 Open Access Article
Open Access ArticleDynamic reversible adhesives based on crosslinking network via Schiff base and Michael addition†
Junyu Rena,
Hongxing Yanga,
Yingchen Wua,
Sichen Liu a,
Kelu Nia,
Xin Rana,
Xiaojian Zhoua,
Wei Gaoa,
Guanben Du*ab and
Long Yang
a,
Kelu Nia,
Xin Rana,
Xiaojian Zhoua,
Wei Gaoa,
Guanben Du*ab and
Long Yang *ab
*ab
aInternational Joint Research Center for Biomass Materials, Yunnan Province Key Lab of Wood Adhesives and Glued Products, Southwest Forestry University, Kunming, 650224, China. E-mail: guanben@swfu.edu.cn; long133109070@126.com
bKey Laboratory for Forest Resources Conservation and Utilization in the Southwest Mountains, Ministry of Education, Southwest Forestry University, Kunming, 650224, China
First published on 18th May 2022
Abstract
It is of practical interest to obtain polymers with complex material properties in a simplified synthetic manner for a broader range of practical applications. In this work, we constructed a dynamic reversible adhesive based on branched polyamine (PA) and p-formylphenyl acrylate (FPA) by simultaneously performing Michael addition reaction and Schiff base reaction. Branched polyamines provide a large number of amino groups as reaction sites that can react with both carbon–carbon double bonds and aldehyde groups. This enables the branched polymeric adhesive system to have a large number of Schiff base bonds within it, an important property of Schiff base bonds is that they are dynamically reversible. This allows us to prepare adhesives with hyperbranched crosslinking networks and recycling properties, and we have verified that FPA–PA adhesives do not exhibit significant fatigue after multiple recycling through the gluing-destruction-gluing process. The resulting FPA–PA adhesives produce tough bonding on multi-substrates such as steel, aluminum, glass, PVC, PTFE, birch and moso bamboo, which exhibited by lap shear strength of 2.4 MPa, 1.7 MPa, 1.4 MPa, 1.3 MPa, 0.4 MPa, 1.6 MPa, and 1.8 MPa, respectively. The feasibility of the synthesis idea of simultaneous Michael addition reaction and Schiff base reaction was demonstrated, as well as the excellent performance and great application potential of FPA–PA adhesives to be recyclable on multi-substrates.
1. Introduction
Modern functional adhesives often face more complex application environments, which means that adhesives need increasingly complex properties and often require multiple functions.1–5 Numerous researchers have developed and prepared adhesives with underwater adhesion,6–11 multiple substrate adhesion,12–16 stretchability,17,18 self-healing,19 biosustainability,20–22 moldability,23 etc. However, this not only requires a more complex structure of the polymer but also means that the conditions and steps required for synthesis become more cumbersome.24,25 How to prepare multi-functional adhesives by a more simplified route is of greater importance for the research development of adhesive polymers.26 Obviously, the choice of reaction substrate and the type of reaction chosen are important guidelines for the preparation of multifunctional adhesives. The preparation of multifunctional adhesives by Michael addition reaction27–31 and Schiff base reaction32–36 synthesis becomes an attractive solution.Michael addition is a conjugate addition reaction through an electrophilic electron acceptor with a nucleophilic electron donor and is one of the more established classes of common methods for growing carbon chains. For example, the hyperbranched polymers with hydrophobic main chains and hydrophilic side chains prepared by Liu et al.37 form cohesion by self-aggregation of hydrophobic interactions and replace water molecules on the adhering surfaces in the aqueous environment thus achieving wet adhesion. Schiff bases are formed by the condensation of amines and active carbonyl groups, which are a class of aldehydes and ketones containing carbonyl groups that undergo nucleophilic addition to amines, and have a wide range of applications in the field of adhesives. Such as, Chen et al.38 enabled the rapid adhesion of hydrogels and organogels at the interface of macroscopic gel sheets to form macroscopic organic hydrogel mixtures by Schiff base bonding. Interestingly their reaction conditions are similar, which leads us to speculate that the polymers produced by the Michael addition reaction and the Schiff base reaction can be obtained in a single step.
Meanwhile, the modification of adhesive polymers by the introduction of hyperbranched polymers39–41 has recently become a new research hotspot due to the excellent properties of hyperbranched polymers. Hyperbranched polymers are very different from linear polymers in terms of physicochemical properties. For example, hyperbranched polymers have a viscosity that does not change with molecular weight, are end-rich in reactive functional groups, are relatively simple to prepare, and can often be synthesized in a “one-step” process. Many studies,42–44 including our previous work,45 have shown that hyperbranched polymers have great potential for applications in adhesives.
