Open Access Article
Open Access ArticleDetermination of macro, micro and toxic element concentrations in peanuts from main peanut producing areas of China by ICP-MS: a pilot study on the geographical characterization
Lu Chen ab,
Min Dingab,
Zengmei Liab,
Xia Li*ab and
Ligang Deng*ab
ab,
Min Dingab,
Zengmei Liab,
Xia Li*ab and
Ligang Deng*ab
aShandong Provincial Key Laboratory of Test Technology on Food Quality and Safety, Jinan 250100, China. E-mail: deng_ligang@163.com
bInstitute of Quality Standard & Testing Technology for Agro-Products, Shandong Academy of Agricultural Sciences, Jinan 250100, China. E-mail: lisa-fsd@163.com
First published on 7th June 2022
Abstract
Peanut is an important crop grown worldwide. The geographic origin of peanuts has been a topic of substantial attention since their prices can vary according to their geographic origins. This study evaluated the main macro (K, Ca, Mg, Na, and Al), micro (Fe, Zn, Mn, Ni, Sr, Mo, Cu, Se, V, Co), and toxic (As, Cd, Cr, and Pb) element concentrations in peanuts collected from six different Chinese provinces. Multi-element analysis of peanuts from different regions was carried out to develop a reliable method to trace the origin of peanuts. After microwave digestion, the element concentrations were determined through inductively coupled plasma mass spectrometry (ICP-MS). Certified reference material (CRM, GBW10011) was used to ensure accurate results. The profile of contents of major elements obtained in the current study showed the order: K > Mg > Ca > Al > Na > Zn > Fe > Mn > Ni > Sr > Mo. The average concentrations of toxic elements such as Pb, Cd, As, and Cr were very low and within the safe limits. Correlation analysis showed that there was a strong correlation between individual elements in peanut samples. The data were processed by means of the chemometric approach of linear discriminant analysis (LDA) and 97.0% of samples were correctly predicted.
1. Introduction
Peanut is an important crop grown worldwide. In China, it is one of the most important oil crops. In addition to oil, peanuts are also widely used in the production of peanut butter, confections, roasted peanuts and snack products.1 China is the largest producer of peanuts in the world. In 2017, China's peanut production was 17.09 million tons, accounting for 35% of the world's peanut production (FAO). China has several important peanut production regions, each with its own environmental conditions, cultivars and cropping systems: Yellow River, Yangtze River, Southeast Coast and Northeast regions.2 Peanuts contain 44% to 56% oil and 22% to 30% protein on a dry seed basis and is a rich source of minerals.3,4 At the same time, some heavy metals also exist in peanuts. The presence of heavy metals in higher concentration is toxic and causes disease.5 Whether focusing on the essential minerals or toxic heavy metals, it is necessary to explore the element contents of peanuts in different regions.At present, atomic absorption spectrometry (AAS), atomic fluorescence spectrometry (AFS), inductively coupled plasma optical emission spectrometry (ICP-OES) and inductively coupled plasma mass spectrometry (ICP-MS) are commonly used for element analysis.6–10 Anzano et al. determined iron and copper of peanuts by AAS.11 Yang et al. analyzed 6 heavy metals in peanut and peanut soil by ICP-MS.12 Phan-Thien et al. investigated the use of ICP-OES and ICP-MS, with and without the use of a dynamic reaction cell (DRC), to analyze 15 essential minerals in peanut kernels.13 Compared with other detection methods, ICP-MS has some distinct advantages, including simultaneous multi-element measurement capability coupled with very low detection limits.14 So ICP-MS is widely used for the analysis of elements in foods.15,16
With the development of international markets and the demanding of high-quality agricultural products, the safety and authenticity of foodstuffs have become major concerns for consumers.17 Determining the geographic origin of agricultural food is attracted much more concerns since there is an increasing interest by consumers for high quality food products with a clear geographical origin.18 The discrimination of the geographical origin of agricultural products based on instrumental analysis is the technical foundation for the protection of geographical origin and improve the quality and safety of agricultural products. Studies regarding determination of the geographical origin of foods, are important in commodity science.19–23 Wang et al. identification identified sub-regional of peanuts from Shandong Province of China based on Fourier transform infrared (FT-IR) spectroscopy. However, few studies delved into identifying the geographical origins of peanuts by the multi-element analysis combined with statistical analysis.24 Zhang et al. reported that the distribution and toxin production of Aspergillus flavus had marked regional characteristics.25 Peanut quality is intricately related to its origin and plays a significant role in its pricing alongside the influence of other varieties. Tracing the geographical origin of peanuts is of substantial interest as it makes the process of recalling contaminated peanuts by aflatoxins easier, thereby mitigating financial losses and protecting consumer interest.26
The objective of this study was to (1) determine 19 elements, including As, Pb, V, Cd, Cr, Co, Cu, Ni, Zn, Sr, Al, K, Mo, Ca, Mg, Fe, Mn, Na and Se in peanuts of different regions by ICP-MS, and (2) explore a method to distinguish peanuts from different producing areas based on multiple elements and chemometrics.
