Open Access Article
Open Access ArticleAurone-derived 1,2,3-triazoles as potential fluorescence molecules in vitro†
Daniel L. Bryant ab,
Arjun Kafleb,
Scott T. Handy
ab,
Arjun Kafleb,
Scott T. Handy b,
Anthony L. Faronea and
Justin M. Miller
b,
Anthony L. Faronea and
Justin M. Miller *b
*b
aDepartment of Biology, Middle Tennessee State University, 1301 E Main St., Murfreesboro 37132, Tennessee, USA
bDepartment of Chemistry, Middle Tennessee State University, 1301 E Main St., Murfreesboro 37132, Tennessee, USA. E-mail: Justin.Miller@mtsu.edu
First published on 12th August 2022
Abstract
Aurones are a class of well-studied natural compounds primarily responsible for the yellow pigment in flowering plants and have been shown to have fluorescent properties as well as beneficial biological effects. Traditionally, aurones can be easily synthesized through a Knoevenagel condensation of benzofuranones with arylaldehydes. Recently, Kafle et al. unexpectedly synthesized a new aurone derivative containing a 1,2,3-triazole within its backbone. Since, 1,2,3-triazole containing structures have been shown to be useful as fluorophores with large Stokes shifts, we hypothesized that these new aurone-derived triazole compounds (ATs) could be utilized as potential fluorophores. Here we describe a newly-synthesized fluorescent compound which has potential for use as a live-cell probe, having a large Stokes shift of 118.3 ± 1.01 nm in phosphate-buffered saline with the benefit of increased fluorescence in protic environments, which is uncommon in aurone-derived fluorophores.
Introduction
Aurones (2-benzylidene-1-benzofuran-3(2H)-one) are a class of naturally occurring flavonoid derivatives responsible for the pigments in some flowering plants, such as the yellow rocket snapdragon.1 There has been a recent increase in the study of aurones due to their bioactivities;2 including anti-inflammatory,3–6 anti-microbial,5,7,8 and anticancer9–11 properties. In addition, there has been an interest in aurones as fluorescent markers due to their visible range excitation and emission spectra, tunable fluorescence properties, and their potential bioavailability.12–14 Most notably, in 2011, Shanker and Dilek described four different aurones with a 4′ amine substitution on the benzylidene group (aminoaurones).12 These aurones had increased Stokes shifts in more polar-protic solvents.12 Two of the aminoaurones were shown to have increased emissions intensities in nonpolar solvents.12 Other studies, both computational and experimental, have determined that the fluorescence spectra and Stokes shift of aurones are also dependent on side chain and solvent polarity.13,14 For example, Espinosa-Bustos et al. observed increased emissions intensities in less polar solvents and that location of the sidechains on the benzylidene group of the aurone modulated the absorbance and emissions spectra, which supported the observations by Shanker and Dilek.12,13 Xue et al. computationally examined the aurones presented by Shanker and Dilek, and found that the increased Stokes shifts and red-shifted emissions were due primarily to intramolecular charge transfer.14 Muñoz-Becerra et al. in 2019 described computationally how the addition of a polar side chain on the 4 carbon of the benzofuranone backbone led to an increased Stokes shifts as well as red-shifted absorbance and emissions spectra.15Aurones have also been examined for potential as fluorescent sensors. Starting in 2013, Chen and associates synthesized multiple aurone-based sensors for cyanide.16–18 In 2018, Zhang et al. described a membrane permeable aurone based sensor for Hg2+.19 Most recently, Kafle et al. in 2020 developed an aurone-derived sensor for hydrogen sulfide.20 Though the potential for aurones as bioavailable fluorescent molecules has been reported, aurones traditionally only fluoresce in non-polar solvents and are quenched in aqueous environments.12,13,20 Kafle et al. were able to bypass the quenching effect of aqueous environments through the use of hexadecyltrimethylammonium bromide (CTAB).20
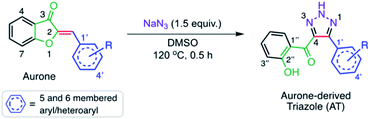
Finding new methods of aurone synthesis has been an area of active research for many years. Two common methods of aurone synthesis were described by Geissman and Harborne in 1955 where they used a condensation reaction between a coumaranone and various aldehydes,21 and by Varma and Varma in 1992 in which they used an alumina catalyzed reaction of benzofuranones with arylaldehydes.22 Alternatively, others have utilized palladium and formic acid,23 silver nitrate,24 gold,25 or choline chloride and urea26 in various condensation and cyclization approaches to the aurone skeleton. In the course of an effort to install an azido group on an intact aurone system, Kafle et al. noted instead the synthesis of a new class of aurone-derived salicyl substituted 1,2,3-triazole (AT) (Fig. 1).27
1,2,3-Triazoles have also been shown to possess fluorescent properties.28–30 In 2015, Ghosh et al. described a “click” synthesized 1,2,3-triazole which showed fluorescence in the presence of a fluoride ion.28 In 2018, Meisner et al. established that 1,2,3-triazole containing compounds have larger Stokes shifts with their fluorescent properties affected by the triazole substitution and the ability to form intramolecular hydrogen bonds.30 Unlike the other 1,2,3-triazoles, the aurone-derived ATs incorporate a salicylic group substituted at C4 on the triazole ring (Fig. 1). With this in mind, it was thought that ATs would have potential as fluorescent compounds. It was hypothesized that 1,2,3-triazoles derived from aurones would have fluorescent properties similar to both triazoles and aurones. Here, we describe ATs with increased fluorescence in polar protic aqueous environments, allowing for increased fluorescence in cellular environments with marked potential as a biologically relevant fluorescent probe.
