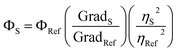Open Access Article
Open Access ArticleEumelanin pigment precursor 2-carboxy-5,6-dihydroxyindole and 2-amino-6-methylbenzothiazole chromophore integration towards melanin inspired chemoresponsive materials: the case of the Zn2+ ion†
Fabio Mocerinoab,
Alessandro Pezzella *bc and
Ugo Carusoa
*bc and
Ugo Carusoa
aDepartment of Chemical Sciences, University of Naples “Federico II” Via Cintia 4, I-80126 Naples, Italy
bBioelectronics Task Force at University of Naples Federico II, Italy
cDepartment of Physics “Ettore Pancini” Complesso Universitario Monte S. Angelo, Via Cintia, I-80126 Naples, Italy. E-mail: alessandro.pezzella@unina.it; Tel: +39 081 674130
First published on 21st July 2022
Abstract
The 2-amino-6-methylbenzothiazole chromophore is introduced at the carboxyl group of the melanin precursor 2-carboxy-5,6-dihydroxyindole achieving a novel dihydroxyindole derivative with metal chelation properties not depending on the catechol moiety. In view of potential exploitation in charge storage systems, systematic investigation of the interaction of the new amide derivative with metal ions is carried out, in comparison with that of the parent 2-carboxy-5,6-dihydroxyindole, and the stoichiometry of the zinc-amide complex is determined.
Introduction
The change in the optical properties of a material following the action of an electric field, as well as chemical interactions, is gaining more and more interest in view of the design and fabrication of active organic layers capable of integrating their actual1 function into devices, with the monitoring of the operational conditions.2–4The investigation of chemical physical properties of functional materials when acting into working device, as well as the measurement of their redox state or even the modulation the material conductivity, may take advantage on the selective introduction of chromophores and/or fluorophores into the active material.5 More generally, the developing of multicomponent materials to integrate different and independent properties into the hybrid system, as well as to obtain additional properties emerging from the interaction of the components, is a chief goal of the research in organic electronics.5,6
Eumelanins, the black insoluble pigments of human skin and eyes, are emerging as a valuable source for the design and fabrication of active layers for application in organic electronics.7,8 Their natural origin and a peculiar set of physicochemical properties, i.e. broadband absorption in the UV-visible range, intrinsic free radical character, water-dependent hybrid ionic–electronic conductor behavior, are at the root of this interest.9
From chemical point of view, eumelanins are the product of oxidative polymerization of 5,6-dihydroxyindole (DHI) and its 2-carboxy derivative (DHICA, 1).10 Recent studies disclosed noteworthy potentials of eumelanin integration into organic electronic devices11 and in particular the ability of DHICA derived melanin to act as positive dopant towards PEDOT:PSS conducting layers.12 Possible exploitation of eumelanin and derivatives as electrodes material within batteries is recently gaining interests in view of possible biocompatible applications.13 In this context in particular, because of the difficulty to design an organic polymeric electrode that can act as a Zn2+ acceptor,14 eumelanin derived coatings are under investigation as a cathode material for zinc ion-based batteries.15
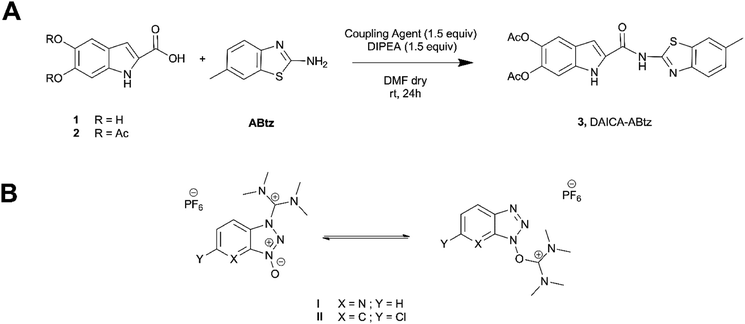
Drawing inspiration from these ideas, herein, we report the synthesis and characterization of a DHICA acetyl derivative (DAICA, 2) featuring amide linkage between the indole and the widely investigated syntone 2-amino-6-methylbenzothiazole (ABtz).16 The reported capability of 2-amino-6-methylbenzothiazole to act as metal (i.e. zinc)17 chelating moiety16 and the deep knowledge of its optical properties make it a choice candidate to investigate as a potential decorating syntone in active eumelanin layers, capable to confer additional properties to the material (i.e. metal chelation, fluorescence) and thus to modulate the polymer properties.
