Open Access Article
Open Access ArticleA uniformly anchored zirconocene complex on magnetic reduced graphene oxide (rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2)) as a novel and reusable nanocatalyst for synthesis of N-arylacetamides and reductive-acetylation of nitroarenes†
Massood
Bayzidi
 and
Behzad
Zeynizadeh
and
Behzad
Zeynizadeh
 *
*
Department of Chemistry, Urmia University, Urmia 5756151818, Iran. E-mail: m.bayzidi@urmia.ac.ir; massoodbayzidi@gmail.com; b.zeynizadeh@urmia.ac.ir; bzeynizadeh@gmail.com
First published on 17th May 2022
Abstract
In this study, a crafted zirconocene complex on rGO@Fe3O4 as a novel magnetic nanocatalyst was synthesized and then characterized using FT-IR, SEM, EDX, VSM, ICP-OES, TGA, BET and MS analyses. Next, catalytic activity of the prepared nanocomposite rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) towards successful reduction of aromatic nitro compounds to arylamines using N2H4·H2O (80%) was investigated. The examined nanocatalyst also showed perfect catalytic activity for reductive-acetylation of aromatic nitro compounds to the corresponding N-arylacetamides without isolation of the prepared in situ amines using the N2H4·H2O/Ac2O system. Furthermore, acetylation of the commercially available arylamines to the corresponding N-arylacetamides was carried out by acetic anhydride in the presence of the rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) nanocomposite. All reactions were carried out in refluxing EtOH as a green solvent to afford the products in high yields. The obtained results exhibited that the nanocomposite of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) showed a great catalytic activity in comparison to rGO and rGO@Fe3O4 as the parent constituents. Recovery and reusability of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) were also examined for 8 consecutive cycles without significant loss of the catalytic activity. This establishes the sustainable anchoring of the zirconocene complex on the surface and mesopores of the rGO@Fe3O4 nanohybrid system.
Introduction
In recent years zirconium complexes have played a key role as catalysts or reagents in organic synthesis.1 One of the first examples of the use of zirconium as a catalyst was zirconocene complexes in Ziegler–Natta polymerization.2 Schwartz reagent as a hydrozirconation and reducing agent was the second application of zirconocene complexes.3 In in situ generation of the Schwartz reagent for hydrozirconation and reduction reactions, the use of different hydride sources such as LiAlH4, NaAlH2(OCH2CH2OCH3)2 (Red-Al),4 t-BuMgCl,5 LiEt3BH6 and DIBAL-H7 has also been reported. In this context, using hydrazine hydrate as a commercially available hydrogen source could be convenient due to green by-products of N2 gas and water.8To overcome problems related to the separation and reusability of the homogeneous catalyst systems, using magnetic nanoparticles are one of the prominent candidates.9,10 Magnetic nanoparticles can be easily separated from the reaction medium using an external magnetic field, no need to filtration, centrifugation or other tedious workup procedures.11 Over the last few years, magnetic cores as well as their coating surfaces have dramatically improved and widely used for different purposes. These improvements have been made in order to increase the accessible sites/zones on the surface of catalyst, greater catalyst reactivity and selectivity, thermal stability and finally easy separation. Fe3O4,12,13 NiFe2O4,14–16 CoFe2O4,17 CuFe2O4,18–20 MnFe2O4,21 Ni–Cu–ZnFe2O4,22 BiFeO3,23 FeAl2O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 and ZnCrFeO4
24 and ZnCrFeO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 as a core, and metals,26–28 silica,29–32 polymers,33–35 carbon nanotubes and sheets,36–40 cellulose,41 small organic molecules,42,43 ionic liquids,44,45 and organosulfonic acids46,47 have been used as a coating.
25 as a core, and metals,26–28 silica,29–32 polymers,33–35 carbon nanotubes and sheets,36–40 cellulose,41 small organic molecules,42,43 ionic liquids,44,45 and organosulfonic acids46,47 have been used as a coating.
Recently, the magnetic hybrid composites were prepared by the combination of reduced graphene oxide (rGO) and magnetic materials. Among the hybrid composite systems, rGO@Fe3O4 is highly regarded for its low price, excellent magnetic property, easy preparation method, high biocompatibility and low toxicity.48 Generally, reduced graphene oxide sheets (rGO) can be easily prepared by oxidative exfoliation of graphite flakes in a large scale followed by chemically or thermally reduction.49 Due to unique electronic and structural properties such as high intrinsic carrier mobility, high mechanical strength and thermal stability, rGO and rGO-based materials have attracted a great deal of attention. Because of the advantages including 2D structure character as well as the large surface area, surface defects and the aromatic scaffold, rGO can also serve as a potential support for the fabrication of hybrid nanomaterials.50–56 Moreover, electronic properties including charge transfer, magnetism, band structure, and density of states are closely interrelated with the metal–support interaction. Charge transfer occurs between the adsorbed/embedded metal and rGO supports due to their different Fermi levels.57,58 In this context, the present study clearly represents that when zirconium as the form of zirconocene is immobilized on the surface and mesopores of magnetite-reduced graphene oxide, the prepared rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) nanohybrid system, due to the facilitated electronic transfer between zirconocene and rGO constituents, was able to perform organic reactions more easily.
On the other hand, aromatic amines are critical starting materials and intermediates for synthesis of various chemicals such as medicines, biomaterials, dyes, agricultural materials and so on.59 One simple and straightforward method for the preparation of aromatic amines is the catalytic reduction of nitroarene compounds by various reducing systems.60 In this context, heterogenization of metal catalysts through the immobilization of transition metals species on solid supporters is the widely used strategy in organic synthesis.61–68
Furthermore, the catalytic formation of amide bonds is one of the most intended procedures in the synthesis of various organic compounds including polymers, agrochemicals, pharmaceuticals and fragrances.69 Due this, acetylation of arylamines and reductive-acetylation of aromatic nitro compounds by acetic anhydride are the commonly used strategies for the preparation of N-arylacetamides. The literature review shows that although the application of transition metals (Mn, Co, Ni, Cu and Zn) based catalyst systems have been reported for the titled transformations,63,68,70,71 however, using any zirconium species did not yet reported in this area.
In line with the outlined strategies and continuation of our research programs towards reduction of aromatic nitro compounds to arylamines and acetylation of arylamines to N-arylacetamides in the presence of transition metals-based nanocatalyst systems,72–77 herein, we report the synthesis of a novel nanohybrid material by the immobilization of zirconocene on magnetic reduced graphene oxide. The prepared rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) nanocomposite was then characterized and its catalytic activity was successfully studied towards reduction and reductive-acetylation of nitroarenes as well as N-acetylation of the commercially available anilines in refluxing ethanol as a green solvent.