2. Experimental
2.1 Samples
In this paper, a total of 66 samples of peanuts were analyzed for their major and trace element contents. The samples included 10 peanuts samples from Jilin Province in the northeast of china, 10 samples from Liaoning Province which is close to Jilin Province, 15 samples from Hebei Province in the north of China, 19 samples from Henan Province which is south of Hebei Province, 5 samples from Guangxi Province, 7 samples from Guangdong Province (Fig. 1). In order to ensure the accuracy of peanut origin, the samples of at least of 3 kg were purchased from acquisition station near peanut harvest land. Peanuts samples were ground by a homogenizer (Midea, MJ-BL80Y21, China) at a speed of 18000 rpm for 5 min for further analysis.2.2 Reagents and instrumentation
All solutions were prepared using ultrapure water (resistivity of 18 MΩ cm−1) obtained from a Milli-Q purification system (Millipore Corp., Bedford, MA, USA). Nitric acid (HNO3, trace metal grade, 67–70%, Thermo Fisher Scientific, USA) and hydrogen peroxide (H2O2; analytical reagent, 30%, Sinopharm Chemical Reagent, China) were used for sample digestion. Stock standard solutions of the elements (1000 mg L−1) were acquired from Inorganic Ventures (Lakewood, NJ, USA). All plastic and glass containers that came into contact with samples or standards were evaluated for contamination to avoid the release of metals. All glass and plastic containers are soaked with nitric acid (20%, v/v) overnight, rinsed with ultrapure water, and then dried. Certified reference materials (CRMs) consisting of soybean (GBW10011) were purchased from the Institute of Geophysical and Geochemical Exploration (China). A microwave digestion instrument (MARS5, CEM, USA) was used for the digestion of samples and CRMs. The simultaneous determination of elements was carried out by ICP-MS (iCAP Q, Thermo, USA). Argon (purity of 99.999%) was used as an auxiliary gas and for plasma generation and nebulization.2.3 Sample preparation and microwave digestion
Samples (0.3 g) or CRMs (0.3 g) were accurately weighed in tetrafluoroethylene digestion vessel, and then 6 mL of nitric acid (65%) and 2 mL of concentrated H2O2 (30%) were added and then kept overnight at room temperature. After tightening the lid of the digestion vessel, digestion was performed according to the following: warmed up to 120 °C in 5 min, held for 5 min, then raised the temperature to 150 °C for 5 min, held for 10 min, and finally raised the temperature to 190 °C for 5 min, held for 20 min. After cooling to room temperature, the samples were transferred into 25 mL polyethylene volumetric flasks and filled with ultrapure water to a final volume of 25 mL before ICP-MS. Blank samples were prepared as described above.2.4 ICP-MS analysis and quality assurance
ICP-MS was used for the determination of As, Pb, V, Cd, Cr, Co, Cu, Ni, Zn, Sr, Al, K, Mo, Ca, Mg, Fe, Mn, Na and Se. The parameter conditions used in ICP-MS are summarized in Table 1. Blank samples were analyzed and subtracted from the sample measurements before the results were calculated. The limits of detection (LOD) were calculated by measuring three times the standard deviation of the blank samples. The limits of quantification (LOQ) were calculated based on the average sample volume and total volume analyzed. Each peanut sample was measured three times, and the average value was used. CRMs (GBW10013 soybean) were used for the assessment of both accuracy and precision in element analysis.| ICP-MS | Parameter conditions |
