decomposition method with 1-dodecanethiol (DDT) acting as both sulfur source and surfactant.32–34 Briefly, manganese(II) chloride tetrahydrate and cobalt(II) chloride hexahydrate with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio were added into three-neck, round bottom flask containing 1-octadecene (30 mL) and evacuated at room temperature for 30 min. The mixture was then heated to 140 °C, following with the quick injection of dodecanethiol (6 mL) as a sulfur source. The temperature of the reaction mixture was raised to 290 °C and kept constant for 60 min under continuous stirring. The solution was then cooled to room temperature. The MCS NPs were washed several times using a mixture of ethanol and methanol by centrifuging at 10
2 molar ratio were added into three-neck, round bottom flask containing 1-octadecene (30 mL) and evacuated at room temperature for 30 min. The mixture was then heated to 140 °C, following with the quick injection of dodecanethiol (6 mL) as a sulfur source. The temperature of the reaction mixture was raised to 290 °C and kept constant for 60 min under continuous stirring. The solution was then cooled to room temperature. The MCS NPs were washed several times using a mixture of ethanol and methanol by centrifuging at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to obtain a black product. Finally, NOBF4 was used as an exchange ligand to modify the surface of MCS NPs prior their dispersion into DMF solvent.
000 rpm for 5 min to obtain a black product. Finally, NOBF4 was used as an exchange ligand to modify the surface of MCS NPs prior their dispersion into DMF solvent.
2.3. Fabrication of MCS–rGO electrode by 3D printing method
The ink solution based on MCS–rGO composites was fabricated by mixing 90 wt% active materials (including MCS NPs and rGO with the weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 10 wt% PVP as a binder in DMF solvent to form a printable solution.30,38 The ink solution was coated on the graphite paper substrate of 2 × 1 cm2 as the current collector by a modified 3D printer and kept for drying at around 100 °C to form MCS/rGO electrode.
1) and 10 wt% PVP as a binder in DMF solvent to form a printable solution.30,38 The ink solution was coated on the graphite paper substrate of 2 × 1 cm2 as the current collector by a modified 3D printer and kept for drying at around 100 °C to form MCS/rGO electrode.
2.4. Characterization
The morphology and elemental composition of the samples were analyzed using a transmission electron microscopy (TEM, JEM1010-JEOL), scanning electron microscopy (SEM, Hitachi S-4800) equipped with an energy-dispersive X-ray (EDX) spectroscopy. Crystalline structures of as-synthesized samples (rGO, MCS, and MCS/rGO) were characterized by X-ray diffraction analysis with Cu-Kα radiation and λ = 1.5406 Å. The surface areas of MCS material was determined by TriStar II 3020 device and calculated by the Brunauer–Emmett–Teller (BET) method.The electrochemical properties of the electrodes were evaluated by cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) techniques. The measurements were performed in a three-electrode configuration system using 6 M KOH solution as the electrolyte. The printed MCS–rGO nanocomposites electrode was used as the working electrode with the size of 2 × 1 cm2, while the counter electrode was a platinum foil, and the reference electrode was a standard calomel electrode (SCE). The electrochemical potential window for all CV measurements at different scan rates was set in the range of −0.9 V to 0.1 V. EIS was also performed to study the synthesized material at a frequency ranging from 10 mHz to 100 kHz.
A symmetric full-cell supercapacitor assembled with two identical MnCo2S4–rGO electrodes, cellulose paper separator and 6 M KOH electrolyte, was tested in a two-electrode system.
3. Results and discussions
3.1. Morphology and phase structure
The TEM image of the MCS NPs (Fig. 2a) shows spherical nanoparticles with a mean size of 10 nm. The small size, high uniformity of MCS NPs with large specific surface area, will be easy to disperse uniformly when combined with rGO material in the ink solution facilitating more electrochemical active sites for faradaic redox reaction process.The MCS/rGO ink solution in the DMF solvent is seen more intensely in Fig. 2b. Also, in Fig. 2c and d SEM images of rGO and MCS/rGO composites can be observed. It can be noticed that the rGO sheets are very thin with a wrinkled silk-like morphology and the MCS NPs are densely deposited on the surface of the rGO sheet after the mixing of MCS NPs and rGO sheets by ultrasonic vibration. The good dispersion of the MCS NPs on the surface of the rGO sheets prevents the re-stacking of the rGO sheets and forms a large amount of void space, creating an interlaced structures in the composite, which is conducive to excess electrolyte interaction and redox reactions at high current densities. This is in accordance with the aforementioned results for the electrochemical properties of the MCS/rGO electrode.