Results and discussion
Catalyst characterization
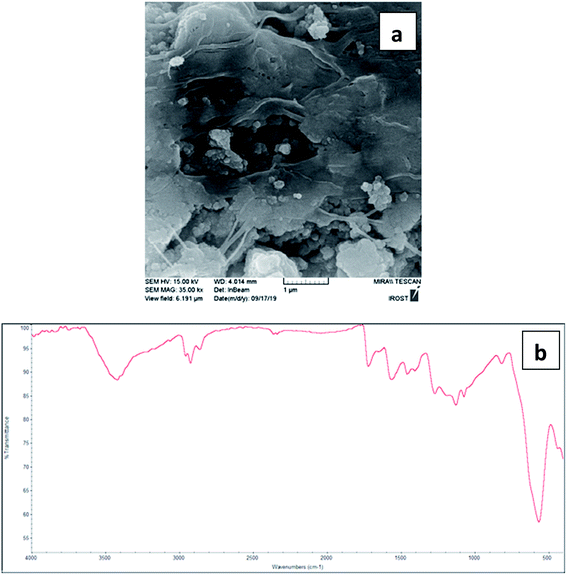
The study was started by the synthesis of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) nanocomposite in a four-step procedure as shown in Fig. 1. This is a general synthetic approach and the details are described in the Experimental section. Primarily graphite flakes were oxidized by H2SO4 and KMnO4 to afford graphene oxide (GO) followed by the reduction with L-ascorbic acid to afford exfoliated reduced graphene oxide (rGO). Next, through the mixing of an aqueous solution of FeCl3·6H2O and FeCl2·4H2O with the prepared rGO and then dropwise addition of an aqueous solution of ammonia 25% under N2 atmosphere, the magnetic reduced graphene oxide (rGO@Fe3O4) was prepared. Finally, refluxing the benzene solution of ZrCp2Cl2 with nanoparticles of magnetic rGO affords rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) nanohybrid material. The prepared nanocatalyst system was then characterized using FT-IR, SEM, EDX, VSM, ICP-OES, TGA, BET and MS analyses.FT-IR spectra of rGO, rGO@Fe3O4 and rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) composite systems are shown in Fig. 2. In FT‐IR spectrum of rGO (Fig. 2a), the broad band around 3300–3650 cm−1 shows the stretching vibration of O–H groups due to the presence of adsorbed water and carboxyl and hydroxyl functionalities. The bands at 2993 cm−1 and 1716 cm−1 are also attributed to asymmetric/symmetric vibrations of CH2 and stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively.78 As well the broad band around 850–1250 cm−1 shows the bending vibrations of C–O–C and O–H groups. In addition to the all bond frequencies of rGO, the stretching vibration of Fe–O is appeared at 580 cm−1 in FT-IR spectrum of rGO@Fe3O4 (Fig. 2b). In FT-IR spectrum of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) (Fig. 2c), the absorption peak at 1567 cm−1 is attributed to the stretching vibration of C
O groups, respectively.78 As well the broad band around 850–1250 cm−1 shows the bending vibrations of C–O–C and O–H groups. In addition to the all bond frequencies of rGO, the stretching vibration of Fe–O is appeared at 580 cm−1 in FT-IR spectrum of rGO@Fe3O4 (Fig. 2b). In FT-IR spectrum of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) (Fig. 2c), the absorption peak at 1567 cm−1 is attributed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group due to existence of Cp groups and aromatic constituents in the structure of rGO. The weak peak at 1633 cm−1 is assigned to the stretching and bending vibrations of OH groups due to the adsorbed water on the surface of rGO. The peak observed at 1403 cm−1 is attributed to C–O–H deforming vibration. The broad band around 1198 cm−1 is corresponded to C–O–C and C–O stretching vibrations. In addition, the peaks at 732, 809 and 1016 cm−1 are accordance with zirconocene dichloride spectrum. All these are indicated that ZrCp2Clx species were successfully anchored on the mesopores and surface of rGO@Fe3O4 constituents.
C group due to existence of Cp groups and aromatic constituents in the structure of rGO. The weak peak at 1633 cm−1 is assigned to the stretching and bending vibrations of OH groups due to the adsorbed water on the surface of rGO. The peak observed at 1403 cm−1 is attributed to C–O–H deforming vibration. The broad band around 1198 cm−1 is corresponded to C–O–C and C–O stretching vibrations. In addition, the peaks at 732, 809 and 1016 cm−1 are accordance with zirconocene dichloride spectrum. All these are indicated that ZrCp2Clx species were successfully anchored on the mesopores and surface of rGO@Fe3O4 constituents.
 | ||
| Fig. 2 FT‐IR spectra of (a) rGO, (b) rGO@Fe3O4 and (c) rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) composite systems. | ||
Structural elucidation of rGO, rGO@Fe3O4 and rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) composite systems was also carried out using X-ray diffraction (XRD) analysis (Fig. 3). In this context, the existence of individual sharp peaks in the depicted XRD patterns represents that all the examined composite systems have the extent of crystallinity character. In this context, the XRD pattern of Fe3O4 (Fig. 3a) shows that the diffraction peaks at 2θ = 30.2°, 35.5°, 43.3°, 53.7°, 57.2° and 62.9° are attributed to (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1) and (4 4 0) crystal planes of nano Fe3O4. The laboratory sample of nano Fe3O4 has a crystalline cubic spinel structure and it is in agreement with the standard one of JCPDS 65-3107. As well, the XRD pattern of rGO@Fe3O4 represents that besides to the all diffraction peaks of nano Fe3O4, the position of a broad peak at 2θ = 16–28° is attributed to rGO constituents. Fig. 3c also shows that the XRD pattern of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) is similar to that of Fe3O4 and rGO@Fe3O4. This similarity could be attributed to low loading of Zr and due this the diffraction peaks for zirconium species have very low intensity or overlap with the peaks of Fe3O4.
Scanning electron microscopy (SEM) was used to study the morphology and size distribution of rGO, rGO@Fe3O4 and rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) composite systems (Fig. 4). Analysis of the images shows that the immobilization of Fe3O4 MNPs (magnetic nanoparticles) and ZrCp2Clx species on rGO are successful. In this context, Fig. 4a reveals that rGO constituents have the wrinkled and folded morphology, and so this causes the well distribution of Fe3O4 and ZrCp2Clx species on rGO. In addition, the mentioned morphology prevents accumulation and agglomeration of Fe3O4 and ZrCp2Clx species in the composition of rGO. The average particle size of rGO@Fe3O4 and rGO@Fe3O4/ZrCp2Clx are to be 25 and 80 nm, respectively.
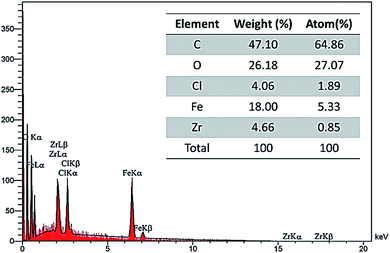
Elemental elucidation of the synthesized rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) nanohybrid material was carried out using energy-dispersive X-ray spectroscopy (EDX) (Fig. 5). The analysis shows that all the required elements including C (47.10%), O (26.18%), Cl (4.06%), Fe (18.00%) and Zr (4.66%) are present in the structure of rGO@Fe3O4/ZrCp2Clx system. In addition, distribution of the elements was investigated using EDX-mapping analysis (Fig. 6). The mapping for Fe, C and Zr elements shows that they were uniformly distributed in all around of rGO@Fe3O4/ZrCp2Clx composite system. SEM and elemental mapping analysis of rGO@Fe3O4/ZrCp2Clx were carried out through the depicted image in Fig. 7.
 | ||
| Fig. 6 Elemental mapping of (a) Fe, (b) C, (c) Zr and (d) Fe, C, and Zr in rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2). | ||
Based on the ICP-OES (inductively coupled plasma optical emission spectrometry), the exact amount of Zr in rGO@Fe3O4/ZrCp2Clx composite system is 4.7%. The measure is exactly in accordance with the obtained data through the EDX-elemental analysis of zirconium (Fig. 5).
Thermogravimetric analysis (TGA) of rGO@Fe3O4/ZrCp2Clx nanohybrid material was used to study the stability and weight loss of the immobilized zirconocene on rGO@Fe3O4 (Fig. 8). The depicted curve represents that 1.69% weight loss in the area of 40–150 °C is attributed to the evaporation of adsorbed water on the surface of rGO@Fe3O4/ZrCp2Clx composite system. As well, the weight loss of 4.7% in the range of 150–320 °C indicates removing of organic moieties such as Cp from the immobilized ZrCp2Clx and RCO2H, RCOR, ROR or ROH species from the rGO constituents. The third weight loss of 3.53% at the area of 350–800 °C is attributed to the fragmentation and decomposition of the composite system. Differential thermal analysis (DTA) clearly shows that the examined nanocomposite has a good thermal stability up to 365 °C with weight loss of 6.39%.