In this work, we report the construction of a novel series of hyperbranched polymeric FPA–PA adhesives by a one-step method using branched polyamines PA4N, PA5N, and PA6N as reaction substrates and p-formylphenyl acrylate (FPA)46–50 with both carbon–carbon double bonds and aldehyde groups (Scheme 1). Branched polyamines obtained from the reaction of methyl acrylate with ethylenediamine, diethylenetriamine and triethylenetetramine have the advantage of being easy to prepare and having a large number of amino groups. Branched polyamines contain a large number of amino groups, which provide abundant active sites for modification, and the FPA molecules contain both carbon–carbon double bonds and aldehyde groups. The carbon–carbon double bond and the amino group can undergo Michael addition reactions, which are easy to carry out and usually do not require additional catalysts or produce additional products. Besides, the aldehyde group and the amino group can undergo Schiff base reactions rapidly. Because of the similarities in the conditions required for these two types of reactions, we can obtain FPA–PA adhesives produced from branched polyamines and FPA by Michael addition reaction and Schiff base reaction in a single step. It exhibits strong bonding performance on the glass, aluminum, steel, wood, bamboo, PTFE, and PVC substrates due to the strong cross-linked structural network established by FPA–PA adhesives through multiple interactions and the tough bonding on different substrates. At the same time, there are a large number of imine bonds in FPA–PA adhesives. Since the Schiff base reaction is an equilibrium reaction, i.e., the aldehyde group and the amino group can easily remove a molecule of water to generate imine bonds, and at the same time, the imine bonds can easily absorb a molecule of water to generate the aldehyde group and the amino group again, so the imine bonds are dynamic and reversible.51–54 The results revealed that the FPA–PA adhesives have dynamic reversible properties, and it has no fatigue in FPA–PA adhesives after cycling the gluing–destroying–gluing process 18 times. The adhesive strength did not vary much and remained at a similar level overall, indicating that FPA–PA adhesives have excellent reusability. Through Michael addition and Schiff base reactions we have prepared hyperbranched polymeric adhesives with more complex structures in a simplified route, enabling tough bonding on a wide range of substrates with dynamic reversibility and excellent bonding performance after multiple damage cycles. This study provides an important route for the preparation of multifunctional adhesives by a simplified route.
 | ||
| Scheme 1 Synthesis of FPA–PA branched polymeric adhesives and illustration of gluing to different substrates. | ||
2. Experimental section
2.1. Materials
Methyl acrylate, ethylenediamine, diethylenetriamine, triethylenetetramine, acryloyl chloride, triethylamine, p-hydroxybenzaldehyde, methanol (MeOH), and dichloromethane (DCM) were purchased from Shanghai Adamas Reagent Co., Ltd. (Shanghai, China). All the chemicals were used without any further purification.2.2. Characterizations methods
Differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), Fourier transform infrared spectrometer (ATR-FTIR), X-ray photoelectron spectroscopy (XPS), 1H NMR, and 13C NMR measurements were provided in the ESI.†2.3. Synthesis of branched polyamines PA4N, PA5N, and PA6N
The synthesis of branched polyamines PA4N, PA5N, and PA6N was carried out concerning the work of Yang et al.452.4. Synthesis and characterization of p-formylphenyl acrylate (FPA)
Adding p-hydroxybenzaldehyde (5 g, 41 mmol), triethylamine (6.5 mL), and DCM (50 mL) to a 250 mL round bottom flask, cool to 0 °C in an ice bath and stir until completely dissolved. Acryloyl chloride (4.5 g, 50 mmol) was dissolved in 10 mL of DCM and added dropwise to a round bottom flask. The reactants were then brought to room temperature overnight. After the reaction was completed, triethylamine hydrochloride was removed by filtration, extracted with saturated NaHCO3, and the organic phase was dried with Na2SO4 and the product was collected by rotary evaporation. After purification by silica gel chromatography (PE/EA = 20/1), the product was collected by rotary evaporation and drying with a yield of about 85%. The 1H NMR and 13C NMR spectra of the FPA were obtained (Fig. S1 and S2†).2.5. Synthesis of FPA–PA adhesives
As an example, PA6N–FPA1:6 was added to a 250 mL round bottom flask with 100 mL of methanol and PA6N (10 g, 12 mmol) and stirred to allow complete dissolution. After FPA (12.7 g, 72 mmol) was completely dissolved in 50 mL of methanol, it was added dropwise to a round bottom flask and heated to reflux overnight. After the reaction, the product was collected by rotary evaporation (yield ∼ 99%). The PA4N–FPA1:4, PA4N–FPA1:2, PA5N–FPA1:5, PA5N–FPA2:5, and PA6N–FPA1:3 adhesives were synthesized and prepared in the same manner as PA6N–FPA1:6 with molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 2
5, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, respectively.
3, respectively.
2.6. Adhesion test
Adhesion tests were all performed with glass, aluminum, steel, wood, bamboo, Teflon, and PVC substrates. All substrates are rectangular in shape 10 × 2.5 × 0.5 cm and have a hole at the end for a pin. The liquid adhesive was deposited on a 2.5 × 1.5 cm area substrate by heating and then covered with another substrate to form a substrate/adhesive/substrate sandwich structure, which was cured at room temperature for one day. We determined the thickness of the adhesive layer by vernier calipers to be about 0.5 mm ± 0.1 mm. The lap shear strength test was performed on an ETM-104B tension machine (Shenzhen Wance Testing Machine Co., Ltd). The lap shear strength test stretches the bonded joint at a displacement rate (2 mm min−1) until the base material separates. After testing, the strength was calculated by dividing the lap area measured with vernier calipers by the fracture load (N). Parallel experiments were performed using six samples and the average values were calculated.Wood-based substrate gluing properties were evaluated by using the birch and moso bamboo. The FPA–PA adhesive (80% or 100% solid content) was applied to the birch or moso bamboo substrate and then covered with another substrate to form a substrate/adhesive/substrate sandwich structure, which cured at room temperature for one day in the absence and presence of a 5 MPa pressure. Parallel experiments were performed using six samples and the average values were calculated.
Dynamic reversible cycle testing was performed using PA5N–FPA2:5 adhesive on steel and glass substrates to test the lap shear strength. The re-curing process was heated at 170 °C for 5 minutes and lap-glued again at room temperature for 30 minutes without pressure. After cooling to room temperature, the lap shear strength test was performed. Six samples were used for parallel experiments and the average value was calculated.