The combination of rGO and MCS nanoparticles aims to enhance the specific surface area of the supercapacitor electrode. Fig. S1† shows the results of the surface analysis with a high BET surface area value of 836 m2 g−1. This demonstrates that the intercalation of MCS nanoparticles between the rGO sheets significantly increases the specific surface area and leads to a higher increase in the double-layer capacitance value.
Fig. 3a shows XRD patterns of the rGO, MCS, and MCS/rGO. The XRD spectrum of rGO samples consists of only one peak at 2θ = 26.5° corresponding to graphene, indicating the effective reduction of GO to rGO.34 Simultaneously, the XRD data of MCS NPs presents multi peaks around 30.9°, 38.2°, 49.9°, and 55.3°, which are indexed to (311), (400), (511), and (440) planes of Co3S4 (JCPDS no. 42-1448).3 In addition, there are no diffraction peaks of manganese sulfide, demonstrating manganese element has partially replaced cobalt ions in the lattice structure. Therefore, the chemical formula of MCS could be speculated to MnxCo3−xS4 where 0 < x < 3. XRD spectrum of MCS/rGO composites reveals the same peak positions with MSC sample, but with lower intensity due to the construction of rGO with MCS NPs.
 | ||
| Fig. 3 (a) XRD patterns of rGO, MCS and MCS/rGO composite; (b) EDS spectrum of the MCS/rGO composite; (c) EDS mapping of the MCS/rGO composite. | ||
Furthermore, the composition and purities of the MCS/rGO composites are confirmed by EDX. Fig. 3b reveals the composition of Co, Mn, S, C, and O. The atomic ratio of Co/Mn = 8.07/3.84 is very close to the Co/Mn ratio in MnCo2S4. In the thermal decomposition reaction, DDT not only acts as a source of sulfur, but also as a capping agent and surfactant. It is even used as a reducing agent in the synthesis of MCS NPs. ODE is employed as the reaction medium for the chloride salt precursors to allow the reactions to be carried out.
In order to investigate the distribution of components in the ink, the ink was printed on the graphite substrate and EDX mapping was performed. In Fig. 3c, the elements (C, Mn, Co and S) are very uniformly dispersed over the entire electrode surface. This proves that the MCS nanoparticles and rGO are well dispersed in the DMF solvent.
3.2. Electrochemical properties
Fig. 4a compares the electrochemical characteristics of four electrodes, i.e. graphite substrate, rGO/graphite substrate (denoted as rGO), MCS/graphite substrate (denoted as MCS), and MCS/rGO/graphite substrate (denoted as MCS/rGO). The capacitance contribution of the graphite paper substrate is negligible and can be ignored. The rGO material contributes almost exclusively to the electric double-layer effect. The CV curve of rGO presents a quasi-rectangular shape and has no obvious redox peaks showing similar behavior with carbon-based materials.39 Meanwhile, the MCS material shows the ability to contribute both electric double-layer and pseudo-capacitive effects. It has a rather high redox peak at a potential of about −0.05 V. The specific capacitances of MCS, rGO and MCS/rGO are 2097, 409, and 3338.5 F g−1 at the scan rate of 5 mV s−1, respectively. It can be seen that the capacitance value increases significantly when combining MCS nanoparticles with rGO. This is possible due to the intercalation of rGO sheets with MCS nanoparticles increasing the contact area of the supercapacitor electrode with the electrolyte and increasing the charge transfer capacity. Therefore, the synergy of rGO and MCS is important to obtain superiority in performance. The CV curve of the MCS/rGO composite shows that the area of the CV curve has been increased and still retains the typical redox peaks of MCS. The redox peaks of MCS and MCS/rGO electrode originate from the interaction between S2−, Co2+/Co3+/Co4+ and Mn2+/Mn3+ ions, which is described by the following reversible reactions:40–46| MS + OH− ↔ MSOH + e− (M = Co, Mn) |