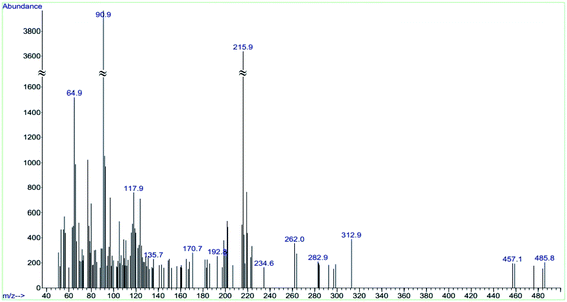
Characterization of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) system was also carried out using mass spectroscopy at the ionization potential of 20 eV. The depicted graph in Fig. 9 shows the fragmentation pattern of the anchored zirconcene on rGO@Fe3O4. In this context, the presence of fragments in m/z = 56 ± 1 [Fe], 65 [Cp], 72 [FeO], 91 [Zr], 160 [Fe2O3], 192 [ZrCpCl], 221–2 [ZrCp2] and 292 [ZrCp2Cl2] exactly confirms the immobilization of ZrCp2Clx species on rGO@Fe3O4 constituents.
N2 adsorption–desorption analysis is further utilized to determine specific surface area and porosity of the surface in rGO@Fe3O4/ZrCp2Clx nanohybrid material. The information for the pore size and total pore volume in the nanomaterial is also accessible using the titled analysis. In this area, Fig. 10 shows the profile of N2 adsorption–desorption for the composite system. Based on BDDT IUPAC classification, the shape of isotherm is closer to the isotherm of type IV with H3 hysteresis loop of desorption profile. This type of isotherm is a characteristic of mesoporous materials. In this context, the surface properties of rGO@Fe3O4/ZrCp2Clx system are summarized as SBET = 81.05 m2 g−1, Vm = 18.622 cm3 g−1, average pore diameter = 11.358 nm and total pore volume = 0.2301 cm3 g−1.
The magnetic property and value of Fe3O4, rGO@Fe3O4 and rGO@Fe3O4/ZrCp2Clx composite systems were further studied using vibrating sample magnetometer (VSM) analysis in an external magnetic field up to 20 kOe at room temperature. The depicted graphs in Fig. 11 represent non-linear and reversible magnetic property for Fe3O4 (curve a), rGO@Fe3O4 (curve b) and rGO@Fe3O4/ZrCp2Clx (curve c). The saturation magnetization (Ms) values of Fe3O4, rGO@Fe3O4 and rGO@Fe3O4/ZrCp2Clx are respectively 75, 45 and 35 emu g−1. The values clearly show that through combining Fe3O4 with rGO and then immobilization of ZrCp2Clx, the magnetization value of rGO@Fe3O4/ZrCp2Clx was intensively diminished. Nevertheless, the magnetization value is still large enough for magnetic separation.
Catalytic activity of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) system
| Entry | Catalyst | Amount (mg) | N2H4·H2O (mmol) | Condition | Time (min) | Yield (%) |
|---|---|---|---|---|---|---|
| a All reactions were carried out with 1 mmol of nitrobenzene in 2 mL solvent. | ||||||
| 1 | rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) | 20 | 2 | EtOH, r.t. | 60 | 55 |
| 2 | rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) | 10 | 2 | EtOH, reflux | 60 | 60 |
| 3 | rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) | 20 | 2 | EtOH, reflux | 10 | 100 |
| 4 | rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) | 30 | 2 | EtOH, reflux | 10 | 70 |
| 5 | rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) | 20 | 1 | EtOH, reflux | 10 | 60 |
| 6 | rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) | 20 | 2 | H2O, 70 °C | 60 | 60 |
| 7 | rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) | 20 | 2 | H2O–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 70 °C 1), 70 °C |
60 | 50 |
| 8 | rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) | 20 | 2 | EtOAc, reflux | 60 | 20 |
| 9 | rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) | 20 | 2 | CH3CN, reflux | 60 | 30 |
| 10 | rGO@Fe3O4 | 20 | 2 | EtOH, reflux | 60 | 8 |
| 11 | ZrCp2Cl2 | 20 | 2 | EtOH, reflux | 60 | 20 |
| 12 | ZrCp2Cl2 | 20 | 2 | H2O, reflux | 60 | 10 |
| 13 | — | — | 2 | EtOH, reflux | 120 | Trace |
Investigation of the results exhibited that among the examined solvents including H2O, EtOH, EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), EtOAc and CH3CN, the model reaction shows the perfect efficiency in refluxing EtOH using 20 mg of the nanocatlyst per 1 mmol of PhNO2 (Table 1, entry 3). These examinations clearly represent that progress of the reduction reaction in protic solvents has higher rates and efficiency than aprotic ones. Although the exact mechanism for this influence is not clear, however, the better solvation of the nanocatalyst and nitro functionality as well as the presence of power hydrogen bonding (N⋯H) between N2H4·H2O and the protic solvent (EtOH and H2O) maybe play a role. All these cause the better transferring of hydrogen (from hydrazine) to nitro group to do the reduction reaction more easily. In the case of H2O, low solubility of nitrobenzene in H2O as a solvent proceeds the reaction with lower rate and efficiency.
1), EtOAc and CH3CN, the model reaction shows the perfect efficiency in refluxing EtOH using 20 mg of the nanocatlyst per 1 mmol of PhNO2 (Table 1, entry 3). These examinations clearly represent that progress of the reduction reaction in protic solvents has higher rates and efficiency than aprotic ones. Although the exact mechanism for this influence is not clear, however, the better solvation of the nanocatalyst and nitro functionality as well as the presence of power hydrogen bonding (N⋯H) between N2H4·H2O and the protic solvent (EtOH and H2O) maybe play a role. All these cause the better transferring of hydrogen (from hydrazine) to nitro group to do the reduction reaction more easily. In the case of H2O, low solubility of nitrobenzene in H2O as a solvent proceeds the reaction with lower rate and efficiency.
Identifying the role of rGO@Fe3O4/ZrCp2Clx nanocomposite system for catalyzing the reduction reaction was also carried out by examining the model reaction in the presence of rGO@Fe3O4 and ZrCp2Cl2 (Table 1). Entries 10–12 clearly show that using rGO@Fe3O4 and ZrCp2Cl2 catalyst systems did not progress the reaction efficiently. It is therefore concluded that the multicatalysts activity of Fe3O4, zirconium species as well as rGO and the extreme surface area and mesopore structural characteristic of rGO@Fe3O4/ZrCp2Clx synergistically accomplish the reduction reaction in shorter reaction time.
The examination for doing reduction of PhNO2 with sodium borohydride or isopropanol as the other commercially available reductants in the presence of rGO@Fe3O4/ZrCp2Clx composite system did not show the reasonable results. The study was therefore continued by performing the reduction reactions with hydrazine hydrate 80% at the optimized conditions (Table 1, entry 3).
The scope and capability of rGO@Fe3O4/ZrCp2Clx composite system to catalyze reduction of nitro functionality was further studied by performing the reduction of diverse aromatic nitro compounds with N2H4·H2O 80% at the optimized reaction conditions. The results of this investigation are summarized in Table 2. The table represents that various nitroarenes containing electron-releasing and withdrawing groups as well as aldehyde and ketone functionalities were reduced using 2 mmol of N2H4·H2O 80% in refluxing EtOH. All reactions were carried out with 15–60 min to afford the aniline products in high yields. In the case of nitro compounds containing carbonyl functionality (entries 5, 9 and 10), the reduction reactions were carried out without any selectivity and both of nitro and carbonyl groups were reduced using 2 mmol of N2H4·H2O 80% in the presence of rGO@Fe3O4/ZrCp2Clx (20 mg) giving the corresponding aniline products with the alcohol functionality.
| Entry | R | Product | Time (min) | Yield (%) |
|---|---|---|---|---|
| a All reactions were carried out with 1 mmol of nitroarene, 2 mmol of N2H4·H2O 80% and 20 mg of rGO@Fe3O4/ZrCp2Clx in refluxing EtOH (2 mL). b In this reaction 3 mmol of N2H4·H2O 80% was used. | ||||
| 1 | H |