Materials and methods
All the reactions and handling of the chemicals were done under an air atmosphere using ACS-grade reagents. Organic solvents were directly employed without further purification. 1H and 13C NMR of all the compounds were recorded on JEOL AS (500 and 125 MHz respectively), using CDCl3, DMSO-D6, or acetone-D6 as a standard reference (Fig. S1†).31 Chemical shifts and the coupling constants (J) for 1H and 13C NMR are reported in parts per million (ppm) and in hertz (Hz), respectively. The following conventions are used for multiplicities: s, singlet; d, doublet; t, triplet; m, multiplet; dd, doublet of doublet; tt, triplet of triplet; dt, doublet of triplet; ddd, doublet of doublets of doublets; br, broad. All high-resolution mass spectra were acquired on a Waters Synapt HDMS QToF with Ion Mobility. Reactions were monitored by Thin Layer Chromatography (TLC) on silica-coated plates using short-wavelength (254 nm) UV light. All extracts were concentrated under reduced pressure using Buchi Rotary Evaporator. The compounds were purified by flash silica gel (32–64 μm) column chromatography.General procedure for the synthesis of aurone-derived triazoles (AT1–AT5)
The aurones and the corresponding triazoles, AT1–AT5 (structures in Fig. S2†) were synthesized and characterized based on the literature.21,27 The characterization data for AT2 and AT5 are provided below. Following preparation of the aurone,21 0.50 mmol of aurone was mixed with 0.75 mmol (1.5 eq.) of sodium azide (NaN3) using 1.5 mL DMSO in a 3-dram glass vial and heated at 120 °C for 30 min (unless otherwise specified) in a sand bath. The progress of the reaction was monitored by TLC. After the completion of the reaction, the reaction mixture was cooled to room temperature and transferred to a 50 mL centrifuge tube. It was then diluted with DI water and extracted several times with ethyl acetate (150 mL). Next, the obtained organic fraction was washed with DI water followed by brine. Finally, the organic fraction was dried over anhydrous MgSO4 and concentrated in vacuo to obtain the crude product, which was purified by flash column chromatography using mixtures of hexane and ethyl acetate (20–50% EtOAc/hexane) to afford the desired triazole.General protocols
All ATs were reconstituted from a purified powder at 100 mM in 100% dimethyl sulfoxide (DMSO; Fisher Scientific, MA; Cat#: D128-500/MP Biomedicals; Cat#: 196![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 055). In all experiments the ATs were diluted to 100 μM in the indicated solution with 0.1% (v/v) DMSO as a cosolvent. To account for DMSO as a cosolvent, all experiments were carried out with 0.1% (v/v) DMSO as a vehicle control. For experiments measuring fluorescence spectra, the absorbance and emissions of the vehicle control were subtracted as a background.
055). In all experiments the ATs were diluted to 100 μM in the indicated solution with 0.1% (v/v) DMSO as a cosolvent. To account for DMSO as a cosolvent, all experiments were carried out with 0.1% (v/v) DMSO as a vehicle control. For experiments measuring fluorescence spectra, the absorbance and emissions of the vehicle control were subtracted as a background.
Cell culture
Raw 264.7 murine macrophage-like cells (ATCC Cat#: TIB-71) and HeLa cells (ATCC Cat#: CCL-2) were cultured in either Dulbecco's modification of Eagle's Medium (DMEM; Corning, NY; Cat#: 10-013-CV) or Roswell Park Memorial Institute 1640 medium (RPMI; Fisher Scientific, MA; Cat#: 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 875
875![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093), respectively. Both media were supplemented with 10% heat-inactivated fetal bovine serum and 100 units/mL penicillin/streptomycin cocktail (Fisher Scientific, MA; Cat#: 15-140-122). The cells were grown in a humidified incubator at 37 °C with 5% CO2 and passaged prior to reaching 90% confluency every 3–5 days. All cell culture experiments were incubated in this manner unless otherwise stated.
093), respectively. Both media were supplemented with 10% heat-inactivated fetal bovine serum and 100 units/mL penicillin/streptomycin cocktail (Fisher Scientific, MA; Cat#: 15-140-122). The cells were grown in a humidified incubator at 37 °C with 5% CO2 and passaged prior to reaching 90% confluency every 3–5 days. All cell culture experiments were incubated in this manner unless otherwise stated.
Cytotoxicity assay
Cytotoxicity of each AT was determined using the PrestoBlue cell viability assay (Invitrogen, CA; Cat# A13262). RAW 264.7 cells, or HeLa cells, were seeded at a density of 5 × 104 cells per well, or 1 × 104 cells per well, respectively, in a sterile 96-well plate and allowed to attach overnight. The cells were washed with 1X phosphate-buffered saline (PBS; Gibco – ThermoFisher Scientific, MA; Cat#: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010023), then treated with 100 μM of each AT, or 0.1% (v/v) DMSO as a vehicle control, for 24 h. Immediately following the treatment, the cells were washed with PBS, and medium containing 1X PrestoBlue was added to the cells for one hour. The absorbance at 570 nm was recorded, and the absorbance at 600 nm was subtracted according to the manufacturer's protocol. The data is presented as a percentage to the average absorbance of the untreated wells for each plate ± SEM.
010023), then treated with 100 μM of each AT, or 0.1% (v/v) DMSO as a vehicle control, for 24 h. Immediately following the treatment, the cells were washed with PBS, and medium containing 1X PrestoBlue was added to the cells for one hour. The absorbance at 570 nm was recorded, and the absorbance at 600 nm was subtracted according to the manufacturer's protocol. The data is presented as a percentage to the average absorbance of the untreated wells for each plate ± SEM.
Confocal microscopy
Absorbance and emissions microplate procedure
Absorbance and emissions spectra for AT5 at varying concentrations were determined by mixing 400 μM of AT5 or 0.4% (v/v) DMSO in PBS (Growcells, CA; Cat#: MRFG-6235) in a 96-deepwell plate (ThermoFisher Scientific, MA; Cat#: 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 251). AT5, or vehicle, was then diluted serially to 6.25 μM in PBS. 100 μL of each concentration was transferred to a UV/Vis 96-well microplates (Fisher Scientific, MA; Cat#: 21-377-832) in triplicate for three independent replicates (n = 9). Both absorbance and emissions spectra were measured using a CLARIOstar microplate reader (BMG-Labtech, Germany). Absorbance was measured at each wavelength from 220–1000 nm using the average of 5 flashes per well. The emissions spectra from 350–621 nm (emission bandwidth of 14 nm) were measured using an excitation wavelength of 320 nm (excitation bandwidth of 12 nm) at a gain of 857 and a focal length height of 5 mm using the top optic. Absorbance and emissions spectra for the vehicle at each concentration were subtracted as background.