3. Results and discussion
3.1. DSC results analysis
The temperature has a large effect on adhesives and is an important factor in evaluating adhesive systems. For example, the temperature has different meanings for thermoset adhesives and thermoplastic adhesives. We used DSC to analyze the thermodynamic behavior of FPA–PA adhesives at a heating rate of 10 °C min−1, and the results are shown in Fig. 1. The thermodynamic behavior of the six adhesives was very similar, with the heat absorption peak of PA4N–FPA1:4 at approximately 228 °C and that of PA4N–FPA1:2 at approximately 232 °C. The heat absorption peak of PA5N–FPA1:5 was at approximately 236 °C and that of PA5N–FPA2:5 at approximately 240 °C. The heat absorption peak of PA6N–FPA1:6 was at approximately 233 °C, while the peak of heat absorption of PA6N–FPA1:3 is about 235 °C. The peak of heat absorption of PA6N–FPA1:3 is about 235 °C. It can be seen that the prepared FPA–PA adhesives have the characteristics of heat absorption and melting, so FPA–PA adhesives are typical hot melt adhesives. Interestingly, the position of the heat absorption peak is lower for all systems with higher FPA content, which indicates that the thermal melting point of the hyperbranched polymer network is lower as the FPA increases in the system, implying that an increase in FPA content may suppress the cross-linking degree of the FPA–PA branched polymer network.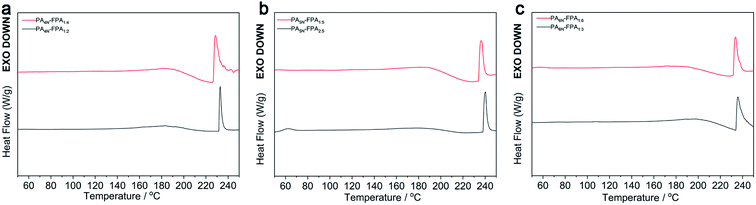 | ||
| Fig. 1 (a) DSC spectra of PA4N–FPA1:4 and PA4N–FPA1:2. (b) DSC spectra of PA5N–FPA1:5 and PA5N–FPA2:5. (c) DSC spectra of PA6N–FPA1:6 and PA6N–FPA1:3. | ||
3.2. TGA results analysis
As shown in Fig. 2, we used a thermogravimetric analyzer (TGA) to study the thermal stability of FPA–PA adhesive. From the results, PA4N–FPA1:4, PA4N–FPA1:2, PA5N–FPA1:5, PA5N–FPA2:5, PA6N–FPA1:6, and PA6N–FPA1:3 polymers exhibited similar weight loss processes during the warming process. A very slow weight loss occurred in the temperature range of 100–200 °C. The FPA–PA adhesive exhibited very good thermal stability. The weight-loss rates of PA4N–FPA1:4, PA5N–FPA1:5, and PA6N–FPA1:6 adhesives were significantly accelerated at around 200 °C and then decreased, and increased again at around 350 °C. The PA4N–FPA1:2, PA5N–FPA2:5, and PA6N–FPA1:3 adhesives, on the other hand, reach their highest weight loss rates at about 300 °C and then level off. All FPA–PA adhesives still have approximately 20% mass remaining. Compared with PA4N, PA5N and PA6N, the FPA–PA adhesives have better thermal stability. This is because the introduction of FPA monomer generates a cross-linked network and a large number of benzene rings form a π–π stacking structure, which gives the polymer a dense network structure and acts as a thermal barrier.Interestingly, PA4N–FPA1:4, PA5N–FPA1:5, and PA6N–FPA1:6 had the highest weight loss rates at around 200 °C, while PA4N–FPA1:2, PA5N–FPA2:5, and PA6N–FPA1:3 had the highest weight loss rates around 300 °C. This may be because excess FPA occupies more amino sites in the polymer network, making the excess carbon–carbon double bonds as well as aldehyde groups unable to continue the reaction thus reducing the cross-linking degree of the cross-linked network. This means that the introduction of more FPA does not better improve the thermal stability of the polymer and may suppress the degree of cross-linking of the hyperbranched polymer network, which is also consistent with the DSC results.
3.3. ATR-FTIR results analysis
Fig. 3 shows the attenuated total reflectance (ATR) FTIR spectra of PA4N–FPA1:4, PA4N–FPA1:2, PA4N, PA5N–FPA1:5, PA5N–FPA2:5, PA5N, PA6N–FPA1:6, PA6N–FPA1:3, and PA6N. This reflects the consumption status of FPA during the reaction with polyamines. The characteristic ATR-FTIR peaks observed in the branched polymers, including ν(N–H) located at 3274 cm−1, and δ (N–H) located at 1551 cm−1 (amide II bands), which is attributed to the abundance of amide bonds in the polymer. The characteristic peaks ν(C–H) located at 2828 and 2943 cm−1 represent the methylene CH2 on the branched chain of the polymer. Compared with the ATR-FTIR spectra of PA4N, PA5N, and PA6N, the ATR-FTIR spectra of FPA–PA adhesives appeared with new characteristic peaks at 839 cm−1 and 1160 cm−1, which are ascribed to γ(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) of the out-of-plane bending vibration (para-substitution) of the benzene ring structure and ν(C–O) of the ester group, respectively. This indicates the successful introduction of FPA in FPA–PA adhesives. Compared to PA4N, PA5N, and PA6N, new characteristic peaks can be seen at about 1644 cm−1 in the ATR-FTIR spectra of FPA–PA adhesives, which is the characteristic peak of the ν(C
C–H) of the out-of-plane bending vibration (para-substitution) of the benzene ring structure and ν(C–O) of the ester group, respectively. This indicates the successful introduction of FPA in FPA–PA adhesives. Compared to PA4N, PA5N, and PA6N, new characteristic peaks can be seen at about 1644 cm−1 in the ATR-FTIR spectra of FPA–PA adhesives, which is the characteristic peak of the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) imine bond formed by the reaction of the amino and aldehyde groups. We observed a new aldehyde group characteristic peak of the ν(C
N) imine bond formed by the reaction of the amino and aldehyde groups. We observed a new aldehyde group characteristic peak of the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 1735 cm−1 for PA4N–FPA1:4, PA5N–FPA1:5, and PA6N–FPA1:6, which was absent from PA4N–FPA1:2, PA5N–FPA2:5, and PA6N–FPA1:3. This is due to the excess of FPA in PA4N–FPA1:4, PA5N–FPA1:5, and PA6N–FPA1:6, where the aldehyde group is not completely consumed. Also, a more pronounced peak appears at 1250 cm−1, which is due to the addition reaction of carbon–carbon double bonds and amino groups to produce more ν(C–N). This preliminarily confirmed that the carbon–carbon double bond in FPA had a Michael addition reaction with the amino group in PA, and the formation of the imine bond also proved that the aldehyde group in FPA had a Schiff base reaction with the amino group in PA.