|
10 | 98 |
| 2 | 4-OH |

|
30 | 90 |
| 3 | 4-NH2 |

|
40 | 98 |
| 4 | 2-OH |
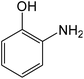
|
15 | 97 |
| 5 | 4-COMe |
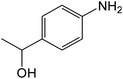
|
20 | 92 |
| 6 | 3-Me |

|
35 | 90 |
| 7 | 4-Me |

|
30 | 91 |
| 8 | 2-NH2 |

|
60 | 95 |
| 9 | 3-COMe |

|
45 | 94 |
| 10 | 3-CHO |

|
60 | 93 |
| 11 | 3-OH |

|
30 | 96 |
| 12b | 3-NH2-5-NO2 |
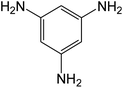
|
20 | 92 |
| Entry | R | Product | Time (min) | Yield (%) |
|---|---|---|---|---|
| a All reactions were carried out with 1 mmol of nitroarene, 2 mmol of N2H4·H2O 80%, 2 mmol Ac2O and 20 mg of rGO@Fe3O4/ZrCp2Clx in refluxing EtOH (2 mL). b In this reaction, 3 mmol of N2H4·H2O 80% and Ac2O was used. | ||||
| 1 | H |

|
15 | 97 |
| 2 | 4-OH |
|
100 | No reaction |
| 3 | 4-NH2 |

|
50 | 98 |
| 4 | 2-OH |

|
25 | No reaction |
| 5 | 4-COMe |

|
30 | 50 |
| 6 | 3-Me |
|
40 | 95 |
| 7 | 4-OMe |
|
40 | 94 |
| 8 | 2-NH2 |

|
70 | 97 |
| 9 | 3-COMe |

|
55 | 60 |
| 10 | 3-CHO |

|
70 | 70 |
| 12b | 3-NH2-5-NO2 |

|
30 | 91 |
Based on the obtained accessibility, therefore, the generality of this synthetic method was further investigated by performing reductive-acetylation of the all examined nitroarenes (at the previous section) to the corresponding N-arylacetamides through adding 2 mmol acetic anhydride to the reaction mixture. The results of this investigation are summarized in Table 3. The table shows that most of the examined nitro functionalities were successfully converted to the corresponding amides in high yields at the optimized reaction conditions. In addition, the compounds containing phenolic functionality in the presence of amino group did not take place acetylation of the amino or phenolic groups, and therefore the produced aminophenol material was recovered from the reaction mixture. Although the exact explanation for this inability is not known, however, this maybe due to the formation of zwitter ion through the acidic and basic characteristic of phenol and amine groups which prevent the progress of the acetylation reaction towards formation of the amide product. Moreover, in the case of the materials containing amino and alcoholic groups, only acetylation of the amine functionality was carried out and accordingly the hydroxy group was remained intact. It is therefore concluded that N2H4·H2O/rGO@Fe3O4/ZrCp2Clx/Ac2O system has a selectivity among alcoholic and amino groups towards the acetylation reaction.
| Entry | R | Product | Time (min) | Yield (%) |
|---|---|---|---|---|
| a All reactions were carried out with 1 mmol of arylamine, 2 mmol Ac2O and 20 mg of rGO@Fe3O4/ZrCp2Clx in refluxing EtOH (2 mL). | ||||
| 1 | H |

|
5 | 97 |
| 2 | 4-MeO |
|
10 | 98 |
| 3 | 4-Cl |
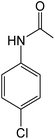
|
5 | 96 |
| 4 | 4-Me |

|
10 | 97 |
| 5 | 3-NH2 |

|
10 | 94 |
| 6 | 2-Me-3-Cl |
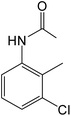
|
5 | 93 |
| 7 | 2-NH2-4-Me |