251). AT5, or vehicle, was then diluted serially to 6.25 μM in PBS. 100 μL of each concentration was transferred to a UV/Vis 96-well microplates (Fisher Scientific, MA; Cat#: 21-377-832) in triplicate for three independent replicates (n = 9). Both absorbance and emissions spectra were measured using a CLARIOstar microplate reader (BMG-Labtech, Germany). Absorbance was measured at each wavelength from 220–1000 nm using the average of 5 flashes per well. The emissions spectra from 350–621 nm (emission bandwidth of 14 nm) were measured using an excitation wavelength of 320 nm (excitation bandwidth of 12 nm) at a gain of 857 and a focal length height of 5 mm using the top optic. Absorbance and emissions spectra for the vehicle at each concentration were subtracted as background.
Solvent polarity
To determine the effect of solvent polarity, 100 mM AT5 in DMSO was mixed with 1 mL of each indicated solvent to a final concentration of 100 μM. Peak excitation was determined by screening emissions of AT5 at 330 nm on a Hitachi F-4500 fluorescence spectrophotometer. Optimal excitation wavelength was determined for each compound by systematically varying excitation wavelength in 0.1 nm increments until the emissions maximum was observed. Emissions spectra were then recorded using the peak excitation for each solvent. Emissions data for 0.1% (v/v) DMSO was gathered for each solvent and subtracted as a baseline.pH sensitivity
The effect of changes in pH of 1X PBS was determined by using 10X PBS (Growcells CA, Cat#: MRFG-6235) diluted with either hydrochloric acid or sodium hydroxide and water to 1X PBS. The acidic or basic 1X PBS was then used to change the pH of 1X PBS to either 5.0, 6.0, 7.0, 7.4, 8.0, 9.0, or 10.0. AT5 was then diluted to 100 μM in the PBS at each pH. Each well was mixed in a 96-deep-well plate and 100 μL of each was loaded into UV/Vis 96-well microplates (Fisher Scientific, MA; Cat#: 21-377-832) in triplicate for three independent replicates. Emissions were measured using a CLARIOstar microplate reader as previously described. Emissions spectra for 0.1% (v/v) DMSO at each pH was subtracted as background.Detergent interactions
To determine the effect of different detergents on AT5, 100 mM of AT5 was diluted to 100 μM in the indicated concentrations of either CTAB (Sigma Aldrich, MO; Cat#: H 6269; CMC = 0.9 mM33), SDS (Fisher Scientific, MA; Cat#: 166-100; CMC ≈ 8 mM34,35), Triton X-100 (Fisher Scientific, MA; Cat#: BP151-500; CMC = 0.18–0.24 mM36–39), or Tween 20 (Fisher Scientific, MA; Cat#: BP337-100; CMC ≈ 0.050–0.057 mM37,40), as well as PBS pH 7.4 (Growcells, CA; Cat#: MRFG-6235) and 18 mΩ H2O. To determine the correct order of mixing, 100 mM AT5 was diluted to 100 μM in PBS and H2O either 30 min before or after the addition of Triton X-100 at a final indicated concentration and mixed via pipetting. To create a broad range of emissions for AT5, 100 μM of AT5 in PBS was mixed with the indicated ratios of both Triton X-100 and SDS to a final detergent concentration of 100 mM. Absorbance and emissions spectra were measured for each well using a CLARIOstar microplate reader as previously mentioned.Results and discussion
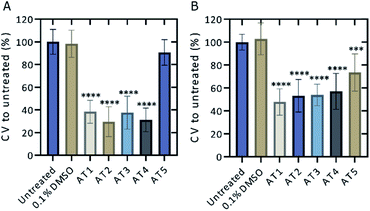
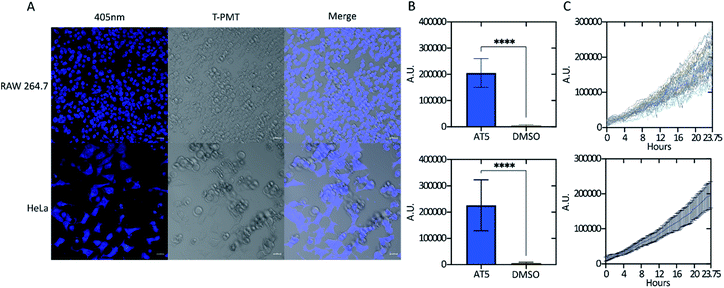
Five ATs; AT1, AT2, AT3, AT4, and AT5 were initially screened for potential fluorescence in vitro using confocal microscopy (Fig. 2). RAW 264.7 cells were treated with the various ATs for 24 h prior to imaging. These results demonstrated that cells treated with 100 μM of either AT2 or AT5 showed notable amounts of fluorescence (Fig. 2A and B). AT5 exhibited emissions when excited by the 405 nm (blue) laser while AT2 did so when excited using both the 488 nm (green) and 555 nm (red) lasers, but not the 405 nm laser (Fig. 2A, B, and S2†). The observation of minor AT5 emissions intensities, relative to DMSO, in the green- and red-channels may be the consequence of crosstalk between channels based on microscope experimental parameters. This conclusion is supported by the lack of AT5 absorbance at these wavelengths as noted in Fig. 5 below. In addition to their differences in fluorescence, both ATs seemed to localize to different areas within the cell; although, from the data collected it is difficult to determine the exact locations of each fluorophore (Fig. 2C). Of these ATs only AT5 showed no statistically significant cytotoxic effect on RAW 264.7 murine macrophage-like cells after 24 h, with an average of 90.7 ± 11.3% cell viability (Fig. 3A). It should be noted that all examined ATs were cytotoxic to HeLa cells after 24 h (Fig. 3B). AT5 was the least cytotoxic with an average of 73.5 ± 16.3% cell viability (Fig. 3B). This apparent lower cytotoxicity may be due to the large methyl acetamide side chain of AT5 compared to the side chains of AT1–4 (Fig. 2 and S2†). Although AT2 showed potential as a fluorescent probe, the cytotoxicity prohibited its use in live-cell imaging experiments. Additionally, preliminary characterization of AT2 showed little fluorescence in PBS, DMSO, and cyclohexane (data not shown). Though this may imply AT2 is interacting with something within the cell to increase its fluorescence. Further research outside the scope of this paper is needed to fully understand this phenomenon. Due to the observed fluorescence and lack of cytotoxicity, AT5 was further pursued for additional characterization.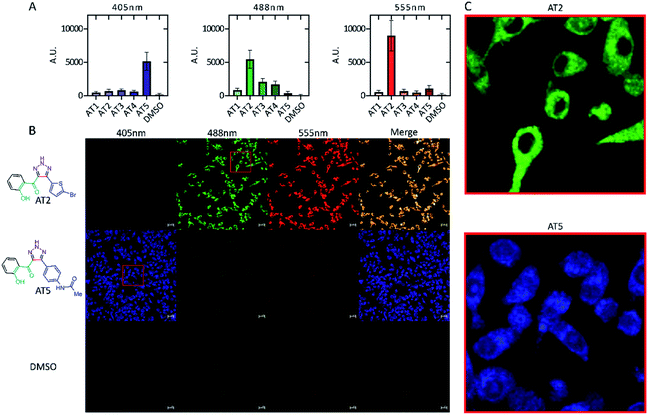 | ||