Results and discussion
In order to achieve the amide formation (Fig. 1(A)) two different coupling agents were exploited, HATU and HCTU (respectively I and II in Fig. 1(B))18–20 to activate the carboxyl group of DAICA (2). After optimization of reaction condition, HATU catalyzed DAICA amidation was chosen allowing to obtain the pure product with 69% isolation yield by simple water induced precipitation of the adduct from the reaction medium and subsequent acidic removal of residual ABtz, as detailed in experimental procedure (ESI†).The structure of 3 was confirmed by 1D and 2D NMR and mass analysis (Fig. S1a and b, ESI†) and the disappearance of the NH2 signal observed in the 1H spectrum of ABtz paired by the appearance of the amide signal (11.3 ppm) in the spectrum of 3. In the 1H NMR spectrum, worthy of note is also the observed resonance shift for H-3 on indole moiety from 7.24 in 2 to 7.78 ppm in final product 3 following a deshielding contribution of the formed amide. The MALDI-MS spectrum revealed the presence of signals at m/z 446 and 462, ascribable respectively to the ions [M + Na]+, [M + K]+ of 3.
The UV-vis spectra of 3 were collected in different solvents (Fig. S2, ESI†).
In acetonitrile, a solvent selected in view of further development of the study in the field of energy storage,21 the absorption profile showed two main bands at λ 218 and 327 nm with a small shoulder in the region around at 250 nm. The optical behavior of 3 toward several metal ions, such as Zn2+, Fe3+, Fe2+, Ag+, Cu2+, was evaluated, also in acetonitrile, at different concentrations of the cations up to 2 equiv. (Fig. S3–S6, ESI†).
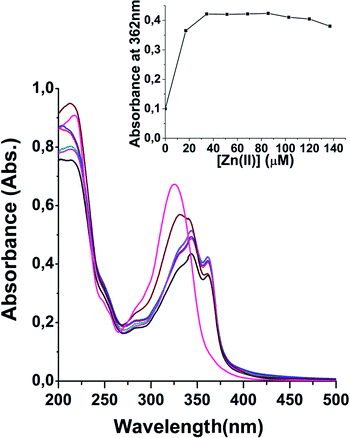
Exposition to Zn2+ ions produced marked changes of UV-vis spectrum profile of 3 (Fig. 2) and the phenomenon was investigated at different Zn2+/3 ratios. Indeed after increasing the Zn2+ concentration, the 218 nm band remained unaffected while the peak at 327 nm decreased in intensity and, at same time, two new bands at 344 and 362 nm were observed suggesting the attitude of 3 to chelate Zn2+ ions and the formation of a complex.
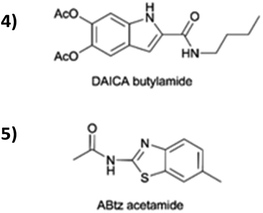
For comparison, the impact Zn2+ on the absorption profiles of two related amides containing the precursors of 3 (acetamide of ABtz, 4, and butylamide of DAICA, 5, represented in Fig. 3) was investigated.
The lack of changes in the spectral features of both 4 and 5 after exposition to Zn2+ ions (Fig. S7 and S8, ESI†) concurs to sustain the central role of the peculiar amide-mediated linking of the two heterocyclic systems in determining the affinity of 3 towards Zn2+. This observation pushed the deepening of the study of 3-Zn2+ interaction.
After the Job's plot analysis22 (Fig. S9, ESI†) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry was proposed as binding mode of amide with Zn2+.23 This stoichiometry was confirmed by MALDI-TOF MS (Fig. S10, ESI†) which provided direct evidence for the formation of a coordinate complex containing two molecules of DAICA-ABtz with one Zn2+ ion whose strength was determined as reported in ESI (Fig. S11 and S12†) obtaining the value of log
2 stoichiometry was proposed as binding mode of amide with Zn2+.23 This stoichiometry was confirmed by MALDI-TOF MS (Fig. S10, ESI†) which provided direct evidence for the formation of a coordinate complex containing two molecules of DAICA-ABtz with one Zn2+ ion whose strength was determined as reported in ESI (Fig. S11 and S12†) obtaining the value of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = 4.88.