|
15 | 91 |
| 8 | 2-NO2 |

|
10 | 96 |
| 9 | 4-OH |

|
60 | No reaction |
| 10 | 3-COMe |

|
10 | 88 |
Recycling of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) nanohybrid material
Recovery and reusability of the heterogeneous catalyst systems is one of the crucial subjects from the prospects of economic and environment. Evaluation of this capability for rGO@Fe3O4/ZrCp2Clx nanocomposite system was carried out by recovering of the nanocatalyst and then reusing it for the further examinations. In this context, when reduction and reductive-acetylation of nitrobenzene as well as acetylation of aniline in the presence of rGO@Fe3O4/ZrCp2Clx composite system was completed, the nanocatalyst was magnetically recovered from the reaction mixture and then washed with EtOH. After drying under air atmosphere, the recovered nanocatalyst was reused for the next runs of the mentioned transformations. The depicted graphs in Fig. 12 show that after 8 consecutive recycling, rGO@Fe3O4/ZrCp2Clx composite system has the acceptable activity to promote the transformation reactions. In this context, FT-IR spectrum and SEM analysis of the recovered nanocatalyst system after 8 reusing cycles represent a slightly change of the morphology and mesoporosity of the surface (Fig. 13). | ||
| Fig. 12 Recycling of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) nanohybrid material in reduction and reductive-acetylation of nitrobenzene and N-acetylation of aniline. | ||
Hot filtration
From the aspects of sustainability and green chemistry, stability and recyclability of the catalyst systems are the desirable characteristics. To illustrate the heterogeneous and structural stability of rGO@Fe3O4/ZrCp2Clx nanohybrid material, a hot filtration examination was carried out through performing the reduction of PhNO2 with hydrazine hydrate within 10 min at the optimized reaction conditions (1 mmol PhNO2, 2 mmol N2H4·H2O 80% and 20 mg of the nanocatalyst in refluxing EtOH). Monitoring of the model reaction showed that in the meantime of 5 min (half of the reaction time), the reduction reaction was progressed for 70%. Next, the magnetic nanohybrid catalyst system was removed from the reaction mixture (using an external magnetic field) and then the reaction was allowed to continue in refluxing EtOH with reducing agent. Progress of the reaction was monitored after 120 min to evaluate the reduction of nitrobenzene in the absence of the nanocatalyst system. It was found that only a trace reduction of PhNO2 (<2%) was carried out upon refluxing ethanolic solution and catalyst-free condition of the reaction mixture. This examination clearly reveals that rGO@Fe3O4/ZrCp2Clx nanohybrid material has the negligible leaching which proves its high stability. In this context, after removing rGO@Fe3O4/ZrCp2Clx composite system in a meanwhile of the hot-filtration examination, ICP-OES analysis for the remaining Zr species in the reaction mixture showed 3% leaching of the nanocatalyst material.In addition, catalytic activity of rGO@Fe3O4/ZrCp2Clx nanocatalyst system for the reduction of nitroarenes with hydrazine hydrate 80% was highlighted by comparison of the obtained results with those of previously reported other catalysts systems (Table 5). A case study shows that in terms of reaction time and conditions, yield of the products, the current protocol represents a more or comparable efficiency than the previous systems.
| Entry | Catalyst (mg) | Reductant | Time | Yield (%) | Conditions | Ref. |
|---|---|---|---|---|---|---|
| 1 | Present work | N2H4·H2O | 10 min | 95–100 | 20 mg, EtOH, 70 °C | — |
| 2 | Fe-phen/C-800 | H2 (g) | 15 h | 90–96 | 50 bar H2, H2O–THF, 120 °C | 79 |
| 3 | Co3O4@Al2O3/SiO2 | N2H4·H2O | 2 h | 90–100 | 2 mol%, EtOH, 60 °C | 80 |
| 4 | Ru-CNT | N2H4·H2O | 3 h | 95–100 | N2 atm, THF, r.t. | 81 |
| 5 | Iron oxide hydroxide [FeO(OH)] | N2H4·H2O | 5 h | 95–100 | 30 mg, EtOH, 70 °C | 82 |
| 6 | rGO/Cu2O–CuO | N2H4·H2O | 2–3 h | 95–100 | 50 mg, MeOH–CH2Cl2–CH3CN visible light, r.t. | 83 |
| 7 | rGO/BiVO4 | N2H4·H2O | 1–2 h | 95–100 | 50 mg, visible light, r.t. | 84 |
| 8 | Ir/ZrO2·xH2O | H2(g) | 2–3 h | 95–100 | 25 mg, EtOH–H2O, 1 atm | 85 |
| 9 | Au@Zr-phosphonate | NaBH4 | 2–3 h | 80–90 | 2 mol%, EtOH, 30 °C | 86 |
Conclusion
In this paper, the immobilization of zirconocene on magnetite-reduced graphene oxide was investigated to afford the novel rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) nanohybrid material. The prepared composite system was then characterized using FT-IR, SEM, EDX, VSM, ICP-OES, TGA, BET and MS analyses. Next, catalytic activity of the nanocomposite was studied towards reduction and reductive-acetylation of nitroarenes as well as N-acetylation of arylamines. Reduction and reductive acetylation of nitroarenes was carried out by N2H4·H2O 80% and N2H4·H2O 80%/Ac2O, respectively. As well, N-acetylation of arylamines was taken place with acetic anhydride in the presence of the nanocatalyst system. All reactions were accomplished in refluxing EtOH as a green solvent giving arylamines and N-arylacetamides in high yields. Recovery and reusability of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) was also carried out for 8 consecutive cycles without the significant loss of the catalytic activity.Experimental section
Chemicals and instruments