O) at 1735 cm−1 for PA4N–FPA1:4, PA5N–FPA1:5, and PA6N–FPA1:6, which was absent from PA4N–FPA1:2, PA5N–FPA2:5, and PA6N–FPA1:3. This is due to the excess of FPA in PA4N–FPA1:4, PA5N–FPA1:5, and PA6N–FPA1:6, where the aldehyde group is not completely consumed. Also, a more pronounced peak appears at 1250 cm−1, which is due to the addition reaction of carbon–carbon double bonds and amino groups to produce more ν(C–N). This preliminarily confirmed that the carbon–carbon double bond in FPA had a Michael addition reaction with the amino group in PA, and the formation of the imine bond also proved that the aldehyde group in FPA had a Schiff base reaction with the amino group in PA.
 | ||
| Fig. 3 (a) ATR-FTIR spectra of PA4N–FPA1:4, PA4N–FPA1:2 and PA4N. (b) ATR-FTIR spectra of PA5N–FPA1:5, PA5N–FPA2:5 and PA5N. (c) ATR-FTIR spectra of PA6N–FPA1:6, PA6N–FPA1:3 and PA6N. | ||
3.4. XPS results analysis
We further investigated the chemical composition and structure of FPA–PA adhesives using XPS. The PA4N–FPA1:2 adhesive showed C, N, O signals in XPS spectra as shown in Fig. 4a, where the C, N, and O atomic contents of PA4N–FPA1:2 were 73.79%, 16.86%, and 9.35%, respectively. As shown in Fig. 4b, the C 1s spectrum of PA4N–FPA1:2 has five peaks at 284.8 eV, 285.6 eV, 286.6 eV, 287.6 eV, and 288.7 eV, corresponding to the C–C, C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C
O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively.55 The N 1s spectrum of PA4N–FPA1:2 (Fig. 4c) has three peaks at 398.9 eV, 399.9 eV, and 401.2 eV, which are attributed to N
O groups, respectively.55 The N 1s spectrum of PA4N–FPA1:2 (Fig. 4c) has three peaks at 398.9 eV, 399.9 eV, and 401.2 eV, which are attributed to N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, N–C, and N–H groups, respectively.45 The O 1s spectrum of PA4N–FPA1:2 (Fig. 4d) has two peaks at 531.1 eV and 532.3 eV, attributed to the C
C, N–C, and N–H groups, respectively.45 The O 1s spectrum of PA4N–FPA1:2 (Fig. 4d) has two peaks at 531.1 eV and 532.3 eV, attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O groups, respectively.39 It can be found that the C
O and C–O groups, respectively.39 It can be found that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group can be observed in the C 1s spectrum, and the C
N group can be observed in the C 1s spectrum, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group can be observed in the N 1s spectrum. The results indicate that the Schiff base reaction occurred with the smooth formation of imine bonds between FPA and branched polyamines. The XPS spectra results of the PA4N–FPA1:4 adhesive (Fig. S3†) was similar to those of PA4N–FPA1:2.
N group can be observed in the N 1s spectrum. The results indicate that the Schiff base reaction occurred with the smooth formation of imine bonds between FPA and branched polyamines. The XPS spectra results of the PA4N–FPA1:4 adhesive (Fig. S3†) was similar to those of PA4N–FPA1:2.