|---|---|
| Radio frequency power (W) | 1550 |
| Cool gas flow (L min−1) | 14 |
| Auxiliary gas flow (L min−1) | 0.8 |
| Nebulizer gas flow (L min−1) | 1.05 |
| Peristaltic pump speed (rpm) | 40 |
| Sampling depth (mm) | 5 |
| Spray chamber temperature (°C) | 2.7 |
| Measurement mode | Kinetic energy discrimination |
| Dwell time (ms) | 20 |
| Isotopes measured | 27Al, 75As, 44Ca, 111Cd, 52Cr, 65Cu, 57Fe, 39K, 24Mg, 55Mn, 23Na, 60Ni, 208Pb, 82Se, 88Sr, 66Zn, 59Co, 95Mo, 51V |
2.5 Statistical analysis
Mean concentration of the elements were evaluated for significance using Duncan's multiple range test and one-way analysis of variance (ANOVA) using SPSS Statistics Software Version 23.0 (IBM, New York, USA). Linear discriminant analysis (LDA) using the stepwise method was carried out to evaluate whether peanuts from different regions could be mathematically distinguished on the basis of elements which had significant differences among the regions. The stepwise procedure was carried out to extract the best discriminant variables separating peanuts from different origins. Wilks' lambda criterion was adopted in this study to select the significant variables.26 The predictive ability of the LDA model was evaluated by leave-one-out cross-validation. The procedure works by omitting each observation one at a time, recalculating the classification function by using the remaining data and then classifying the omitted observation.27 The advantage is that the leave-one-out method allows estimating the prediction error avoiding the overfitting of the model.28 A correlation analysis was conducted to assess the relationship between the peanuts' constituent elements. The correlations of all elements in the peanut samples were evaluated by a Pearson's correlation test. P values were two-tailed, and two significant levels were using P = 0.05 and 0.01. The correlation analysis and LDA were also performed by SPSS Statistics Software Version 23.0. Radar plot analysis of element concentrations were processed by Microsoft Excel 2010.3. Results and discussion
3.1 Method validation
Table 2 summarizes the data on linearity and accuracy of the method employed. The evaluation of the linearity was based on injections of the 6 standard solutions. Each solution was injected five times (n = 5). Good linearity was observed in each concentration range, with R2 > 0.9993. LOD values ranged from 0.0001 to 0.82 mg kg−1. LOQ values ranged from 0.0003 to 2.47 mg kg−1. Accuracy was assessed by agreement between measured values and the certified value of CRMs. It can be seen from the data presented in Table 2 that good agreement was achieved between the certified values and those determined by ICP-MS for the 19 elements reported. The recovery ranged from 91.4–109.1%.| Element | Calibration range (μg L−1) | R2 | LOD (mg kg−1) | LOQ (mg kg−1) | GBW10013 | ||
|---|---|---|---|---|---|---|---|
| Certified value | Measured value | Recovery (%) | |||||