All reagents and materials were purchased from chemical sources and they were used without further purification. A Bruker Vertex 70 spectrometer was used to record Fourier transform infrared spectra of the materials. 1H and 13C NMR spectra were recorded on 250/400 MHz Bruker spectrometer. X-ray diffraction (XRD) measurements were fulfilled on X'Pert Pro Panalytical diffractometer in 40 kV/30 mA with a Cu Kα radiation (λ = 1.5418 Å). Signal data were recorded in 2θ = 10–80° with a step interval of 0.05°. A FESEM‐TESCAN MIRA3 apparatus was utilized to determine the morphology and size distribution of particles by scanning electron microscopy (SEM) method. This apparatus was also used to reveal the chemical composition and elemental mapping of the nanocatalyst through energy-dispersive X-ray spectroscopy (EDX) technique. The BET (Brunauer–Emmett–Teller) surface area, pore volume and pore diameter of the samples were measured using Nova Station B, Quantachrome Instrument, USA. The thermogravimetric analysis (TGA) pattern was recorded using Shimadzu DTG-60 instrument. Vibrating sample magnetometer (VSM, Meghnatis Daghigh Kavir Co., Iran) analysis under magnetic field up to 20 kOe was applied to determine the magnetic property of the samples. Mass spectrometry measurement was recorded using Agilent Technology (HP) Model: 5973 network mass selective detector. Elemental analysis of Zr was carried out by inductively coupled plasma-optical emission spectrometry (ICP-OES) model ARCOS, Germany. Thin layer chromatography (TLC) was utilized to determine the purity of the products and to monitor the progress of the reactions using SILG/UV 254 aluminum sheets.Synthesis of reduced graphite oxide (rGO) nanosheets
rGO was synthesized from graphite by the already reported procedure.87 In a typical procedure, 1 g of graphite flakes was added to 50 mL concentrated sulfuric acid 98%, while stirring in an ice-water bath. 3 g potassium permanganate was gradually added by maintaining the temperature under 10 °C. Then, the suspension was stirred at room temperature for 25 min followed by 5 min sonication in an ultrasonic bath. After repeating the stirring-sonication process for 12 times, the reaction was quenched by the addition of 200 mL distilled water and 2 h more sonication. After adjusting the pH at 6, the suspension was further sonicated for 1 h. 10 g L-ascorbic acid was dissolved in 100 mL distilled water and then was slowly added to the exfoliated graphite oxide suspension at room temperature. The reduction was performed at 95 °C for 1 h. The resultant black precipitates were simply filtered by cellulose filter paper and were further washed with hydrochloric acid (1 M) and distilled water to gain neutral pH. The filtrate was finally freeze-dried to obtain rGO powder.Preparation of rGO@Fe3O4 nanohybrid
0.8 g of the obtained rGO was dispersed in 50 mL distilled water by sonication for 2 min. Then, an aqueous solution of FeCl3·6H2O (1.9 g) and FeCl2·4H2O (1.4 g) in 30 mL of distilled water was added to the dispersion of rGO followed by sonication for 5 min. Next, a solution of 40 mL ammonia 25% and 20 mL distilled water was added to the prepared mixture in dropwise manner and continuous stirring under N2 atmosphere for 1 h. The resulting mixture was stirred for 30 min and then filtered, washed with distilled water and dried in oven at 60 °C for 2 h.Preparation of rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) nanocomposite
0.5 g of magnetically nanoparticles of rGO@Fe3O4 was dispersed in 50 mL of benzene by sonication for 1 min. Then, a solution of the dissolved ZrCp2Cl2 (0.1 g) in 50 mL of benzene was added to the solution of rGO@Fe3O4 and the resulting mixture was stirred for 24 h at 50 °C. The prepared rGO@Fe3O4/ZrCp2Clx nanocomposite system were magnetically separated and washed with ethanol and deionized water followed by drying at room temperature under vacuum.A general procedure for reduction of nitroarenes with N2H4·H2O and rGO@Fe3O4/ZrCp2Clx (x = 0, 1, 2) system
In a round-bottom flask equipped with a magnetic stirrer, a mixture of nitroarene (1 mmol), rGO@Fe3O4/ZrCp2Clx (20 mg) and ethanol (2 mL) was prepared. Then, N2H4·H2O (80%, solution in water, 2 mmol) was added and the resulting mixture was stirred under reflux conditions. Progress of the reaction was monitored by TLC. After completion of the reaction, the magnetic nanocatalyst was separated using an external magnetic field and the mixture was then extracted with EtOAc. The combined organic layers were dried over anhydrous Na2SO4. Evaporation of the solvent under reduced pressure affords the corresponding arylamine product.A general procedure for reductive-acetylation of nitroarenes with N2H4·H2O/rGO@Fe3O4/ZrCp2Clx/Ac2O system
In a round-bottom flask equipped with a magnetic stirrer, a mixture of nitroarene (1 mmol), N2H4·H2O (80%) (2 mmol) and rGO@Fe3O4/ZrCp2Clx (20 mg) in ethanol (2 mL) was prepared. Progress of the reduction reaction was carried out for an appropriate time (mentioned in Table 2) under reflux conditions. After that, Ac2O (2 mmol) was added and the resulting mixture was stirred to accomplish N-acetylation of the in situ prepared arylamines in refluxing EtOH. Magnetically nanoparticles of rGO@Fe3O4/ZrCp2Clx were separated using an external magnetic field and the mixture was then extracted with EtOAc. The combined organic layers were dried over anhydrous Na2SO4. Evaporation of the solvent under reduced pressure gave the corresponding N-arylacetamides.A general procedure for acetylation of arylamines with rGO@Fe3O4/ZrCp2Clx/Ac2O system
In a round-bottom flask equipped with a magnetic stirrer, a mixture of arylamine (1 mmol), Ac2O (2 mmol) and rGO@Fe3O4/ZrCp2Clx (20 mg) in ethanol (2 mL) was prepared. The resulting mixture was stirred under reflux conditions for an appropriate time mentioned in Table 4. After completion of the reaction (monitored by TLC), magnetically nanoparticles of rGO@Fe3O4/ZrCp2Clx were separated using an external magnetic field and the mixture was then extracted with EtOAc. The combined organic layers were dried over anhydrous Na2SO4. Evaporation of the solvent under reduced pressure gave the corresponding N-arylacetamides.Funding
Research Council of Urmia University.Author contributions