Ka = 4.88.
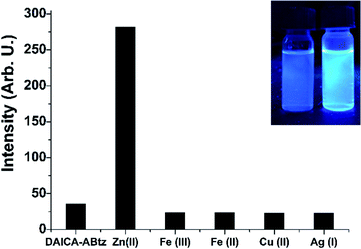
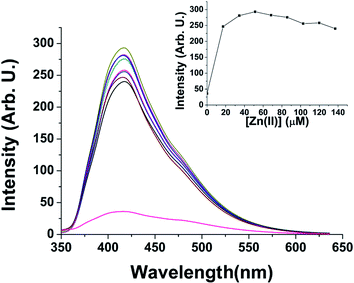
Upon excitation at 325 nm, the amide displays an emission at 417 nm in acetonitrile. After the addition of different metal ions, a relevant enhancement in emission intensity, in the case of Zn2+ with respect to other cations, was observed as reported in Fig. 4.
Fluorescence measurements were carried out up to 2 equiv. of the Zn2+ ion showing a larger than eight-fold increase of the emission compared to the free amide (inset Fig. 5), reaching a maximum at 0.75 equiv. of the cation (Fig. 5).
The quantum yield of 3 was measured in the absence and in the presence of zinc ions in acetonitrile (see ESI† for details) obtaining 0.035 and 0.12 as the quantum yields of 3 and 3 + Zn2+ (0.75 equiv.), respectively. According to the following formula:
The rationale of functionalizing a melanin precursor includes future developments addressing the exploitation of polymer forms of the new amide as cation (Zn2+) exchanger for energy storage applications. Such a long-term goal requires the ability of a solid layer of the polymer to reversibly exchange Zn2+ when interfacing with appropriate electrolytes. In this perspective preliminary studies were carried out to investigate the ability to of 3 and its polymers to complex Zn2+ in solid phase.
Thin films of deacetylated form of 3 were allowed to oxidize according to literature protocols11,12 and oxidation progress was followed by the evolution of the UV-vis profile which presented typical features of melanin-like polymer formation24 (Fig. S13, ESI†). Qualitative inspection of the composition of the oxidized film, was achieved by LC-MS analysis of the methanol soluble fraction, observing signals at the m/z values expected for of oligomeric species (Fig. S14, ESI†).
Basing on these preliminary results concerning the oxidative polymerization of new amide, thin films of the deacetylated form of 3 were investigated, as prepared as well as after exposition to oxidizing atmosphere, to achieve information about the polymer-Zn2+ interaction.
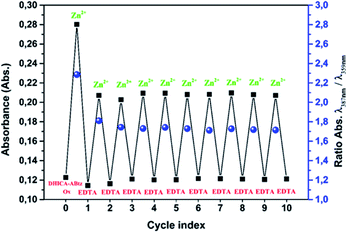
Indeed, after Zn2+ ions exposition the films of the amide polymer did exhibit a marked modification of the UV-vis profile akin the one observed in solution for the monomer (Fig. S15, ESI†). Moreover, a reversible exchange of Zn2+ ions was demonstrated by alternating treatment of a thin film with ethylenediaminetetraacetic acid (EDTA) in water and Zn2+ in acetronitrile. For comparison the polymer–Zn2+ interaction was also investigated for thin films of the sole DHICA polymer which did not show any appreciable modification after exposition to Zn2+ ions.
In Fig. 6 the observed reversibility is highlighted reporting the absorbance of the film at the maxima of the amide (359 nm) and of the complex (387) at each treatment cycle (Zn2+/EDTA). After an initial larger increase, the exposition of the film to the Zn2+ solution results in a constant increase of the absorbance at 387 nm followed, after EDTA treatment, by a parallel decrease featuring complete reversibility up to ten cycles. In this picture the absorbance difference observed in presence of Zn2+ form the 0 cycle to the next ones has to be attributed to some absorbed EDTA in the film and thus competing with the polymer for zinc chelation.