| Al | 0.5–100 | 0.9999 | 0.036 | 0.109 | (430) | 416 ± 22 | 96.7 |
| As | 0.1–5 | 0.9999 | 0.0007 | 0.0021 | 0.035 ± 0.012 | 0.032 ± 0.005 | 91.4 |
| Ca* | 1–2000 | 0.9995 | 0.59 | 1.78 | 0.153 ± 0.008 | 0.157 ± 0.006 | 102.6 |
| Cd | 0.1–5 | 0.9999 | 0.0001 | 0.0003 | (0.011) | 0.011 ± 0.002 | 100.0 |
| Cr | 0.1–20 | 0.9993 | 0.001 | 0.003 | 0.28 ± 0.04 | 0.271 ± 0.010 | 96.8 |
| Co | 0.1–5 | 0.9999 | 0.005 | 0.015 | 0.125 ± 0.012 | 0.127 ± 0.010 | 101.6 |
| Cu | 1–200 | 0.9996 | 0.015 | 0.045 | 10.2 ± 0.5 | 10.4 ± 0.3 | 102.0 |
| Fe | 5–1000 | 0.9993 | 0.088 | 0.265 | 139 ± 4 | 135 ± 7 | 97.1 |
| K* | 5–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.9996 | 0.82 | 2.47 | 1.86 ± 0.09 | 1.82 ± 0.08 | 97.8 |
| Mg* | 2–3000 | 0.9994 | 0.067 | 0.201 | 0.230 ± 0.014 | 0.233 ± 0.011 | 101.3 |
| Mn | 1–500 | 0.9994 | 0.011 | 0.034 | 28 ± 1 | 28.7 ± 0.8 | 102.5 |
| Mo | 0.5–100 | 0.9999 | 0.023 | 0.069 | 0.71 ± 0.04 | 0.733 ± 0.032 | 103.2 |
| Na | 1–1000 | 0.9995 | 0.159 | 0.478 | (15) | 15.5 ± 0.8 | 103.3 |
| Ni | 0.5–50 | 0.9997 | 0.005 | 0.016 | 4.0 ± 0.3 | 4.12 ± 0.18 | 103.0 |
| Pb | 0.1–5 | 0.9999 | 0.003 | 0.009 | 0.07 ± 0.02 | 0.065 ± 0.013 | 92.9 |
| Se | 0.2–10 | 0.9993 | 0.008 | 0.025 | (0.022) | 0.024 ± 0.004 | 109.1 |
| Sr | 1–100 | 0.9998 | 0.024 | 0.072 | 9.9 ± 0.6 | 9.5 ± 0.5 | 96.0 |
| V | 0.1–5 | 0.9994 | 0.005 | 0.015 | (0.08) | 0.077 ± 0.005 | 96.2 |
| Zn | 5–500 | 0.9993 | 0.082 | 0.247 | 38 ± 2 | 37.4 ± 0.7 | 98.4 |
3.2 Element concentration in peanuts
The average contents of elements in peanut samples of different geographical origins on fresh weight basis are presented in Table 3, the results being expressed in mg kg−1. There were differences (p <0.05) in the concentrations of all elements with the exception of Al (p = 0.222), Fe (p = 0.069), As (p = 0.077), Se (p = 0.125), Sr (p = 0.357), Cr (p = 0.263) and Pb (p = 0.516).| Element | Jilin | Liaoning | Henan | Hebei | Guangxi | Guangdong |
|---|---|---|---|---|---|---|
| Macro element | ||||||
| K | 6230 ± 484ab | 6537 ± 475ab | 6692 ± 576b | 6743 ± 854bb | 6197 ± 516ab | 6034 ± 374a |
| Mg | 1637 ± 56ab | 1765 ± 88c | 1730 ± 131bc | 1625 ± 81a | 1613 ± 97a | 1653 ± 83ab |
| Ca | 664 ± 138c | 624 ± 42bc | 589 ± 86b | 589 ± 90b | 436 ± 33a | 570 ± 32b |
| Al | 86.4 ± 29.9 | 113 ± 23 | 119 ± 93 | 94.7 ± 31.2 | 75.5 ± 4.3 | 64.5 ± 30.3 |
| Na | 12.1 ± 4.0a | 6.32 ± 2.32a | 58.0 ± 33.2b | 62.7 ± 21.4b | 24.3 ± 3.8a | 13.8 ± 11.2a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Micro element | ||||||
| Cu | 4.14 ± 0.64a | 4.92 ± 0.38abc | 6.42 ± 1.00d | 5.99 ± 1.45cd | 5.47 ± 2.16bcd | 4.66 ± 0.81ab |
| Fe | 24.8 ± 6.2 | 24.4 ± 3.2 | 25.0 ± 7.7 | 23.5 ± 3.7 | 19.1 ± 2.3 | 38.7 ± 33.2 |
| Co | 0.048 ± 0.008ab | 0.077 ± 0.019abc | 0.093 ± 0.079abc | 0.036 ± 0.009a | 0.098 ± 0.143bc | 0.185 ± 0.038bc |
| Mn | 14.2 ± 1.1ab | 20.5 ± 1.8c | 17.5 ± 9.0bc | 11.9 ± 3.2a | 22.3 ± 5.8c | 19.0 ± 2.4bc |
| Mo | 1.05 ± 0.48abc | 0.621 ± 0.731ab | 2.33 ± 2.25c | 1.83 ± 0.68bc | 0.368 ± 0.060a | 0.468 ± 0.128a |
| Sr | 3.18 ± 0.24 | 3.61 ± 0.78 | 3.24 ± 1.64 | 3.50 ± 1.26 | 2.32 ± 0.95 | 2.88 ± 0.18 |
| V | 0.050 ± 0.041a | 0.094 ± 0.055b | 0.052 ± 0.037a | 0.061 ± 0.018ab | 0.033 ± 0.016a | 0.052 ± 0.030a |
| Zn | 22.7 ± 2.6a | 29.0 ± 3.0 cd | 32.5 ± 5.2d | 30.0 ± 4.4 cd | 27.5 ± 3.4bc | 23.8 ± 3.6ab |