Massood Bayzidi: formal analysis; investigation; methodology; project administration.Behzad Zeynizadeh: conceptualization; funding acquisition; project administration; supervision.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors gratefully appreciated the financial support of this work by the Research Council of Urmia University.References
- M. D. Jones, Sustainable Catalysis with Non-endangered Metals, ed. M. North, Royal Society of Chemistry, Yurk, 2016, vol. 2, pp. 199–212 Search PubMed.
- (a) L. Resconi, F. Piemontesi, G. Franciscono, L. Abis and T. Fiorani, J. Am. Chem. Soc., 1992, 114, 1025–1032 CrossRef CAS; (b) G. J. P. Britovsek, V. C. Gibson and D. F. Wass, Angew. Chem., Int. Ed. Engl., 1999, 38, 428–447 CrossRef CAS.
- (a) K. de la Vega-Hernández, R. Senatore, M. Miele, E. Urban, W. Holzer and V. Pace, Org. Biomol. Chem., 2019, 17, 1970–1978 RSC; (b) J. T. Spletstoser, J. M. White, A. R. Tunoori and G. I. Georg, J. Am. Chem. Soc., 2007, 129, 3408–3419 CrossRef CAS PubMed; (c) V. Pace, K. de la Vega-Hernández, E. Urban and T. Langer, Org. Lett., 2016, 18, 2750–2753 CrossRef CAS PubMed; (d) C. Matt, F. Kölblin and J. Streuff, Org. Lett., 2019, 21, 6983–6988 CrossRef CAS PubMed.
- J. A. Miller and E. Negishi, Tetrahedron Lett., 1984, 25, 5863–5866 CrossRef CAS.
- H. Makabe and E. Negishi, Eur. J. Org. Chem., 1999, 1999, 969–971 CrossRef.
- B. H. Lipshutz and E. L. Ellsworth, J. Am. Chem. Soc., 1990, 112, 7440–7441 CrossRef CAS.
- Z. Huang and E. Negishi, Org. Lett., 2006, 8, 3675–3678 CrossRef CAS PubMed.
- H. Tsuneaki and M. Osamu, Chem. Lett., 1975, 4, 259–260 CrossRef.
- P. Barbaro and F. Liguori, Heterogenized Homogeneous Catalysts for Fine Chemicals Production: Catalysis by Metal Complexes, Springer, Dordrecht, 2010, vol. 33 Search PubMed.
- T. Tamoradi, S. M. Mousavi and M. Mohammadi, New J. Chem., 2020, 44, 8289–8302 RSC.
- (a) M. Kazemi and M. Mohammadi, Appl. Organomet. Chem., 2020, 34, e5400 CrossRef CAS; (b) L. M. Rossi, N. J. S. Costa, F. P. Silva and R. Wojcieszak, Green Chem., 2014, 16, 2906–2933 RSC.
- M. Nasr-Esfahani, S. J. Hoseini, M. Montazerozohori, R. Mehrabi and H. Nasrabadi, J. Mol. Catal. A: Chem., 2014, 382, 99–105 CrossRef CAS.
- C. Yang, J. Wu and Y. Hou, Chem. Commun., 2011, 47, 5130–5141 RSC.
- S. Iraqui, S. S. Kashyap and M. H. Rashid, Nanoscale Adv., 2020, 2, 5790–5802 RSC.
- S. Sharifi, A. Yazdani and K. Rahimi, Sci. Rep., 2020, 10, 10916 CrossRef CAS PubMed.
- C. Simon, M. B. Zakaria, H. Kurz, D. Tetzlaff, A. Blösser, M. Weiss, J. Timm, B. Weber, U.-P. Apfel and R. Marschall, Chem.–Eur. J., 2021, 27, 16990–17001 CrossRef CAS PubMed.
- M. Houshiar, F. Zebhi, Z. J. Razi, A. Alidoust and Z. Askari, J. Magn. Magn. Mater., 2014, 371, 43–48 CrossRef CAS.
- R. Eisavi, S. Ghadernejad, B. Zeynizadeh and F. Mohammad Aminzadeh, J. Sulfur Chem., 2016, 37, 537–545 CrossRef CAS.
- K. Baruah, A. Kant, P. Gaijon, S. Ghosh and M. R. Singh, Appl. Surf. Sci. Adv., 2021, 6, 100130 CrossRef.
- R. Eivazzadeh-Keihan, S. Asgharnasl, M. S. Bani, F. Radinekiyan, A. Maleki, M. Mahdavi, P. Babaniamansour, H. Bahreinizad, A. E. Shalan and S. Lanceros-Méndez, Langmuir, 2021, 37, 8847–8854 CrossRef CAS PubMed.
- N. Akhlaghi and G. Najafpour-Darzi, J. Ind. Eng. Chem., 2021, 103, 292–304 CrossRef CAS.
- S. Taghavi Fardood, A. Ramazani, Z. Golfar and S. W. Joo, Appl. Organomet. Chem., 2017, 31, e3823 CrossRef.
- N. Wang, X. Luo, L. Han, Z. Zhang, R. Zhang, H. Olin and Y. Yang, Nano-Micro Lett., 2020, 12, 81 CrossRef CAS PubMed.
- A. Ghorbani-Choghamarani, M. Mohammadi, L. Shiri and Z. Taherinia, Res. Chem. Intermed., 2019, 45, 5705–5723 CrossRef CAS.
- M. Taei, F. Hasanpour, M. Movahedi and S. Mohammadian, RSC Adv., 2015, 5, 37431–37439 RSC.
- X. W. Lou and L. A. Archer, Adv. Mater., 2008, 20, 1853–1858 CrossRef CAS.
- (a) A. Ghorbani-Choghamarani, M. Mohammadi and Z. Taherinia, J. Iran. Chem. Soc., 2019, 16, 411–421 CrossRef CAS; (b) W. Chiu, P. Khiew, M. Cloke, D. Isa, H. Lim, T. Tan, N. Huang, S. Radiman, R. Abd-Shukor and M. A. A. Hamid, J. Phys. Chem. C, 2010, 114, 8212–8218 CrossRef CAS.
- H. Peng, X. Wang, C. J. Hu and X. Tian, New J. Chem., 2016, 40, 7911–7916 RSC.
- A. Khazaei, M. Khazaei and M. Nasrollahzadeh, Tetrahedron, 2017, 73, 5624–5633 CrossRef CAS.
- Z. Arabpoor and H. R. Shaterian, RSC Adv., 2016, 6, 44459–44468 RSC.
- (a) M. Gilanizadeh and B. Zeynizadeh, Polycyclic Aromat. Compd., 2021, 41, 15–32 CrossRef CAS; (b) L. Ding, L. Yang, J. Xu, J. Zheng and M. Zhang, Inorg. Chem., 2021, 60, 8880–8889 CrossRef CAS PubMed.
- B. Zeynizadeh, M. Sadeghbari and N. N. Pesyan, Curr. Org. Synth., 2019, 16, 1010–1023 CrossRef CAS PubMed.
- S. Xuan, Y.-X. J. Wang, J. C. Yu and K. C.-F. Leung, Langmuir, 2009, 25, 11835–11843 CrossRef CAS PubMed.
- D. Rosario-Amorin, M. Gaboyard, R. Clérac, L. Vellutini, S. Nlate and K. Heuzé, Chem.–Eur. J., 2012, 18, 3305–3315 CrossRef CAS PubMed.
- A. Schätz, T. R. Long, R. N. Grass, W. J. Stark, P. R. Hanson and O. Reiser, Adv. Funct. Mater., 2010, 20, 4323–4328 CrossRef PubMed.
- M. Esmati and B. Zeynizadeh, Appl. Organomet. Chem., 2022, 36, e6496 CrossRef CAS.
- M. Alvand and F. Shemirani, Microchim. Acta, 2016, 183, 1749–1757 CrossRef CAS.
- J. Guo, H. Jiang, Y. Teng, Y. Xiong, Z. Chen, L. You and D. Xiao, J. Mater. Chem. B, 2021, 9, 9076–9099 RSC.
- M. Zhang, L. Ding, J. Zheng, L. Liu, H. Alsulami, M. A. Kutbi and J. Xu, Appl. Surf. Sci., 2020, 509, 145348 CrossRef CAS.
- C. R. Minitha, R. Suresh, U. K. Maity, Y. Haldorai, V. Subramaniam, P. Manoravi, M. Joseph and R. T. Rajendra Kumar, Ind. Eng. Chem. Res., 2018, 57, 1225–1232 CrossRef CAS.
- P. Ghamari Kargar, G. Bagherzade and H. Eshghi, RSC Adv., 2020, 10, 32927–32937 RSC.
- S. Mallakpour and M. Madani, Prog. Org. Coat., 2015, 86, 194–207 CrossRef CAS.