| Fig. 2 Fluorescence intensity of ATs in vitro. RAW 264.7 murine macrophages were incubated for 24 h with 100 μM of the indicated compound. Cells were imaged using a Zeiss LSM 700 confocal microscope with excitation wavelength indicated as 405 nm, 488 nm, or 555 nm. Signal intensity for cells for each laser were measured using FIJI and standardized to the unit area of each cell. Background in each image was subtracted from each measurement. Values where the fluorescence intensity of the cell was less than the background (negative values) were set to zero. (A) Mean emission intensity ± SD n = 150 for each compound. Cells were sampled at random from various fields using the transmitted light image to avoid bias (not shown) across three, independent biological replicates. Emission intensities were determined as a quantification of (B) (and Fig. S2†) showing the fluorescence of each compound in vitro for each specific laser. Scale bars are 20 μm. (C) Enlarged regions of each photo showing distribution of ATs in vitro. Brightness across all images was adjusted equally post analysis. | ||
To determine whether the fluorescence activity of AT5 was only visible in RAW 264.7 cells, both RAW 264.7 cells and HeLa cells were incubated with 100 μM AT5 for 24 h at 37 °C in a 5% CO2 incubator and imaged using the Zeiss LSM 700 confocal microscope. We found that although there is a slight difference in the fluorescent intensity of AT5 in HeLa and RAW 264.7 cells (comparison not shown), AT5 could indeed be used as a live-cell probe in different cell lines (Fig. 4A and B). To determine the timeframe required for use with live cells, RAW 264.7 cells were treated with 100 μM AT5 at 37 °C in 5% CO2 and imaged every 15 min for approximately 24 hours (Fig. 4C). Though fluorescence consistently increased, changes in fluorescence increased approximately 4 h post-treatment, at which point the fluorescence intensity seemed to increase at a greater rate for the remainder of the experiment (Fig. 4C). Additionally, fluorescence intensity of AT5 in RAW 264.7 cells never plateaued indicating the potential for increased fluorescence at time points of 24 h or later (Fig. 4C). There was observable cell death at time points approaching 24 h, which is potentially due to prolonged exposure to the 405 nm laser because in fields adjacent to the final images of this experiment, there was little to no observable cell death (images not shown). This is supported by the previously mentioned cell viability experiments (Fig. 3). In addition to acting as fluorophore in live cells, AT5 was able to stain fixed and permeabilized RAW 264.7 cells (Fig. S3†) further indicating its applicability. In many images, AT5 can be seen inside both RAW 264.7 cells and HeLa cells, though it does not seem to be ubiquitous throughout the cell (Fig. 2B, C, 4A, and S2†). It is possible that AT5 is not simply sequestered, but rather interacting with cellular components. Further research is necessary to validate this idea. Our initial results indicate that pH may also play a role in fluorescence since we observed a slight reduction in emissions of AT5 in PBS with a pH ≤ 6 (Fig. S4†).
Changes in AT5 fluorescence due to solvent polarity
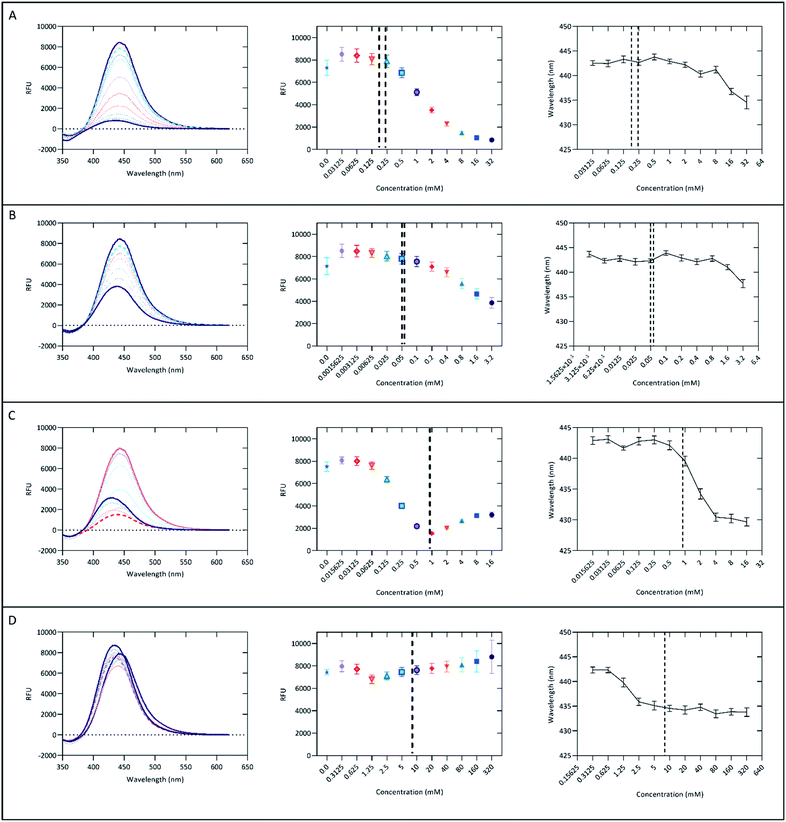
Solvent polarity has been previously reported to affect the emissions of aurones and their derivatives, showing a trend for increased emissions as polarity decreased.12,13 It was hypothesized that polarity may influence the fluorescent properties of AT5 as well. To test this, AT5 was dissolved to a concentration of 100 μM in various solvents. Each solvent was ranked according to the relative normalized molar electronic transition energies of pyridium N-phenolate betaine dye 36 (ET (30)) values (ENT) on a scale between tetramethylsilane (TMS; 0.00) and water (1.00).41,42 Surprisingly, it was found that the fluorescence of AT5 increased in polar-protic environments, with the greatest fluorescence occurring in PBS (Fig. 5A). Indeed, even the weakest fluorescence in a polar-protic solvent (isopropanol, 889.1 ± 10.96 [SD] RFU) exhibited more than 35-fold stronger emissions (p < 0.0001; Unpaired two-tailed t-test with a Welch's correction) compared to chloroform (25.3 ± 0.86 [SD] RFU) which had the strongest emissions of both the polar-aprotic and non-polar solvents (Fig. 5A). Given that the ET (30) scale is well known to be highly sensitive to hydrogen-bonding, these results were also examined in the context of the pi* scale, which reflects more general solvent polarity.42–44 There was effectively no correlation of fluorescence results with the pi* scale indicating that hydrogen-bonding is indeed the dominant feature influencing fluorescence intensity, although it should be noted that no attempt was made to ensure anhydrous conditions during fluorescence measurements, so the influence of residual water in the solvents or on the cuvettes cannot be ruled out.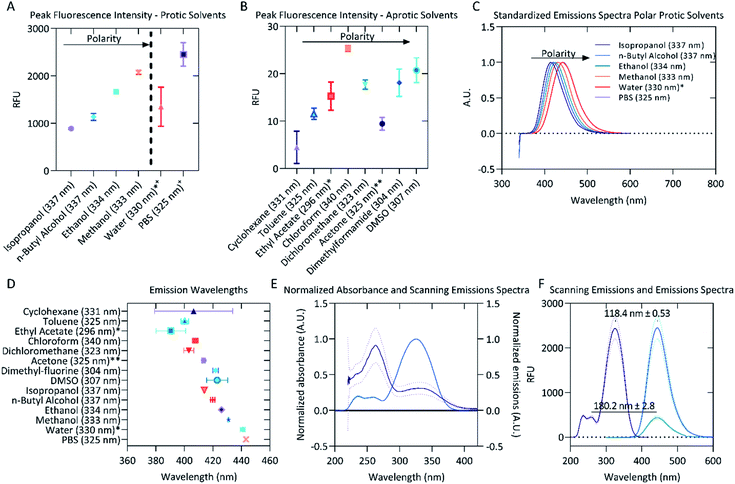 | ||