| Ni | 3.08 ± 1.21ab | 7.53 ± 1.17c | 5.87 ± 6.11bc | 1.34 ± 1.49a | 8.73 ± 1.77c | 8.33 ± 3.70c |
| Se | 0.030 ± 0.012 | 0.027 ± 0.003 | 0.037 ± 0.010 | 0.040 ± 0.019 | 0.038 ± 0.006 | 0.033 ± 0.008 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Toxic element | ||||||
| As | 0.015 ± 0.007 | 0.020 ± 0.008 | 0.020 ± 0.012 | 0.016 ± 0.004 | 0.009 ± 0.0008 | 0.014 ± 0.003 |
| Cd | 0.110 ± 0.049ab | 0.204 ± 0.131c | 0.147 ± 0.080bc | 0.047 ± 0.040a | 0.168 ± 0.063bc | 0.121 ± 0.096c |
| Cr | 0.278 ± 0.092 | 0.234 ± 0.099 | 0.293 ± 0.126 | 0.268 ± 0.069 | 0.280 ± 0.041 | 0.262 ± 0.072 |
| Pb | 0.025 ± 0.045 | 0.060 ± 0.020 | 0.018 ± 0.045 | ND | 0.024 ± 0.041 | 0.007 ± 0.044 |
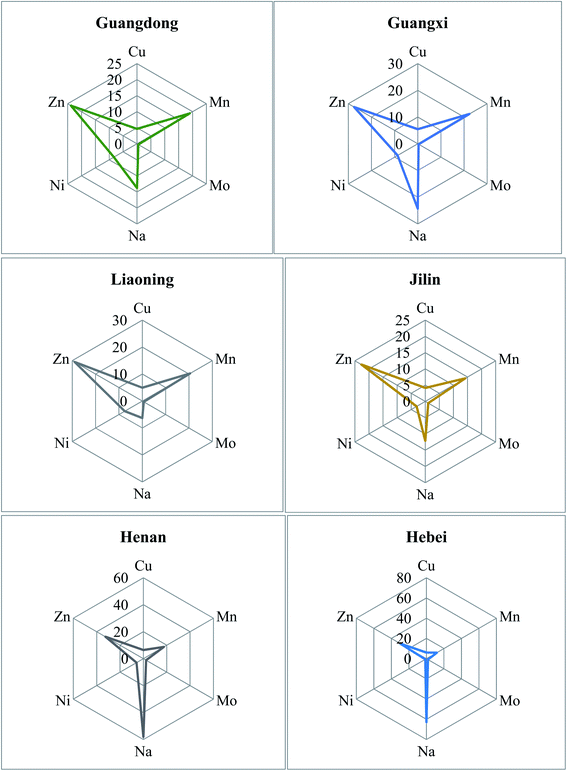
3.3 Radar plot analysis
A radar plot based on some elements has been used to better show the elemental differences between different provinces. At the same time, the method can be used to simply, quickly and conventionally distinguish the regions of peanuts. For ease of comparison, radar plots analysis was performed on the basis of six elements (Na, Cu, Zn, Mo, Mn and Ni). These elements were chosen because their concentrations showed high variation in each peanut sample. The contents of the six elements are similar and the same element has significant differences between different provinces.There are differences in peanut elements between different provinces. In addition, Jilin and Liaoning province, Henan and Hebei province, Guangdong and Guangxi province are similar (Fig. 2). Those findings can be attributed to the fact that Jilin and Liaoning are both parts of northeastern China, and soil types for planting peanuts are mostly sandy soil, gravel soil, and aeolian sand soil. Certain element contents (Cu, Na, and Mo) in the soil were lower than in the other provinces. The cultivation system is generally one harvest per year or three harvests during two years. Henan and Hebei both belong to north-central China. The soil for peanut planting is mainly river alluvial sand and soil loam. Moreover, since this region is a major crop producer, fertilizers were often used during periods of crop growth, enabling the accumulation of certain essential elements such as Na, Mo, and Zn. The cultivation system generally includes two harvests per year or three harvests over two years. Guangdong and Guangxi are both parts of Southern China. The soil types are mainly red and yellow soil. The cultivation system usually includes two or three yearly harvests.38,39
Peanuts can be influenced by the element profile of the environment (soil, water, air, etc.). Soils are the major repository of heavy metals in terrestrial ecosystems.40 Peanut seeds are underground, and the influence of soil on the element content of peanuts is essential. Trace element profiles of soils differ based on several environmental and geological factors such as soil type, soil parent material, soil pH, and climate conditions.41 The natural diffusional movement of elemental traces follows a pattern moving from rocks to soil, and from the soil to the agricultural products.42 And different regions in China have different soil environmental quality due to different natural factors and human activities.