3.5. NMR analysis of the adhesive
We further determined by 1H NMR spectrum whether there are excess carbon–carbon double bonds and aldehyde groups remaining in the polymer (Fig. 5, S4–S9†). We can see a distinct aldehyde group characteristic peak at about 10 ppm and a distinct carbon–carbon double bond characteristic peak at 6.0–6.7 ppm in the 1H NMR spectrum of FPA (Fig. S1†). In contrast, in the 1H NMR spectrum of PA4N–FPA1:4, PA4N–FPA1:2, PA5N–FPA1:5, PA5N–FPA2:5, PA6N–FPA1:6 and PA6N–FPA1:3, we did not observe the characteristic peaks of carbon–carbon double bonds at 6.0–6.7 ppm. In Fig. S5, S7, and S9,† we can observe the obvious characteristic peak of the aldehyde group at 9.7–10 ppm, which is absent in Fig. S4, S6, and S8.† This indicates that the carbon–carbon double bonds and aldehyde groups of FPA are almost completely consumed in PA4N–FPA1:2, PA5N–FPA2:5, and PA6N–FPA1:3. In PA4N–FPA1:4, PA5N–FPA1:5, and PA6N–FPA1:6, the carbon–carbon double bond of FPA is almost completely consumed and the aldehyde group remains. The amino group undergoes more addition reactions with the carbon–carbon double bond during the reaction, resulting in some aldehyde groups not being able to participate in the reaction and forming capped ends. The degree of cross-linking of PA4N–FPA1:4, PA5N–FPA1:5 and PA6N–FPA1:6 adhesives with excess FPA was reduced, which is consistent with the results of DSC and TG. | ||
| Fig. 5 1H NMR spectrum (600 MHz, CDCl3, rt) of PA4N–FPA1:4, PA4N–FPA1:2, PA5N–FPA1:5, PA5N–FPA2:5, PA6N–FPA1:6, and PA6N–FPA1:3. | ||
3.6. Analysis of the adhesion performance of different substrates
The adhesion of FPA–PA adhesives to a wide range of substrate surfaces is a distinctive feature. We tested the adhesion performance of FPA–PA adhesives by using various types of typical substrates such as steel, aluminum, glass, PVC, and PTFE. And the results are shown in Fig. 6a (Table S1†). From the results, it is known that FPA–PA adhesives have a tough adhesion on steel, aluminum, glass, PVC, and PTFE substrates. The introduction of FPA constructed a highly cross-linked polymer network structure via Schiff base and Michael addition reactions. Multiple groups in the polymer enable multiple interactions between the branched polymer and the substrate, resulting in high lap shear strength (Fig. 6b). For example, functional groups such as benzene rings, ester groups, and aldehyde groups can form interactions such as hydrogen bonds, electrostatic interactions, and metal chelating with the substrate.1 Compared to FPA unsaturated adhesives, FPA saturated adhesives such as PA4N–FPA1:4 achieve higher adhesion strength on steel and glass substrates. The NMR and FTIR results show that FPA saturated adhesives contain more aldehyde residues, which allows a large number of aldehyde groups to chelate with the steel surface and form more hydrogen bonds on the glass surface. Therefore, higher adhesion strength is achieved compared to FPA unsaturated adhesives. On PVC substrate, the FPA–PA adhesive crosses the interface with the substrate, spreading and twisting around each other to produce adhesion strength. The hydrogen atoms of the amino group can form coordination bonds with the surface of the PTFE substrate, resulting in strong bonding strength. It is the ability of FPA–PA adhesives to interact with a wide range of substrates in various covalent and non-covalent ways that gives FPA–PA adhesives their strong bonding strength on different substrate surfaces.1,45At the same time, we tested the glue stability and adhesion performance of FPA–PA adhesive soaked in water (Fig. S10†). FPA unsaturated adhesives such as PA5N–FPA2:5 completely lost bond strength after 24 h, while FPA saturated adhesives such as PA5N–FPA1:5 lost strength completely after 48 h. Obviously, the increase in the amount of FPA enhances the water-resistance of the adhesive. This is because a large number of hydrophobic structures in FPA, such as the large stacking of ester groups and benzene rings, form a barrier and prolong the time required for hydrolysis. It shows the possibility of improving water-resistance by introducing hydrophobic groups and provides ideas for further modification.
3.7. Analysis of wood substrate adhesion properties
We have investigated the adhesion performance of FPA–PA adhesives on wood materials using birch and moso bamboo as substrates. In Fig. 7a, FPA–PA adhesive (80% solid content) was used and cured at room temperature for 1 day. In Fig. 7b, FPA–PA adhesive (100% solid content) was used and cured at room temperature for 1 day. In Fig. 7c, FPA–PA adhesive (100% solid content) was used and cured for 1 day at room temperature under 5 MPa pressure. The FPA–PA adhesive (80% solid content) contains a 20% mass fraction of methanol present. Compared to FPA–PA adhesive (100% solid content), it has a certain degree of fluidity at room temperature and is easier to apply.In the results of Fig. 7a, there is a tendency to increase the adhesion strength of FPA–PA adhesives with increasing number of terminal amino groups. This may be due to the higher degree of branched crosslinking in the crosslinking network structure of FPA–PA adhesives as the number of terminal amino groups increases. Compared to moso bamboo substrate, birch substrate has a rougher surface and more pores. Since FPA–PA adhesive (80% solid content) has better flow ability, it can able to interact sufficiently with rough birch wood substrate to form mechanical mosaic, and the bonding strength is higher than in moso bamboo substrate. However, the results in Fig. 7b demonstrate the trend of decreasing adhesion strength of FPA–PA adhesives (100% solid content) on wood substrates with the number of terminal amino groups and the degree of cross-linking. This is probably because the FPA–PA adhesive (100% solid content) loss of good flowability, making it difficult for the adhesive to interact sufficiently with the substrate. The rough, porous birch substrate has less actual contact with the adhesive, so its adhesion strength is lower on birch substrates than on moso bamboo substrate. Moreover, PA4N–FPA1:4, PA5N–FPA1:5 and PA6N–FPA1:6 adhesives (80% solid content and 100% solid content) failed to finish curing after one day of curing at room temperature. This is due to the fact that the degree of cross-linking of the polymer is reduced due to the excess of FPA, so the curing process becomes more difficult. When the curing time is extended, it is also able to finish curing on wood and bamboo substrates. This shows that FPA–PA adhesives can be cured on steel, aluminum, glass, PVC, PTFE, wood, and bamboo, but the requirements for curing time are different, which is also due to the different surface groups and surface energies (Table S2 and S3†) of different substrates. In the results of Fig. 7c, using FPA–PA adhesives (100% solid content) and applying 5 MPa pressure, we found that the PA4N–FPA1:4, PA5N–FPA1:5 and PA6N–FPA1:6 adhesives were also fully cured. Without pressure, we measured the thickness of the adhesive layer to be 0.5 mm ± 0.1 mm, while the thickness of the pressed adhesive layer was 0.3 mm ± 0.1 mm. This also proves that the contact between the adhesive layer and the substrate will be much closer. This indicates that the adhesive layer and the substrate are in closer contact due to pressure, forcing a tighter bond between the adhesive and the substrate to facilitate the curing of the adhesive.