| Fig. 5 Fluorescent properties of 100 μM AT5 in different solvents. (A) Emission intensities of AT5 in various protic solvents organized by increasing polarity. Excitation wavelengths (in parenthesis) were determined using the scanning emissions spectra. (B) Emission intensities of AT5 in various aprotic solvents organized by increasing polarity. Excitation wavelengths (in parenthesis) were determined using the scanning emissions spectra. (C) Red-shift in emission spectra for protic solvents. The mean emissions spectra for each solvent were standardized, 1 being the highest emission intensity and 0 set to 0 emissions. Excitation wavelengths (in parenthesis) were determined using the scanning emissions spectra. (D) Peak emission wavelengths for AT5 in various solvents. Excitation wavelengths (in parenthesis) were determined using the scanning emissions spectra. (E) Normalized absorbance (solid purple line) and normalized scanning emission (solid blue line) spectra ± SD, n = 9 across three independent replicates measured using a CLARIOstar microplate reader. Absorbance and scanning emissions spectra for wavelengths > 420 nm not shown. (F) Scanning emissions spectra (solid purple line) and emission (solid blue line) spectra of PBS ± SD measured using the spectrophotometer, n = 3. Excitation wavelengths (in parenthesis) were determined using the scanning emissions spectra. For all panels (A–F), each emission spectra for the various solvents were measured and blank corrected using 0.1% (v/v) DMSO as a vehicle background. Peak emissions and peak emission wavelengths were determined as n = 3 independent replicates for the indicated excitation wavelength (in parenthesis) with mean values represented. Vertical lines represented standard deviation calculated for each set of measurements. *Low solubility. **Negative values for one replicate led to only using an n = 2. +Aqueous solvents not statistically different due to high variability. 0Polarity data not known for PBS. 1Polarity based on the relative normalized ET (30) [ENT] polarity.41 | ||
Water is the most polar-protic solvent on this scale, so it was expected that water would cause AT5 to have the highest fluorescence emissions, but due to the low solubility of AT5 in water, it was unable to go fully into solution potentially limiting its detectable fluorescence. The data also suggests that polarity in aprotic solvents has little to no effect on the fluorescence emissions of AT5, but rather is dependent on the protic nature of the solvent (Fig. 5A and B and Table 1). This suggests a potential use of AT5 in selective fluorescence visualization experiments based on solvent environment. This is supported by the similar, but not equivalent, findings of Meisner et al. which noted that the emissions of 1,2,3-triazoles were quenched in the polar-aprotic solvent dichloromethane compared to DMSO, which was attributed to the interruption of intramolecular hydrogen bonds.30 The authors described that the triazoles were acting as the hydrogen bond donor.30 Conversely, the higher fluorescence (RFU > 800) in AT5 in polar protic solvents (Isopropanol, n-butyl alcohol, ethanol, methanol, PBS, and water), compared with very slight fluorescence (RFU < 30) in either polar aprotic solvents (DCM, toluene, ethyl-acetate, DMF, and DMSO) or aprotic solvents (acetone, chloroform, and cyclohexane) indicate that AT5 has increased fluorescence in solvents favoring AT5 behaving as a hydrogen bond acceptor (Fig. 5A and B and Table 1). In support of this as solvent polarity increases in protic solvents, there is a slight red-shift in emissions spectra ranging from 414.1 ± 0.702 nm (isopropanol) to 443.267 ± 0.643 nm (PBS) (Fig. 5C and D), though there is a less uniform red-shift as polarity increases in aprotic solvents as well (Fig. 5D). It is possible that the hydroxyl group present on C2′′ could potentially form an intramolecular hydrogen bond with the ketone-group. Both the ketone-group and the amide group on C4′ can act as hydrogen bond acceptors, while the hydrogen ion on N2 of the triazole could potentially act as a hydrogen bond donor.