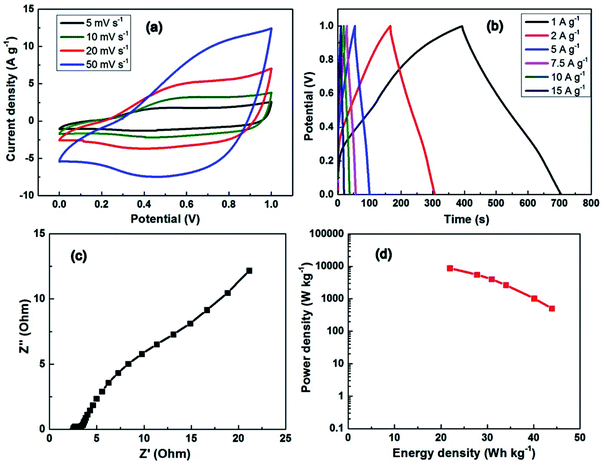
A lot of pseudo-capacitive materials for supercapacitors are usually quite sensitive to the effects of voltage and current changes during their operation because these are related to the speed and efficiency of the reversible reactions. The CV curves of MCS/rGO (Fig. 4b) at different scan rates from 5 to 50 mV s−1 in the potential window of −0.9–0.1 V (vs. SCE) present a similar quasi-rectangular shape with the distinct pairs of redox peaks, indicating that the capacitance is attributed to the combination of EDLC mechanism of rGO and pseudocapacitance mechanism of manganese cobalt sulfide material. The curves also reveal the increase of the current densities with increasing scan rates, proving excellent diffusion of ions at the interface between the electrode and the electrolyte. Furthermore, the positions of the oxidation and reduction peaks move to higher and lower potentials respectively when the scan rate increases, which is mainly attributed to the polarization effect of the electrodes.5,47 Additionally, the shape of CV curves is maintained even at the high scan rate of 50 mV s−1, indicating superior electron transport kinetics, and excellent rate performance of MCS/rGO electrode.
Fig. 4c illustrates the GCD plots of the as-synthesized MCS/rGO electrode at various current densities ranging from 2 to 50 A g−1. According to the GCD curves, the specific capacitance (Csp) can be acquired as shown below:48
Cycle endurance is a key parameter to evaluate the quality of SCs. As a result, cycling stability test for MCS/rGO electrode was carried out using constant current density of 50 A g−1 as shown in Fig. 5. MCS/rGO electrode displays great stability of 92.2% after 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles. The enhancement in capacitance retention has been attributed to the electrochemical activation of the electrode surface, which is facilitated by the wettability of electrode material and electrolyte ions.49 In addition, the hybrid MCS–rGO materials expanded the interlayer structure showing faster ion diffusion.50,51 The gradual decrement in specific capacitance is mainly due to the conversion of metal sulfide to hydroxide under alkaline medium as well as the shedding and dissolution of the active material. In our recent study, the MCS-single nanosheet material only maintained 93% capacitance retention after 5000 duty cycles.16 Therefore, the MCS material is degraded after undergoing a reversible redox reaction. Fig. S2† shows the CV curve of MCS/rGO before and after 22
000 charge–discharge cycles. The enhancement in capacitance retention has been attributed to the electrochemical activation of the electrode surface, which is facilitated by the wettability of electrode material and electrolyte ions.49 In addition, the hybrid MCS–rGO materials expanded the interlayer structure showing faster ion diffusion.50,51 The gradual decrement in specific capacitance is mainly due to the conversion of metal sulfide to hydroxide under alkaline medium as well as the shedding and dissolution of the active material. In our recent study, the MCS-single nanosheet material only maintained 93% capacitance retention after 5000 duty cycles.16 Therefore, the MCS material is degraded after undergoing a reversible redox reaction. Fig. S2† shows the CV curve of MCS/rGO before and after 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 duty cycles. It can be seen that the redox peak has been reduced, but the double layer capacitance is almost unchanged.