3.8. Dynamic reversible cycle test analysis
We tested the lap shear strength by using PA5N–FPA2:5 adhesive on steel and glass substrates and then heated the separated substrates and the residual adhesive to bond them again. The lap shear strength was tested after curing at room temperature for 30 minutes, and the results of repeating the previous operation are shown in Fig. 8a. We can see that PA5N–FPA2:5 adhesive has no fatigue phenomenon after many cycles, and the strength of 18 cycles does not change much, which indicates its good reusability. And we analyzed the variation in storage modulus (G′) and loss modulus (G′′) of PA5N–FPA2:5 adhesive over a temperature range of 80 °C to 140 °C, and the heating rate of 5 °C min−1 (Fig. 8b). Both G′′ and G′ increased when the temperature decreased from 140 °C to 90 °C, and G′′ started to override G′ at 99 °C, which means that curing behavior occurred. At the same time, the variation of storage modulus (G′) and loss modulus (G′′) was studied for strains from 0.01 to 1000 (%) at 160 °C (Fig. S11†).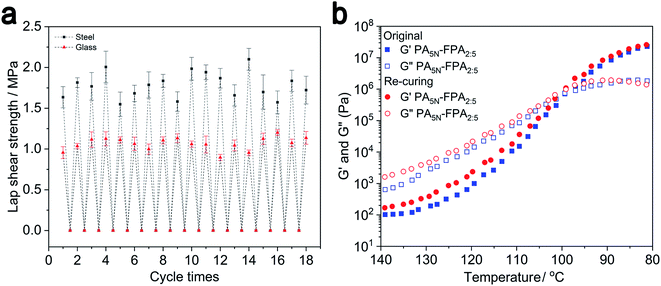 | ||
| Fig. 8 (a) Schematic diagram of the strength of PA5N–FPA2:5 glued–destroyed–glued cycles on steel and glass substrates. (b) Temperature-dependent variation in G′ and G′′ of PA5N–FPA2:5 adhesive. | ||
Clearly, the G′ and G′′ values of the adhesive increase rapidly with decreasing temperature, with a clear intersection at approximately 99 °C. The G′ and G′′ values decrease rapidly with increasing temperature, implying owing to the dynamic breakage of noncovalent interactions in polymer networks, such as hydrogen bonds and Schiff base bonds. As the temperature decreased, the noncovalent interactions in the polymer network recovered again such that the G′ and G′′ values rose rapidly and eventually recovered almost completely. As the strain increases from 0.01% to 100%, the G′ and G′′ values of the adhesive remain almost constant. Even when very high strains of γ = 1000% were applied to induce fracture or detrimental damage to the adhesive, efficient healing was observed after the adhesive was allowed to relax back to low strain. This is due to the fact that even though a large number of dynamic bonds have been broken at high temperatures, the polymer itself still has an entangled network structure. When the strain is too high, the disruption of chain-to-chain entanglement and friction leads to a rapid decrease in G′ and G′′. When the strain decreases, it is clear that the degree of entanglement between the chains is not fully recovered, and the values of G′ and G′′ are recovered by about half. At the same time at 80 °C, we see that the G′ and G′′ of the adhesive can reach 2.57 × 107 Pa and 1.8 × 106 Pa, respectively, which are much higher than the values of G′ and G′′ at 160 °C. This indicates that the main source of the mechanical strength of the adhesive is the noncovalent interactions force that exists abundantly in the cross-linked network structure. This makes FPA–PA adhesives have strong reusability and have important application value.
4. Conclusions
In this study, we constructed a new kind of FPA–PA adhesives by using branched polyamines (PA4N, PA5N, and PA6N) to react with FPA monomers by performing Michael addition reactions and Schiff base reactions. The ability to bond on a wide range of substrates such as steel, aluminum, glass, PVC, PTFE, birch, and moso bamboo is due to the complex and diverse functional groups in FPA–PA adhesives that provide a variety of covalent and non-covalent bonding forces. Interestingly, the Michael addition reaction and the Schiff base reaction can occur simultaneously under the same conditions, allowing the final synthetic preparation to be obtained in a single reaction, which has obvious advantages in terms of industrial production. The bonding performance of various substrates and FPA–PA adhesives with different solid contents were studied. The results show that FPA–PA adhesives have a good bonding effect on steel, aluminum, glass, PVC, and PTFE substrates. Due to enough imine bonds present in its structure, the FPA–PA adhesives exhibited excellent recyclability, which has no fatigue after 18 cycles of gluing–destroying–gluing on glass and steel substrates. It demonstrates the excellent performance and great application potential of FPA–PA adhesives for recyclability on a wide range of substrates.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Applied Basic Research Program of Yunnan Province (No. 202001AW070017, 202101AT070047), National Natural Science Foundation of China (No. 32171884, 32060323, 32071688), and the Key Project of National Natural Science Foundation of China (No. 30930074). G. D. acknowledges Program for Leading Talents, Department of Science and Technology of Yunnan Province (No. 2017HA013). L. Y. acknowledges Candidates of the Young and Middle-Aged Academic Leaders of Yunnan Province (202105AC160048), the Ten Thousand Talent Program for Young Top-notch Talents of Yunnan Province (YNWR-QNBJ-2020-136), the National Post-Doctoral Innovative Talent Support Program of China (No. BX20190291), and the 111 project (D21027).References
- C. Cui, R. Gu, T. Wu, Z. Yuan, C. Fan, Y. Yao, Z. Xu, B. Liu, J. Huang and W. Liu, Adv. Funct. Mater., 2022, 32, 2109144 CrossRef CAS.