Experimental
All commercially available reagents were used as received and all the solvents were of analytical grade. 2-Carboxy-5,6-dihydroxyindole (DHICA) were prepared according to a reported procedure7Details about the analytical techniques used to characterize the materials and about the tests are reported in the ESI section.†
Synthesis of 1H-indole-2-carboxamide-5,6-bis(acetyloxy)-N-(6-methyl-2-benzothiazolyl) (3)
DAICA (50 mg, 0.1804 mmol) was dissolved in 650 μL DMF dry, then coupling agent (0.2708 mmol) were added simultaneously with 47 μL of DIPEA. The reaction is carried out in inert atmosphere. After about 30 minutes, a DMF dry solution of ABtz was added.The progress of the reaction was monitored by TLC. After completion of the reaction, water was added to the solution to promote the precipitation of the adduct; the precipitate is then taken up with ethyl acetate and it was treated with acetic acid. Without the amine, the product precipitates from the organic phase as a white solid and isolated by filtration (76.3 mg, 69%). Mp, 277–279 °C. 1H NMR (CD3COCD3-d6, 400 MHz, δ, ppm): 2.31 (s, 3H), 2.32 (s, 3H), 2.47 (s, 3H), 7.29 (dd, J = 10 Hz, J = 1.5 Hz, 1H), 7.48 (s, 3H), 7.58 (s, 3H), 7.66 (d, J = 10 Hz, 1H), 7.77 (t, J = 1.5 Hz, 1H), 7.78 (dd, J = 2.75, 0.75 Hz, 1H), 11.00 (s, 1H), 11.29 (s, 1H).
13C NMR (CD3COCD3-d6, 100 MHz, δ, ppm), 20.09, 20.15, 20.95, 106.45, 107.01, 116.02, 120.74, 121.46, 125.43, 127.89, 131.25, 132.81, 134.01, 135.48, 138.32, 141.78, 147.26, 157.58, 159.77, 168.58 and 168.85. MS (MALDI) m/z: 446 (75% [M + Na]+), 462 (25% [M + K]+). FTIR: ν = 1200, 1268, 1380 1460, 1560, 1715, 1759, 3235, 3383.
Conclusion
The integration of melanin precursor DHICA and the 2-aminobenzothiazole synthon allowed to achieve a chelation depending fluorophore exhibiting noteworthy selectivity to Zn2+ ions capable to form a complex in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The value of Ka (7.6 × 104) indicates the high stability of 3–Zn complex.
1 molar ratio. The value of Ka (7.6 × 104) indicates the high stability of 3–Zn complex.
Notably, although the amide bond linking the two aromatic π-conjugated systems could have been associated to a cross-conjugation involving the indole and benzothiazole rings,25 the presence of a band at 327 nm in the absorption profile of 3 reflects a unexpected communication between two moieties. Most likely this can be the consequence of a synergistic delocalization through the amide bond, assisted by the potential hydrogen bond with the atom of N and/or S of the benzothiazole ring.26 Moreover, the amide bond linking the two aromatic bicycle systems acts as intramolecular rotation joint capable to provide a dissipation channel for excited state decay. These structural features are responsible for a very high sensitivity in the micromolar range (0.7 μM) towards Zn2+ ions and for the remarkable chelating enhanced fluorescence,27 which allowed to achieve up to eight fold increase of the emission intensity after the exposition of the amide to Zn2+ ions.
The presence in the structure of 3 of the dihydroxyindole melanin precursor and the characteristic of the amide to operate zinc chelation, not involving the catechol system, preserves the oxidative polymerization attitude of DHICA moiety within 3. This feature opens to the fabrication of decorated melanin polymers, for biointerfaces as well as bioelectronics applications, integrating the expected functionalities of the polymer and the sensoring ones of the novel amide expanding scope of eumelanin exploitation in bioelectronics.
Moreover, on a wider perspective, the capability of the polymer of 3 to chelate Zn2+ was investigated verifying the attitude of thin films of polymer to reversibly chelate Zn2+ ions. This finding provides a key element supporting the developments of a melanin derived solid polymer electrolyte28 for design and fabrication of non aqueous Zn batteries29 as well as COFs-based electrochemical energy storage.30
Author contributions
A. P. and U. C. conceptualization, A. P. and F. M. methodology and investigation, A. P., U. C. and F. M. writing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Financial support from ENEA (Italian Project RELIGHT), European Commission (PolyMed project in the FP7-PEOPLE-2013-IRSES frame, r.n. PIRSES-GA-2013-612538), MIUR and MISE is acknowledged.Notes and references
- V. Stavila, A. A. Talin and M. D. Allendorf, MOF-based electronic and opto-electronic devices, Chem. Soc. Rev., 2014, 43(16), 5994–6010 RSC.