000 duty cycles. It can be seen that the redox peak has been reduced, but the double layer capacitance is almost unchanged.
Electrochemical impedance spectrum analysis was also performed and an equivalent circuit model (ECM) for the MCS/rGO electrode was built accordingly. Fig. 6 shows the EIS Nyquist plot of the MCS/rGO electrode (including graphite paper substrate) and compares its EIS curve with equivalent circuit parameters before and after 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 duty cycles. The Nyquist curve is composed of a semicircle in the high-frequency region and a near linear in the low-frequency region. The smaller the radius of a semicircle, the lower the charge transfer resistance, and the larger the slope of the line results to better performance.5,39 The inset in Fig. 6a shows an ECM, which has been matched to the measured EIS spectrum. The model includes: an equivalent series resistance (Rs); a resistance due to the redox reactions at the electrode/electrolyte interface (Rct); a leak resistance (RL); a double-layer capacitance (CDL); a constant phase element (CPEL) of non-ideal capacitor made from the junction between the active material and the graphite substrate; and a Warburg diffusion element (W) – representing the resistance of ionic diffusion in the electrolyte.35 With this model, very low values of Rs (1.087 Ω) and Rct (0.025 Ω) indicate good conductivities of MCS/rGO, along with easy ion exchange for reversible redox reactions. Thereby, the reversible redox reactions occur easily and repeat steadily during the charge/discharge process. In Fig. 6b, EIS of MCS/rGO electrode after 22
000 duty cycles. The Nyquist curve is composed of a semicircle in the high-frequency region and a near linear in the low-frequency region. The smaller the radius of a semicircle, the lower the charge transfer resistance, and the larger the slope of the line results to better performance.5,39 The inset in Fig. 6a shows an ECM, which has been matched to the measured EIS spectrum. The model includes: an equivalent series resistance (Rs); a resistance due to the redox reactions at the electrode/electrolyte interface (Rct); a leak resistance (RL); a double-layer capacitance (CDL); a constant phase element (CPEL) of non-ideal capacitor made from the junction between the active material and the graphite substrate; and a Warburg diffusion element (W) – representing the resistance of ionic diffusion in the electrolyte.35 With this model, very low values of Rs (1.087 Ω) and Rct (0.025 Ω) indicate good conductivities of MCS/rGO, along with easy ion exchange for reversible redox reactions. Thereby, the reversible redox reactions occur easily and repeat steadily during the charge/discharge process. In Fig. 6b, EIS of MCS/rGO electrode after 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles shows that Rs and Rct increased insignificantly (Rs is from 1.087 Ω to 1.172 Ω and Rct is from 0.025 Ω to 0.078 Ω). This further confirms the contribution of rGO in the material system. rGO had high strength and low loss during the working life of the electrode.
000 charge/discharge cycles shows that Rs and Rct increased insignificantly (Rs is from 1.087 Ω to 1.172 Ω and Rct is from 0.025 Ω to 0.078 Ω). This further confirms the contribution of rGO in the material system. rGO had high strength and low loss during the working life of the electrode.
 | ||
Fig. 6 EIS of (a) MCS/rGO electrode and its equivalent circuit model, (b) MCS/rGO electrode before and after 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charging/discharging cycles. 000 charging/discharging cycles. | ||
The asymmetric supercapacitor with graphite powder as the negative electrode, MCS/rGO as the positive electrode and a cellulosic separator in 6 M KOH electrolyte was used to evaluate the electrochemical properties of the two-electrode system with working area of 2 × 1 cm2. Fig. 7 shows the CV curve, GCD charge–discharge characteristic, EIS Nyquist plot, and the relationship between the power and energy of the full-cell supercapacitor. The CV curve is taken over the voltage range from 0 V to 1 V. The CV curve swells at the high potential range showing the redox properties of the MCS/rGO materials. From the GCD curves of the asymmetric device (Fig. 7b), the calculated capacitances are 316.2, 288.7, 244.7, 222.6, 200, and 157.7 F g−1 at the current densities of 1, 2, 5, 7.5, 10, and 15 A g−1, respectively. The low specific capacitance and coulombic efficiency may be due to the reversible chemical reaction and ion exchange being limited by the separator and the two electrodes being tightly pressed together.21,52 The EIS spectrum also shows that the internal resistance of the device increased (about 2.5 Ω, Fig. 7c). And, the maximum energy density of device achieves 43.91 W h kg−1 at a power density of 505 W kg−1, and remains 21.9 W h kg−1 at 8762 W kg−1.