| Solvent (excitation) | Relative polarity1 | Stokes shift (nm) | Std dev. | Peak emission (RFU) | Std dev. |
|---|---|---|---|---|---|
| Isopropanol (337 nm) | 0.546 | 77.1 | 0.7 | 889.1 | 13.4 |
| n-Butyl alcohol (337 nm) | 0.586 | 82.9 | 1.5 | 1135.3 | 70.4 |
| Ethanol (334 nm) | 0.654 | 92.1 | 0.2 | 1660.3 | 31.9 |
| Methanol (333 nm) | 0.762 | 98.1 | 0.3 | 2072.7 | 45.0 |
| Water (330 nm)*+ | 1.000 | 110.9 | 1.70 | 1349.03 | 413.8 |
| PBS (325 nm)+ | — | 118.3 | 0.6 | 2451.0 | 250.1 |
The strongest emissions of AT5 were observed in PBS, therefore subsequent experiments and characterization were performed in PBS. The absorbance spectra showed two major peaks, with the largest peak absorbance occurs between 262 and 263 nm (Fig. 5E). There was an additional smaller absorbance peak with a maximum occurring between 328 and 330 nm (Fig. 5E). The absorbance and emission intensity of both peaks was linked to concentration of AT5 (Fig. S5†). Excitation at 262 nm yielded slight emissions of 443.4 ± 40.99 RFU with a mean peak emission wavelength of 442 ± 2.8 nm (Fig. 5F). To determine which wavelength of excitation was ideal, the scanning excitation spectra (SES) for emissions at 442 nm, which systematically records the intensity of emissions at a given wavelength for each excitation wavelength, was recorded. Though excitation occurs at 262 nm, peak excitation for emissions at 442 nm most likely occurs at 324.7 ± 0.3 nm (Fig. 5F). This was confirmed by measuring emissions from AT5 excited at 325 nm and found peak emissions at 443.3 ± 0.64 nm with peak emissions of 2451 ± 250.1 RFU (Fig. 5F). Because the highest absorbance peak did not correspond with peak fluorescence, the SES was used to determine peak excitation in all solvents. Using the peak SES and peak emissions (SE) to determine Stokes shift, a large Stokes shift of 118.3 ± 0.6 nm (n = 3) was observed for AT5 in PBS (Fig. 5F). Large Stokes shifts are a common feature of other triazoles.29,30
The SE Stokes shift of AT5 in various protic solvents (calculated as the difference in peak emissions, n = 3, compared to the peak excitation wavelength initially determined through SES) was observed to be linearly dependent on relative solvent polarity such that increasing polarity promotes a correlated increase in SE Stokes shift from 77.7 ± 0.7 nm in isopropanol to 118.36 ± 0.6 nm in PBS buffer (Table 1). The poor signal intensities presented in Fig. 5B for AT5 emissions in aprotic solvent systems introduce large error in estimation of the Stokes shift under these conditions. Indeed, the SE Stokes shift of AT5 in cyclohexane was determined to be 75.5 ± 27.5 nm, where the large standard deviation is likely due to low signal intensity with emissions of only 6.1 ± 5.9 RFU. Similar results were observed in all aprotic solvent systems examined. Moreover, identical experimental parameters (slit widths, integration times, etc.) were utilized for measurements collected in protic and aprotic solvent systems to allow for comparison of photophysical behaviors among all solvent systems examined. In contrast to aprotic conditions, protic conditions yield maximal AT5 emissions intensities, thereby allowing for estimation of SE Stokes shifts values with less than 2% experimental error (Table 1). The large SE Stokes shift of AT5 in PBS further indicates its potential as a useful biological probe.
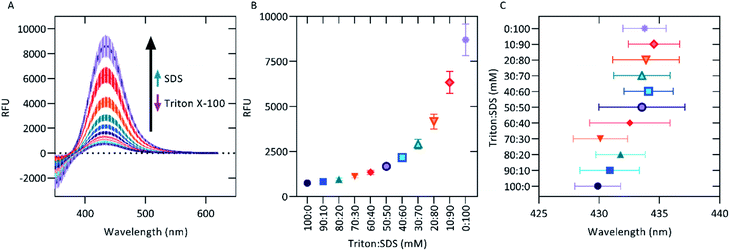
Detergent micelle models
Since ionic detergents such as CTAB and sodium dodecyl-sulfate (SDS) have been shown to modulate the strength of fluorescent probes20,45,46 and since detergent micelles have been used as membrane mimics for the study of membrane proteins,47,48 it was decided that the interactions of AT5 with both ionic and neutral detergents should be characterized. To do this, various concentrations flanking the critical micelle concentration of CTAB, Triton X-100 (Triton), SDS, or Tween 20 (Tween) were separately mixed with 100 μM AT5 in PBS. For all detergents, the lowest concentration seemed to increase the fluorescence intensity of AT5 (Fig. 6). As the concentration of both neutral detergents (Triton and Tween) increased, the emissions of AT5 had an average percent decrease of 90.10 ± 0.73% and 54.72 ± 3.28%, respectively, demonstrating that neutral detergents had a strong quenching effect at concentrations above the CMC (Fig. 6A and B). Additionally, it was found that in 10 mM Triton X-100, addition of AT5 prior to detergent addition slightly increased fluorescence (Fig. S6†). Based on this, for all subsequent experiments, AT5 was added prior to detergent. Unlike both neutral detergents, in the cationic detergent, CTAB, the emission of AT5 was quenched at concentrations greater than 0.125 mM. For this condition, the average percent decrease was 80.8 ± 0.7% (of peak fluorescence at 0.03125 mM) with midpoint occurring at 0.25 mM CTAB (Fig. 6C). In SDS, the intensity of AT5 was quenched at 1.25 mM to approximately 14.7 ± 1.1% lower than its initial peak fluorescence (at 0.3125 mM, Fig. 6D). In all detergents as concentrations increased, there was a slight but noticeable blue-shift in peak emissions wavelength, with the most drastic change occurring in CTAB shifting from a peak emission wavelength around 443 nm (all concentrations < 0.25 mM) to a peak emissions wavelength around 430 nm (all concentrations > 4 mM) (Fig. 6C).Due to the quenching effect of