- N. Fuentes, A. Martin-Lasanta, L. Alvarez de Cienfuegos, M. Ribagorda, A. Parra and J. M. Cuerva, Organic-based molecular switches for molecular electronics, Nanoscale, 2011, 3(10), 4003–4014 RSC.
- G. A. Leith, C. R. Martin, A. Mathur, P. Kittikhunnatham, K. C. Park and N. B. Shustova, Dynamically Controlled Electronic Behavior of Stimuli-Responsive Materials: Exploring Dimensionality and Connectivity, Adv. Energy Mater., 2022, 12(4), 2100441 CrossRef CAS.
- A. Seeboth, D. Lotzsch, R. Ruhmann and O. Muehling, Thermochromic polymers–function by design, Chem. Rev., 2014, 114(5), 3037–3068 CrossRef CAS PubMed.
- Y. Kalachyova, O. Guselnikova, P. Postnikov, P. Fitl, L. Lapcak, V. Svorcik and O. Lyutakov, Reversible switching of PEDOT:PSS conductivity in the dielectric–conductive range through the redistribution of light-governing polymers, RSC Adv., 2018, 8(20), 11198–11206 RSC.
- Y. Wakayama, R. Hayakawa, K. Higashiguchi and K. Matsuda, Photochromism for optically functionalized organic field-effect transistors: a comprehensive review, J Mater Chem C, 2020, 8(32), 10956–10974 RSC.
- M. d'Ischia, K. Wakamatsu, F. Cicoira, E. Di Mauro, J. C. Garcia-Borron, S. Commo, I. Galvan, G. Ghanem, K. Kenzo, P. Meredith, A. Pezzella, C. Santato, T. Sarna, J. D. Simon, L. Zecca, F. A. Zucca, A. Napolitano and S. Ito, Melanins and melanogenesis: from pigment cells to human health and technological applications, Pigm. Cell Melanoma Res., 2015, 28(5), 520–544 CrossRef.
- A. W. Pezzella and J. Eumelanin: An Old Natural Pigment and a New Material for Organic Electronics – Chemical, Physical, and Structural Properties in Relation to Potential Applications. John Wiley & Sons 2013 Search PubMed.
- P. Meredith and T. Sarna, The physical and chemical properties of eumelanin, Pigm. Cell Res., 2006, 19(6), 572–594 CrossRef CAS PubMed.
- M. d'Ischia, K. Wakamatsu, A. Napolitano, S. Briganti, J. C. Garcia-Borron, D. Kovacs, P. Meredith, A. Pezzella, M. Picardo, T. Sarna, J. D. Simon and S. Ito, Melanins and melanogenesis: methods, standards, protocols, Pigm. Cell Melanoma Res., 2013, 26(5), 616–633 CrossRef PubMed.
- L. Migliaccio, D. Altamura, F. Scattarella, C. Giannini, P. Manini, F. Gesuele, M. G. Maglione, P. Tassini and A. Pezzella, Impact of Eumelanin-PEDOT Blending: Increased PEDOT Crystalline Order and Packing-Conductivity Relationship in Ternary PEDOT:PSS:Eumelanin Thin Films, Adv. Electron. Mater., 2019, 5(3), 1800585 CrossRef.
- L. Migliaccio, F. Gesuele, P. Manini, M. G. Maglione, P. Tassini and A. Pezzella, Eumelanin Precursor 2-Carboxy-5,6-Dihydroxyindole (DHICA) as Doping Factor in Ternary (PEDOT:PSS/Eumelanin) Thin Films for Conductivity Enhancement, Materials, 2020, 13(9), 2108 CrossRef CAS PubMed.
- C. J. Bettinger, Edible hybrid microbial-electronic sensors for bleeding detection and beyond, Hepatobiliary Surg. Nutr., 2019, 8(2), 157–160 CrossRef PubMed.
- M. A. Khayum, M. Ghosh, V. Vijayakumar, A. Halder, M. Nurhuda, S. Kumar, M. Addicoat, S. Kurungot and R. Banerjee, Zinc ion interactions in a two-dimensional covalent organic framework based aqueous zinc ion battery, Chem. Sci., 2019, 10(38), 8889–8894 RSC.