4. Conclusions
In summary, MCS NPs, rGO, and MCS/rGO composites have been successful fabricated. The ink solution based on MCS/rGO composites was produced by simple and effective synthetic method, and it was used in 3D printing technique to prepare the electrodes. These techniques are promising large-scale and rapid production for practical applications. The as-prepared MCS/rGO electrode with anchoring of MCS NPs on the surface of the rGO sheets forms an interwoven structure facilitating access and diffusion of the electrolyte ions during charge/discharge process. The electrochemical behavior test results of MCS/rGO electrode show an ultrahigh specific capacitance of 3812.5 F g−1 at a current density of 2 A g−1 and a remaining high value of 1780.8 F g−1 at a current density of 50 A g−1. The electrode also reveals the admirable cycling life time of 92.2% retention though 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at high current density (50 A g−1). This work demonstrates that MCS/rGO composites are promising electrode materials for energy storage devices such as supercapacitors in the future.
000 cycles at high current density (50 A g−1). This work demonstrates that MCS/rGO composites are promising electrode materials for energy storage devices such as supercapacitors in the future.
Author contributions
H. M. N., L. T. T. T. and N. T. N. L. synthesized the MCS nanoparticles. L. T. T. T. and D. T. T. prepared the printing ink, and were major contributors in writing the manuscript. N. V. D. and H. T. D. modified the 3D printer, designed and printed the supercapacitor electrodes. N. T. D. and L. A. T. performed the analysis and assessment of the morphology and structure. L. T. T. T. and D. T. T. performed the measurements and analyzed the electrochemical results. L. T. L. and N. V. Q. designed experiments. D. V. and T. K. L. worked on the manuscript. L. T. L. and P. N. M. helped to supervise the project and improved the manuscript. All the authors read and approved the final manuscript.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
This work was supported by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under Grant Number 103.02-2018.66.References
- J. Gao, X. Wang, X. Wang, R. Que, Y. Fang, B. Shi and Z. Wang, RSC Adv., 2016, 6, 68460–68467 RSC.
- H. Li, L. Shen, J. Wang, S. Fang, Y. Zhang, H. Dou and X. Zhang, J. Mater. Chem. A, 2015, 3, 16785–16790 RSC.
- X. Han, H. Xuan, J. Gao, T. Liang, J. Yang, Y. Xu, P. Han and Y. Du, Electrochim. Acta, 2018, 288, 31–41 CrossRef CAS.
- S. K. Sami, S. Siddiqui, M. T. Feroze and C. H. Chung, Mater. Res. Express, 2017, 4, 116309 CrossRef.
- F. Wang, K. Zhou, J. Zheng and J. Ma, Synth. Met., 2019, 256, 116113 CrossRef CAS.
- P. Sivasakthi, G. N. K. Ramesh Bapu, K. Murugavel and S. Mohan, J. Alloys Compd., 2017, 709, 240–247 CrossRef CAS.
- N. S. Arul, L. S. Cavalcante and J. In Han, J. Solid State Electrochem., 2017, 22, 303–313 CrossRef.
- J. E. Zuliani, C. Q. Jia and D. W. Kirk, J. Appl. Electrochem., 2017, 47, 1213–1226 CrossRef CAS.
- H. Li, H. Xuan, Y. Guan, G. Zhang, R. Wang, X. Liang, Z. Xie, P. Han and Y. Wu, Electrochim. Acta, 2020, 345, 136260 CrossRef CAS.