Triton and the lack of quenching observed with higher concentrations of SDS, we decided to use these detergents to mimic a mixed lipid composition environment. We hypothesized that, as the molar ratio shifted from a large Triton concentration to a large SDS concentration, we would observe increasing emission intensities. Indeed, a broad range of emission intensities from 100 μM AT5 from 753.4 ± 49.5 RFUs (100 mM Triton: 0 mM SDS) to 8705 ± 872 RFUs (0 mM Triton: 100 mM SDS) (Fig. 7A and B). This implies that in regions of anionic lipids (as opposed to neutral lipids), AT5 would have increased fluorescence. Peak emission wavelengths were also shown to slightly shift as the detergent concentration shifted from Triton to SDS (Fig. 7C), matching those observed in high concentrations of Triton and SDS (Fig. 6A, D, 7C). Ionic detergents such as CTAB and SDS can affect fluorescence through their ability to form, or disrupt, hydrogen bonds.49,50 These data potentially indicate a possible interruption of the hydrogen bonding of water to AT5. It is also possible for ionic interactions between the anionic head group of SDS with the amide nitrogen of AT5 on its side chain leading to an effect the fluorescent properties of AT5, while the blue-shift observed in all detergents may be due to increasing hydrophobic interactions.51
Conclusions
Aurones are a widely researched class of flavonoid with many bioactivities. Recently there has been increased interest in their use as fluorescent probes. “Click” 1,2,3-triazole structures have also been shown to have potential use as fluorophores as well. Recently, our group has synthesized and characterized aurone-derived triazoles. Five of these aurone-derived triazoles (AT1–5) were examined for their potential use as live-cell fluorescent probes. Of these five, AT2 and AT5 fluoresced in cells, but AT5 was the only compound which was not substantially cytotoxic, potentially due to its R′ group. AT5 was shown to have high fluorescence intensity in polar protic solvents, potentially implying the need for AT5 to act as a hydrogen bond acceptor, though further study is warranted. In micellular models, emissions of AT5 were quenched in detergents at most concentrations below the CMC, though the fluorescence recovery observed in ionic detergents indicates the potential for ionic interactions with AT5, and their potential effect on the fluorescent properties of AT5. Here AT5 was shown to have potential as a fluorophore in live-cell microscopy.Author contributions
Daniel L. Bryant: conceptualization, data curation, formal analysis, investigation, project administration, validation, visualization, and writing - original draft. Arjun Kafle: conceptualization, data curation, formal analysis, methodology, resources, and writing – original draft. Anthony L. Farone: funding acquisition, supervision, and writing – review & editing. Scott T. Handy: conceptualization, funding acquisition, supervision, and writing – review & editing. Justin M. Miller: conceptualization, funding acquisition, project administration, supervision, visualization, and writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would all like to acknowledge the Molecular Biosciences program at Middle Tennessee State University for financial support as well as David Nelson PhD for insight and training. The authors would also like to acknowledge Shrijana Bhattarai for her contributions and support.Notes and references
- S. Asen, K. H. Norris and R. N. Stewart, Phytochemistry, 1972, 11, 2739–2741 CrossRef CAS.
- G. Sui, T. Li, B. Zhang, R. Wang, H. Hao and W. Zhou, Bioorg. Med. Chem., 2021, 29, 115895 CrossRef CAS.
- H. S. Park, D. E. Nelson, Z. E. Taylor, J. B. Hayes, K. D. Cunningham, B. A. Arivett, R. Ghosh, L. C. Wolf, K. M. Taylor, M. B. Farone, S. T. Handy and A. L. Farone, Int. Immunopharmacol., 2017, 43, 116–128 CrossRef CAS PubMed.
- S. Y. Shin, M. C. Shin, J.-S. Shin, K.-T. Lee and Y. S. Lee, Bioorg. Med. Chem. Lett., 2011, 21, 4520–4523 CrossRef CAS PubMed.
- B. P. Bandgar, S. A. Patil, B. L. Korbad, S. C. Biradar, S. N. Nile and C. N. Khobragade, Eur. J. Med. Chem., 2010, 45, 3223–3227 CrossRef CAS PubMed.
- M.-Y. Song, G.-S. Jeong, H.-S. Lee, K.-S. Kwon, S.-M. Lee, J.-W. Park, Y.-C. Kim and B.-H. Park, Biochem. Biophys. Res. Commun., 2010, 400, 83–88 CrossRef CAS PubMed.
- F. M. Alqahtani, B. A. Arivett, Z. E. Taylor, S. T. Handy, A. L. Farone and M. B. Farone, PLOS ONE, 2019, 14, e0226068 CrossRef CAS PubMed.
- O. Kayser, A. F. Kiderlen and R. Brun, Planta Med., 2001, 67, 718–721 CrossRef CAS PubMed.
- G. Alsaif, N. Almosnid, I. Hawkins, Z. Taylor, D. L. T. Knott, S. Handy, E. Altman and Y. Gao, Curr. Pharm. Biotechnol., 2017, 18, 384–390 CAS.
- R. Václavíková, E. Kondrová, M. Ehrlichová, A. Boumendjel, J. Kovář, P. Stopka, P. Souček and I. Gut, Bioorg. Med. Chem., 2008, 16, 2034–2042 CrossRef PubMed.
- N. J. Lawrence, D. Rennison, A. T. McGown and J. A. Hadfield, Bioorg. Med. Chem. Lett., 2003, 13, 3759–3763 CrossRef CAS.
- N. Shanker, O. Dilek, K. Mukherjee, D. W. McGee and S. L. Bane, J. Fluoresc., 2011, 21, 2173–2184 CrossRef CAS PubMed.
- C. Espinosa-Bustos, D. Cortés-Arriagada, M. A. Soto-Arriaza, J. Robinson-Duggon, N. Pizarro, A. R. Cabrera, D. Fuentealba and C. O. Salas, Photochem. Photobiol. Sci., 2017, 16, 1268–1276 CrossRef CAS.
- Y. Xue, Y. Dou, L. An, Y. Zheng, L. Zhang and Y. Liu, RSC Adv., 2016, 6, 7002–7010 RSC.