- S. Rouhani, A. Rostami and A. Salimi, RSC Adv., 2016, 6, 26709–26718 RSC.
- M. Yarie, M. A. Zolfigol, Y. Bayat, A. Asgari, D. A. Alonso and A. Khoshnood, RSC Adv., 2016, 6, 82842–82853 RSC.
- R. Taheri-Ledari, J. Rahimi and A. Maleki, Ultrason. Sonochem., 2019, 59, 104737 CrossRef PubMed.
- L. Shiri, S. Zarei, M. Kazemi and D. Sheikh, Appl. Organomet. Chem., 2018, 32, e3938 CrossRef.
- B. Zeynizadeh, S. Rahmani and E. Eghbali, Polyhedron, 2019, 168, 57–66 CrossRef CAS.
- (a) Y. Zhang, S. Liu, W. Lu, L. Wang, J. Tian and X. Sun, Catal. Sci. Technol., 2011, 1, 1142–1144 RSC; (b) D. Shan, S. Deng, C. Jiang, Y. Chen, B. Wang, Y. Wang, J. Huang, G. Yu and M. R. Wiesner, Environ. Sci.: Nano, 2018, 5, 1650–1660 RSC; (c) S. Mahalingam and Y.-H. Ahn, New J. Chem., 2018, 42, 4372–4383 RSC; (d) C. Ding, W. Wei, H. Sun, J. Ding, J. Ren and X. Qu, Carbon, 2014, 79, 615–622 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- J. Pyun, Angew. Chem., Int. Ed., 2011, 50, 46–48 CrossRef CAS PubMed.
- D. R. Dreyer, H.-P. Jia and C. W. Bielawski, Angew. Chem., Int. Ed., 2010, 49, 6813–6816 CAS.
- B. Majumdar, D. Sarma, T. Bhattacharya and T. K. Sarma, ACS Sustainable Chem. Eng., 2017, 5, 9286–9294 CrossRef CAS.
- D. Sengupta, S. Ghosh and B. Basu, Curr. Org. Chem., 2017, 21, 834–854 CrossRef CAS.
- D. Higgins, P. Zamani, A. Yu and Z. Chen, Energy Environ. Sci., 2016, 9, 357–390 RSC.
- C. Feng, H. Y. Zhang, N. Z. Shang, S. T. Gao and C. Wang, Chin. Chem. Lett., 2013, 24, 539 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro, P. Concepción, V. Fornés and H. Garcia, Chem. Commun., 2012, 48, 5443–5445 RSC.
- H.-Y. Zhuo, X. Zhang, J.-X. Liang, Q. Yu, H. Xiao and J. Li, Chem. Rev., 2020, 120, 12315–12341 CrossRef CAS PubMed.
- J. Liu, ChemCatChem, 2011, 3, 934–948 CrossRef CAS.
- (a) A. M. Tafesh and J. Weiguny, Chem. Rev., 1996, 96, 2035–2052 CrossRef CAS PubMed; (b) V. Goyal, N. Sarki, B. Singh, A. Ray, M. Poddar, A. Bordoloi, A. Narani and K. Natte, ACS Appl. Nano Mater., 2020, 3, 11070–11079 CrossRef CAS.
- (a) F. Yang, C. Chi, C. Wang, Y. Wang and Y. Li, Green Chem., 2016, 18, 4254–4262 RSC; (b) V. Goyal, J. Gahtori, A. Narani, P. Gupta, A. Bordoloi and K. Natte, J. Org. Chem., 2019, 84, 15389–15398 CrossRef CAS.
- Z. Wei, S. Mao, F. Sun, J. Wang, B. Mei, Y. Chen, H. Li and Y. Wang, Green Chem., 2018, 20, 671–679 RSC.
- Y. Sheng, X. Wang, S. Yue, G. Cheng, X. Zou and X. Lu, ChemCatChem, 2020, 12, 4632–4641 CrossRef CAS.
- B. Zeynizadeh, Z. Shokri and M. Hasanpour Galehban, Appl. Organomet. Chem., 2019, 33, e4771 CrossRef.
- S. Karami, B. Zeynizadeh and Z. Shokri, Cellulose, 2018, 25, 3295–3305 CrossRef CAS.
- D. Cantillo, M. M. Moghaddam and C. O. Kappe, J. Org. Chem., 2013, 78, 4530–4542 CrossRef CAS PubMed.
- J. Shabir, C. Garkoti, P. Gupta, M. Sharma, S. Rani, M. Kumari and S. Mozumdar, ACS Omega, 2021, 6, 1415–1425 CrossRef CAS PubMed.
- T. V. Thu, P. J. Ko, T. V. Nguyen, N. T. Vinh, D. M. Khai and L. T. Lu, Appl. Organomet. Chem., 2017, 31, e3781 CrossRef.
- B. Zeynizadeh, S. Rahmani and H. Tizhoush, Polyhedron, 2020, 175, 114201 CrossRef.
- (a) J. M. García, F. C. García, F. Serna and J. L. de la Peña, Prog. Polym. Sci., 2010, 35, 623–686 CrossRef; (b) B. Alcaide, P. Almendros and C. Aragoncillo, Chem. Rev., 2007, 107, 4437–4492 CrossRef CAS PubMed; (c) S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451–3479 CrossRef CAS PubMed.
- (a) Z. Shokri, B. Zeynizadeh and S. A. Hosseini, J. Colloid Interface Sci., 2017, 485, 99–105 CrossRef CAS PubMed; (b) X. Sun, A. I. Olivos-Suarez, L. Oar-Arteta, E. Rozhko, D. Osadchii, A. Bavykina, F. Kapteijn and J. Gascon, ChemCatChem, 2017, 9, 1854–1862 CrossRef CAS.
- (a) R. Nie, J. Wang, L. Wang, Y. Qin, P. Chen and Z. Hou, Carbon, 2012, 50, 586–596 CrossRef CAS; (b) M. Gilanizadeh and B. Zeynizadeh, J. Iran. Chem. Soc., 2018, 15, 2821–2837 CrossRef.
- B. Zeynizadeh, F. M. Aminzadeh and H. Mousavi, Green Process. Synth., 2019, 8, 742–755 CAS.
- B. Zeynizadeh, F. Sepehraddin and H. Mousavi, Ind. Eng. Chem. Res., 2019, 58, 16379–16388 CrossRef CAS.
- B. Zeynizadeh, R. Younesi and H. Mousavi, Res. Chem. Intermed., 2018, 44, 7331–7352 CrossRef CAS.
- B. Zeynizadeh, H. Mousavi and S. Zarrin, J. Chin. Chem. Soc., 2019, 66, 928–933 CrossRef CAS.
- B. Zeynizadeh, F. Mohammad Aminzadeh and H. Mousavi, Res. Chem. Intermed., 2019, 45, 3329–3357 CrossRef CAS.
- B. Zeynizadeh and F. Sepehraddin, J. Organomet. Chem., 2018, 856, 70–77 CrossRef CAS.
- G. Cheng, Y.-L. Liu, Z.-G. Wang, J.-L. Zhang, D.-H. Sun and J.-Z. Ni, J. Mater. Chem., 2012, 22, 21998–22004 RSC.
- R. V. Jagadeesh, A.-E. Surkus, H. Junge, M.-M. Pohl, J. Radnik, J. Rabeah, H. Huan, V. Schünemann, A. Brückner and M. Beller, Science, 2013, 342, 1073–1076 CrossRef CAS PubMed.
- P. L. Reddy, M. Tripathi, R. Arundhathi and D. S. Rawat, Chem.–Asian J., 2017, 12, 785–791 CrossRef CAS PubMed.
- D. V. Jawale, E. Gravel, C. Boudet, N. Shah, V. Geertsen, H. Li, I. N. N. Namboothiri and E. Doris, Chem. Commun., 2015, 51, 1739–1742 RSC.
- M. Lauwiner, P. Rys and J. Wissmann, Appl. Catal., A, 1998, 172, 141–148 CrossRef CAS.
- A. O. Moghanlou, M. H. Sadr, A. Bezaatpour, F. Salimi and M. Yosefi, Mol. Catal., 2021, 516, 111997 CrossRef CAS.
- R. Azad, A. Bezaatpour, M. Amiri, H. Eskandari, S. Nouhi, D. H. Taffa, M. Wark, R. Boukherroub and S. Szunerits, Appl. Organomet. Chem., 2019, 33, e5059 CrossRef.
- G.-Y. Fan, L. Zhang, H.-Y. Fu, M.-L. Yuan, R.-X. Li, H. Chen and X.-J. Li, Catal. Commun., 2010, 11, 451–455 CrossRef CAS.
- F. Ferlin, M. Cappelletti, R. Vivani, M. Pica, O. Piermatti and L. Vaccaro, Green Chem., 2019, 21, 614–626 RSC.
- S. Abdolhosseinzadeh, H. Asgharzadeh and H. Seop Kim, Sci. Rep., 2015, 5, 10160 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02293a |
| This journal is © The Royal Society of Chemistry 2022 |