- Y. Liu, J. Zhang, S. Wang, K. Wang, Z. Chen and Q. Xu, New J. Chem., 2014, 38, 4045–4048 RSC.
- J. Pu, F. Cui, S. Chu, T. Wang, E. Sheng and Z. Wang, ACS Sustainable Chem. Eng., 2014, 2, 809–815 CrossRef CAS.
- H. Zhou, S. Zhu, M. Hibino and I. Honma, J. Power Sources, 2003, 122, 219–223 CrossRef CAS.
- K. J. Huang, J. Z. Zhang, G. W. Shi and Y. M. Liu, Mater. Lett., 2014, 131, 45–48 CrossRef CAS.
- X. Han, K. Tao, D. Wang and L. Han, Nanoscale, 2018, 10, 2735–2741 RSC.
- L. Liu, K. P. Annamalai and Y. Tao, N. Carbon Mater., 2016, 31, 336–342 CrossRef CAS.
- H. M. Nguyet, L. T. T. Tam, D. T. Tung, N. T. Yen, H. T. Dung, N. T. Dung, P. N. Hong, L. A. Tuan, P. N. Minh and L. T. Lu, Facile synthesis of MnCo2S4 nanosheets as a binder-free electrode material for high performance supercapacitor applications, New J. Chem., 2022 10.1039/D1NJ05809F.
- H. Wang, K. Zhang, Y. Song, J. Qiu, J. Wu and L. Yan, Carbon, 2019, 146, 420–429 CrossRef CAS.
- Y. M. Chen, Z. Li and X. W. D. Lou, Angew. Chem., Int. Ed., 2015, 54, 10521–10524 CrossRef CAS PubMed.
- Y. Zhao, Z. Shi, H. Li and C. A. Wang, J. Mater. Chem. A, 2018, 6, 12782–12793 RSC.
- F. Zhang, M. Cho, T. Eom, C. Kang and H. Lee, Ceram. Int., 2019, 45, 20972–20976 CrossRef CAS.
- S. Sahoo and C. S. Rout, Electrochim. Acta, 2016, 220, 57–66 CrossRef CAS.
- S. Liu and S. C. Jun, J. Power Sources, 2017, 342, 629–637 CrossRef CAS.
- L. G. Beka, X. Li and W. Liu, Sci. Rep., 2017, 7, 2105 CrossRef PubMed.
- H. Zhang, X. Zhang, D. Zhang, X. Sun, H. Lin, C. Wang and Y. Ma, J. Phys. Chem. B, 2013, 117, 1616–1627 CrossRef CAS PubMed.
- X. Zhu, H. Dai, J. Hu, L. Ding and L. Jiang, J. Power Sources, 2012, 203, 243–249 CrossRef CAS.
- T. Yao, X. Guo, S. Qin, F. Xia, Q. Li, Y. Li, Q. Chen, J. Li and D. He, Nano-Micro Lett., 2017, 9(4), 38 CrossRef PubMed.
- X. Cai, X. Shen, L. Ma, Z. Ji and L. Kong, RSC Adv., 2015, 5, 58777–58783 RSC.
- W. Peng, H. Chen, W. Wang, Y. Huang and G. Han, Curr. Appl. Phys., 2020, 20, 304–309 CrossRef.
- Y. M. Fan, Y. Liu, X. Liu, Y. Liu and L. Z. Fan, Electrochim. Acta, 2017, 249, 1–8 CrossRef CAS.
- Q. Li, N. Mahmood, J. Zhu, Y. Hou and S. Sun, Nano Today, 2014, 9, 668–683 CrossRef CAS.
- K. Fu, Y. Wang, C. Yan, Y. Yao, Y. Chen, J. Dai, S. Lacey, Y. Wang, J. Wan, T. Li, Z. Wang, Y. Xu and L. Hu, Adv. Mater., 2016, 28, 2587 CrossRef CAS PubMed.