- X. Yue, H. Liu and P. Liu, Polymer grafted on carbon nanotubes as a flexible cathode for aqueous zinc ion batteries, Chem. Commun., 2019, 55(11), 1647–1650 RSC.
- U. Caruso, B. Panunzi, A. Roviello, M. Tingoli and A. Tuzi, Two aminobenzothiazole derivatives for Pd(II) and Zn(II) coordination, Inorg. Chem. Commun., 2011, 14(1), 46–48 CrossRef CAS.
- R. Diana and B. Panunzi, The Role of Zinc(II) Ion in Fluorescence Tuning of Tridentate Pincers: A Review, Molecules, 2020, 25(21), 4984 CrossRef CAS PubMed.
- P. Alewood, D. Alewood, L. Miranda, S. Love, W. Meutermans and D. Wilson, Rapid in situ neutralization protocols for Boc and Fmoc solid-phase chemistries, Methods Enzymol., 1997, 289, 14–29 CAS.
- L. A. Carpino, l-Hydroxy-7-azabenzotriazole. An Efficient Peptide Coupling Additive, J. Am. Chem. Soc., 1993, 115, 3 CrossRef.
- O. Marder, Y. Shvo and F. Albericio, HCTU and TCTU: New Coupling Reagents — Development and Industrial Aspects, ChemInform, 2003, 34(32) DOI:10.1002/chin.200332258.
- N. Borchers, S. Clark, B. Horstmann, K. Jayasayee, M. Juel and P. Stevens, Innovative zinc-based batteries, J. Power Sources, 2021, 484, 229309 CrossRef CAS.
- C. Y. Huang, Determination of binding stoichiometry by the continuous variation method: the Job plot, Methods Enzymol., 1982, 87, 509–525 CAS.
- J. S. Renny, L. L. Tomasevich, E. H. Tallmadge and D. B. Collum, Method of continuous variations: applications of job plots to the study of molecular associations in organometallic chemistry, Angew. Chem., Int. Ed. Engl., 2013, 52(46), 11998–12013 CrossRef CAS.
- A. Pezzella, L. Panzella, O. Crescenzi, A. Napolitano, S. Navaratnam, R. Edge, E. J. Land, V. Barone and M. d'Ischia, Lack of visible chromophore development in the pulse radiolysis oxidation of 5,6-dihydroxyindole-2-carboxylic acid oligomers: DFT investigation and implications for eumelanin absorption properties, J. Org. Chem., 2009, 74(10), 3727–3734 CrossRef CAS PubMed.
- N. F. P. a. M. Orchin, Cross Conjugation, J. Chem. Educ., 1968, 45(10), 633–637 CrossRef.
- M. Maldonado-Domínguez, R. Arcos-Ramos, M. Romero, B. Flores-Pérez, N. Farfán, R. Santillan, P. G. Lacroix and I. Malfant, The amide bridge in donor–acceptor systems: delocalization depends on push–pull stress, New J. Chem., 2014, 38(1), 260–268 RSC.
- C. A. S. Pothulapadu, A. Jayaraj, S. N, R. N. Priyanka and G. Sivaraman, Novel Benzothiazole-Based Highly Selective Ratiometric Fluorescent Turn-On Sensors for Zn(2+) and Colorimetric Chemosensors for Zn(2+), Cu(2+), and Ni(2+) Ions, ACS Omega, 2021, 6(38), 24473–24483 CrossRef CAS PubMed.
- E. Shembel, Y. Polishchuk, V. Kyrychenko, V. Redko, B. A. Blyuss and T. Pastushkin, Generating Innovative Solid Polymer Electrolyte Based on Melanin and Without Binder for High Energy Li Batteries, ECS Trans., 2021, 105(1), 29–34 CrossRef CAS.
- A. S. Etman, M. Carboni, J. Sun and R. Younesi, Acetonitrile-Based Electrolytes for Rechargeable Zinc Batteries, Energy Technol., 2020, 8(9), 2000358 CrossRef CAS.
- V. Singh and H. R. Byon, Advances in electrochemical energy storage with covalent organic frameworks, Mater. Adv., 2021, 2(10), 3188–3212 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02616c |
| This journal is © The Royal Society of Chemistry 2022 |