- B. D. B. Tiu, P. Delparastan, M. R. Ney, M. Gerst and P. B. Messersmith, ACS Appl. Mater. Interfaces, 2019, 11, 28296–28306 CrossRef CAS PubMed.
- Z. Wang, S. Zhang, S. Zhao, H. Kang, Z. Wang, C. Xia, Y. Yu and J. Li, Chem. Eng. J., 2021, 404, 127069 CrossRef CAS.
- Z. Wang, S. Zhao, R. Song, W. Zhang, S. Zhang and J. Li, Sci. Rep., 2017, 7, 1–10 CrossRef.
- X. Li, J. Lai, Y. Deng, J. Song, G. Zhao and S. Dong, J. Am. Chem. Soc., 2020, 142, 21522–21529 CrossRef CAS PubMed.
- Y. Zhou, C. Zhang, S. Gao, W. Li, J.-j. Kai and Z. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 50451–50460 CrossRef CAS PubMed.
- M. Dompé, F. J. Cedano-Serrano, M. Vahdati, L. van Westerveld, D. Hourdet, C. Creton, J. van der Gucht, T. Kodger and M. Kamperman, Adv. Mater. Interfaces, 2020, 7, 1901785 CrossRef.
- Y. Wang, V. Kang, W. Federle, E. Arzt and R. Hensel, Adv. Mater. Interfaces, 2020, 7, 2001269 CrossRef.
- M. Zhao, L. Bai and J. Jang, Appl. Surf. Sci., 2020, 511, 145589 CrossRef CAS.
- C. Liang, Z. Ye, B. Xue, L. Zeng, W. Wu, C. Zhong, Y. Cao, B. Hu and P. B. Messersmith, ACS Appl. Mater. Interfaces, 2018, 10, 25017–25025 CrossRef CAS PubMed.
- H. Y. Yuen, H. P. Bei and X. Zhao, Chem. Eng. J., 2021, 431, 133372 CrossRef.
- Z.-H. Liu, J.-Q. Huang, L.-J. Sun, D. Lei, J. Cao, S. Chen, W.-C. Shih, F.-L. Qing and Z.-W. You, Chinese. J. Polym. Sci., 2018, 36, 58–64 CrossRef CAS.
- J. Zhu, X. Lu, W. Zhang and X. Liu, Macromol. Rapid Commun., 2020, 41, 2000098 CrossRef CAS PubMed.
- H. M. Siebert and J. J. Wilker, ACS Sustain. Chem. Eng., 2019, 7, 13315–13323 CrossRef CAS.
- G. Schmidt, K. H. Smith, L. J. Miles, C. K. Gettelfinger, J. A. Hawthorne, E. C. Fruzyna and J. J. Wilker, Adv. Sust. Syst., 2022, 2100392 CrossRef.
- B. D. James, K. M. Kimmins, M.-T. Nguyen, A. J. Lausch and E. D. Sone, Sci. Rep., 2021, 11, 1–10 CrossRef PubMed.
- R. Zhang, H. Peng, T. Zhou, Y. Yao, X. Zhu, B. Bi, X. Zhang, B. Liu, L. Niu and W. Wang, ACS Appl. Polym. Mater., 2019, 1, 2883–2889 CrossRef CAS.
- D. Gan, W. Xing, L. Jiang, J. Fang, C. Zhao, F. Ren, L. Fang, K. Wang and X. Lu, Nat. Commun., 2019, 10, 1–10 CrossRef CAS PubMed.
- S. Song, H. Yang, Y. Cui, Y. Tang, Y. Chen, B. Yang, J. Yuan and J. Huang, Polymer, 2020, 198, 122528 CrossRef CAS.
- S. Zhao, Z. Wang, Z. Li, L. Li, J. Li and S. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 32414–32422 CrossRef CAS PubMed.
- H. Pang, Q. Yan, C. Ma, S. Zhang and Z. Gao, ACS Appl. Mater. Interfaces, 2021, 13, 59527–59537 CrossRef CAS PubMed.
- Z. Tang, M. Zhang, H. Xiao, K. Liu, X. Li, B. Du, L. Huang, L. Chen and H. Wu, ACS Biomater. Sci. Eng., 2022, 8, 1096–1102 CrossRef CAS PubMed.
- H. Cho, G. Wu, J. C. Jolly, N. Fortoul, Z. He, Y. Gao, A. Jagota and S. Yang, Proc. Natl. Acad. Sci. U.S.A., 2019, 116, 13774–13779 CrossRef CAS PubMed.
- Y. Okaya, Y. Jochi, T. Seki, K. Satoh, M. Kamigaito, T. Hoshino, T. Nakatani, S. Fujinami, M. Takata and Y. Takeoka, Macromolecules, 2019, 53, 374–386 CrossRef.
- G. P. Maier, M. V. Rapp, J. H. Waite, J. N. Israelachvili and A. Butler, Science, 2015, 349, 628–632 CrossRef CAS PubMed.
- Q. Zhang, C.-Y. Shi, D.-H. Qu, Y.-T. Long, B. L. Feringa and H. Tian, Sci. Adv., 2018, 4, eaat8192 CrossRef CAS PubMed.
- S. Omura, Y. Kawazoe and D. Uemura, Materials, 2021, 14, 5479 CrossRef CAS PubMed.