- L. T. T. Tam, D. T. Tung, N. T. Dung, P. N. Hong, P. V. Hoi, P. N. Minh and L. T. Lu, Commun. Phys., 2020, 4, 399–408 Search PubMed.
- L. T. T. Tam, N. V. Hung, D. T. Tung, N. T. Dung, H. T. Dung, P. T. Nam, P. N. Minh, P. N. Hong and L. T. Lu, Vietnam J. Sci. Technol., 2019, 57, 58 CrossRef.
- D. T. Tung, L. T. T. Tam, H. T. Dung, N. T. Dung, N. Tuan-Dung, T. Hoang, T. D. Lam, P. N. Minh, T. V. Thu, P. N. Hong and L. T. Lu, J. Electron. Mater., 2020, 49, 4671 CrossRef CAS.
- D. T. Tung, L. T. T. Tam, H. T. Dung, N. T. Dung, P. N. Hong, H. M. Nguyet, N. Van-Quynh, N. V. Chuc, V. Q. Trung, L. T. Lu and P. N. Minh, Electrochim. Acta, 2021, 392, 138992 CrossRef CAS.
- Z. Tang, Z. Zhang, Z. Han, S. Shen, J. Li and J. Yang, J. Mater. Sci., 2016, 51, 8791–8798 CrossRef CAS.
- W. Wei and X. Qu, Small, 2012, 8, 2138–2151 CrossRef CAS PubMed.
- C. Wu, Q. Cheng, K. B. Wu, G. Wu and Q. Li, Anal. Chim. Acta, 2014, 825, 26 CrossRef CAS PubMed.
- T. K. Enock, C. K. King’ondu, A. Pogrebnoi and Y. A. C. Jande, Mater. Today Energy, 2017, 5, 126–137 CrossRef.
- X. Xia, C. Zhu, J. Luo, Z. Zeng, C. Guan, C. F. Ng, H. Zhang and H. J. Fan, Small, 2014, 10, 766–773 CrossRef CAS PubMed.
- T. Chen, Y. Tang, Y. Qiao, Z. Liu, W. Guo, J. Song, S. Mu, S. Yu, Y. Zhao and F. Gao, Sci. Rep., 2016, 6, 23289 CrossRef CAS PubMed.
- Y. Tang, T. Chen and S. Yu, Chem. Commun., 2015, 51, 9018–9021 RSC.
- Q. Zhang, C. Xu and B. Lu, Electrochim. Acta, 2014, 132, 180–185 CrossRef CAS.
- J. Shi, X. Li, G. He, L. Zhang and M. Li, J. Mater. Chem. A, 2015, 3, 20619–20626 RSC.
- Y. Zhu, Z. Wu, M. Jing, X. Yang, W. Song and X. Ji, J. Power Sources, 2015, 273, 584–590 CrossRef CAS.
- X. Li, J. Shen, N. Li and M. Ye, J. Power Sources, 2015, 282, 194–201 CrossRef CAS.
- R. Chen, L. Liu, J. Zhou, L. Hou and F. Gao, J. Power Sources, 2017, 341, 75–82 CrossRef CAS.
- Y. Zhu, X. Ji, H. Chen, L. Xi, W. Gong and Y. Liu, RSC Adv., 2016, 6, 84236–84241 RSC.
- W. Liu, H. Niu, J. Yang, K. Cheng, K. Ye, K. Zhu, G. Wang, D. Cao and J. Yan, Chem. Mater., 2018, 30, 1055–1068 CrossRef CAS.
- Q. Liu, X. Hong, X. You, X. Zhang, X. Zhao, M. Ye and X. Liu, Energy Storage Mater., 2020, 24, 541–549 CrossRef.
- C. Zhan, W. Liu, M. Hu, Q. Liang, X. Yu, Y. Shen, R. Lv and F. Kang, NPG Asia Mater., 2018, 10, 775–787 CrossRef CAS.
- X. Hu, S. Liu, Y. Chen, J. Jiang, H. Cong, J. Tang, Y. Sun, S. Han and H. Lin, New J. Chem., 2020, 44, 11786 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02818b |
| This journal is © The Royal Society of Chemistry 2022 |