- K. Muñoz-Becerra, N. Villegas-Escobar, C. Zúñiga-Loyola, D. Cortés-Arriagada and A. Toro-Labbé, Mol. Phys., 2019, 117, 1451–1458 CrossRef.
- H. Chen, Y. Sun, C. Zhou, D. Cao, Z. Liu and L. Ma, Spectrochim. Acta, Part A, 2013, 116, 389–393 CrossRef CAS PubMed.
- H. Chen, Z. Liu, D. Cao, S. Lu, J. Pang and Y. Sun, Sens. Actuators, B, 2014, 199, 115–120 CrossRef CAS.
- Y. Xu, H. Chen, R. Guan, D. Cao, Q. Wu and X. Yu, Fibers Polym., 2016, 17, 181–185 CrossRef CAS.
- M. Zhang, Y.-T. Bao, W. Yang, H.-F. Xiao, Z.-X. Han, X. Wu and L. Yang, J. Heterocycl. Chem., 2018, 55, 1130–1135 CrossRef CAS.
- A. Kafle, S. Bhattarai, J. M. Miller and S. T. Handy, RSC Adv., 2020, 10, 45180–45188 RSC.
- T. A. Geissman and J. B. Harborne, J. Am. Chem. Soc., 1955, 77, 4622–4624 CrossRef CAS.
- R. S. Varma and M. Varma, Tetrahedron Lett., 1992, 33, 5937–5940 CrossRef CAS.
- X. Qi, R. Li and X.-F. Wu, RSC Adv., 2016, 6, 62810–62813 RSC.
- T.-T. Jong and S.-J. Leu, J. Chem. Soc., Perkin Trans. 1, 1990, 423–424, 10.1039/P19900000423.
- H. Harkat, A. Blanc, J.-M. Weibel and P. Pale, J. Org. Chem., 2008, 73, 1620–1623 CrossRef CAS PubMed.
- I. Hawkins and S. T. Handy, Tetrahedron, 2013, 69, 9200–9204 CrossRef CAS.
- A. Kafle, S. Bhattarai and S. T. Handy, Synthesis, 2020, 52, 2337–2346 CrossRef CAS.
- D. Ghosh, S. Rhodes, K. Hawkins, D. Winder, A. Atkinson, W. Ming, C. Padgett, J. Orvis, K. Aiken and S. Landge, New J. Chem., 2015, 39, 295–303 RSC.
- Y.-C. Zhang, R. Jin, L.-Y. Li, Z. Chen and L.-M. Fu, Molecules, 2017, 22, 1380 CrossRef.
- Q. J. Meisner, J. V. Accardo, G. Hu, R. J. Clark, D.-e. Jiang and L. Zhu, J. Phys. Chem. A, 2018, 122, 2956–2973 CrossRef CAS.
- H. E. Gottlieb, V. Kotlyar and A. Nudelman, J. Org. Chem., 1997, 62, 7512–7515 CrossRef CAS PubMed.
- J. Schindelin, I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, S. Preibisch, C. Rueden, S. Saalfeld, B. Schmid, J.-Y. Tinevez, D. J. White, V. Hartenstein, K. Eliceiri, P. Tomancak and A. Cardona, Nat. Methods, 2012, 9, 676–682 CrossRef CAS.
- W. Li, M. Zhang, J. Zhang and Y. Han, Front. Chem., 2006, 1, 438–442 Search PubMed.
- Y. Moroi, K. Motomura and R. Matuura, J. Colloid Interface Sci., 1974, 46, 111–117 CrossRef CAS.
- B. Hammouda, J. Res. Natl. Inst. Stand. Technol., 2013, 118, 151–167 CrossRef CAS PubMed.
- K. Kalyanasundaram and J. K. Thomas, J. Am. Chem. Soc., 1977, 99, 2039–2044 CrossRef CAS.
- S. K. Hait and S. P. Moulik, J. Surfactants Deterg., 2001, 4, 303–309 CrossRef CAS.
- D. Koley and A. J. Bard, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 16783–16787 CrossRef CAS.
- G. E. Tiller, T. J. Mueller, M. E. Dockter and W. G. Struve, Anal. Biochem., 1984, 141, 262–266 CrossRef CAS.
- A. Patist, S. S. Bhagwat, K. W. Penfield, P. Aikens and D. O. Shah, J. Surfactants Deterg., 2000, 3, 53–58 CrossRef CAS.
- C. Reichardt and T. Welton, in Solvents and Solvent Effects in Organic Chemistry, Ed. C. Reichardt and T. Welton, 2010425–508, 549–586, DOI: DOI:10.1002/9783527632220.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- M. J. Kamlet, J. L. Abboud and R. W. Taft, J. Am. Chem. Soc., 1977, 99, 6027–6038 CrossRef CAS.
- Y. Marcus, The Properties of Solvents, John Wiley & Sons Ltd, West Sussex, England, 1998 Search PubMed.
- H. Tian, J. Qian, H. Bai, Q. Sun, L. Zhang and W. Zhang, Anal. Chim. Acta, 2013, 768, 136–142 CrossRef CAS PubMed.
- D. Tai and J. Liu, Luminescence, 2015, 30, 358–361 CrossRef CAS PubMed.
- R. M. Garavito and S. Ferguson-Miller, J. Biol. Chem., 2001, 276, 32403–32406 CrossRef CAS PubMed.
- A. M. Seddon, P. Curnow and P. J. Booth, Biochim. Biophys. Acta, Rev. Biomembr., 2004, 1666, 105–117 CrossRef CAS.
- O. S. Nnyigide, S.-G. Lee and K. Hyun, Sci. Rep., 2019, 9, 10643 CrossRef.
- S. Javadian, V. Ruhi, A. Heydari, A. Asadzadeh Shahir, A. Yousefi and J. Akbari, Ind. Eng. Chem. Res., 2013, 52, 4517–4526 CrossRef CAS.
- H. Li, T.-Z. Xie, Z. Liang, D. Dahal, Y. Shen, X. Sun, Y. Yang, Y. Pang and T. Liu, Chem. Commun., 2019, 55, 330–333 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02578g |
| This journal is © The Royal Society of Chemistry 2022 |