- A. Rather, N. Jana, S. Begum, H. Srivastava and U. Manna, Green Chem., 2017, 19, 4527–4532 RSC.
- Z. Zeng, X.-m. Mo, C. He, Y. Morsi, H. El-Hamshary and M. El-Newehy, J. Mater. Chem. B, 2016, 4, 5585–5592 RSC.
- B. D. Mather, K. Viswanathan, K. M. Miller and T. E. Long, Prog. Polym. Sci., 2006, 31, 487–531 CrossRef CAS.
- T. Xie, J. Ding, X. Han, H. Jia, Y. Yang, S. Liang, W. Wang, W. Liu and W. Wang, Mater. Horiz., 2020, 7, 605–614 RSC.
- Y. Wu, L. Yuan, N.-a. Sheng, Z.-q. Gu, W.-h. Feng, H.-y. Yin, Y. Morsi and X.-m. Mo, Front. Mater. Sci., 2017, 11, 215–222 CrossRef.
- J. Liu, J. Li, F. Yu, Y.-x. Zhao, X.-m. Mo and J.-f. Pan, Int. J. Biol. Macromol., 2020, 147, 653–666 CrossRef CAS PubMed.
- X. Gong, Z. Cheng, S. Gao, D. Zhang, Y. Ma, J. Wang, C. Wang and F. Chu, Carbohydr. Polym., 2020, 250, 116846 CrossRef CAS PubMed.
- J. Pang, S. Bi, T. Kong, X. Luo, Z. Zhou, K. Qiu, L. Huang, X. Chen and M. Kong, Carbohydr. Polym., 2020, 237, 116138 CrossRef CAS PubMed.
- H. Guo, S. Huang, A. Xu and W. Xue, Chem. Mater., 2022, 34, 2655–2671 CrossRef CAS.
- C. Cui, C. Fan, Y. Wu, M. Xiao, T. Wu, D. Zhang, X. Chen, B. Liu, Z. Xu and B. Qu, Adv. Mater., 2019, 31, 1905761 CrossRef CAS PubMed.
- G. Deng, Q. Ma, H. Yu, Y. Zhang, Z. Yan, F. Liu, C. Liu, H. Jiang and Y. Chen, ACS Macro Lett., 2015, 4, 467–471 CrossRef CAS.
- S. Liu, G. Du, H. Yang, H. Su, X. Ran, J. Li, L. Zhang, W. Gao and L. Yang, ACS Sustain. Chem. Eng., 2021, 9, 16849–16861 CrossRef CAS.
- T. Li, B. Zhang, S. Jiang, X. Zhou, G. Du, Z. Wu, M. Cao and L. Yang, ACS Sustain. Chem. Eng., 2020, 8, 5209–5216 CrossRef CAS.
- S. Liang, Y. Zhang, H. Wang, Z. Xu, J. Chen, R. Bao, B. Tan, Y. Cui, G. Fan and W. Wang, Adv. Mater., 2018, 30, 1704235 CrossRef PubMed.
- Y. Tang, C. K. Jim, Y. Liu, L. Ye, A. Qin, J. W. Lam, C. Zhao and B. Z. Tang, ACS Appl. Mater. Interfaces, 2010, 2, 566–574 CrossRef CAS PubMed.
- R. Mezzenga, L. Boogh and J.-A. E. Månson, Compos. Sci. Technol., 2001, 61, 787–795 CrossRef CAS.
- D. Lu, Y. Zhang, Y. Li, H. Wang, Z. Shen, Q. Wei and Z. Lei, Polym. Chem., 2016, 7, 1963–1970 RSC.
- H. Yang, G. Du, Z. Li, X. Ran, X. Zhou, T. Li, W. Gao, J. Li, H. Lei and L. Yang, ACS Appl. Polym. Mater., 2021, 3, 1638–1651 CrossRef CAS.
- E. Subramanyam, S. Mohandoss and H. W. Shin, J. Appl. Polym. Sci., 2009, 112, 2741–2749 CrossRef CAS.
- D. Xu, Z. Cao, T. Wang, J. Zhong, J. Zhao, F. Gao, X. Luo, Z. Fang, J. Cao and S. Xu, Prog. Org. Coat., 2019, 135, 510–516 CrossRef CAS.
- Y. Wang, H. Yu, H. Yang, X. Hao, Q. Tang and X. Zhang, Macromol. Chem. Phys., 2017, 218, 1700348 CrossRef.
- J. Salaklang, V. Maes, M. Conradi, R. Dams and T. Junkers, React. Chem. Eng., 2018, 3, 41–47 RSC.
- F. Xin, Y. Li, C. Fu, I. Javed, X. Huang, A. Schaschkow, R. S. Garcia Ribeiro, E. N. Gurzov, T. P. Davis and X. Zhang, Chem. Mater., 2020, 32, 1080–1088 CrossRef CAS.
- C. D. Meyer, C. S. Joiner and J. F. Stoddart, Chem. Soc. Rev., 2007, 36, 1705–1723 RSC.
- M. E. Belowich and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 2003–2024 RSC.
- A. Chao, I. Negulescu and D. Zhang, Macromolecules, 2016, 49, 6277–6284 CrossRef CAS.
- H. Zhang, D. Wang, W. Liu, P. Li, J. Liu, C. Liu, J. Zhang, N. Zhao and J. Xu, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2011–2018 CrossRef CAS.
- X. Ran, Q. Qu, X. Qian, W. Xie, S. Li, L. Li and L. Yang, Sens. Actuators, B, 2018, 257, 362–371 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02299k |
| This journal is © The Royal Society of Chemistry 2022 |