3.4 Correlation analysis
Correlation analysis was performed to identify possible relationships between the elements. The correlation coefficient can range from −1 to +1 and is independent of the units of measurement. A value near 0 indicates virtually no correlation between two attributes, whereas a value near +1 or −1 indicates a high level of correlation. The results from the correlation coefficients between elements are listed in Table 4. Ni was positively correlated with Mn and Co (r = 0.908 and r = 0.900; P < 0.01). Mo was negative correlated with Mn and Ni (r = −0.887 and r = −0.858; P < 0.01). A previous study also observed that plants with a high requirement for Mo may have a low requirement for Mn.43 Positive correlations were found between Mn and Co (r = 0.898; P < 0.01). A significant and negative correlation between Ca and K (r = −0.78) in the peanuts was reported also by Branch et al.44 However, contrary to our results, the correlation coefficient between K and Ca is 0.014. The relationship between elements demonstrates significant negative correlations between some elements. This finding was probably due to the interaction between ions whose chemical properties were sufficiently similar, and they compete for site of absorption, transport, and function in plant tissues.45 The significant positive correlation could be due to the ability of the ions to form chemical bonds. In this case, the formation of complexes may lead to the synergistic effect of peanut on the absorption of elements.| Na | Mg | Al | K | Ca | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | As | Se | Sr | Mo | Cd | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a **: Correlation is significant at the 0.01 level (2-tailed).b *: Correlation is significant at the 0.05 level (2-tailed). | |||||||||||||||||||
| Na | 1.000 | ||||||||||||||||||
| Mg | −0.203 | 1.000 | |||||||||||||||||
| Al | 0.166 | 0.262* | 1.000 | ||||||||||||||||
| K | 0.429** | 0.142 | 0.216 | 1.000 | |||||||||||||||
| Ca | −0.138 | 0.412** | 0.165 | 0.014 | 1.000 | ||||||||||||||
| V | −0.241 | 0.014 | 0.013 | −0.042 | 0.104 | 1.000 | |||||||||||||
| Cr | −0.154 | 0.156 | −0.255* | −0.114 | 0.111 | 0.150 | 1.000 | ||||||||||||
| Mn | −0.569** | 0.338** | −0.072 | −0.320** | −0.140 | −0.084 | 0.129 | 1.000 | |||||||||||
| Fe | 0.114 | 0.152 | 0.604** | 0.265* | 0.221 | 0.142 | −0.014 | −0.093 | 1.000 | ||||||||||
| Co | −0.552** | 0.263* | −0.116 | −0.225 | −0.179 | −0.022 | 0.214 | 0.898** | 0.051 | 1.000 | |||||||||
| Ni | −0.580** | 0.256* | −0.089 | −0.392** | −0.124 | −0.001 | 0.173 | 0.908** | −0.065 | 0.900** | 1.000 | ||||||||
| Cu | 0.514** | 0.212 | 0.066 | 0.314* | 0.050 | −0.260* | 0.021 | 0.072 | 0.056 | −0.033 | −0.063 | 1.000 | |||||||
| Zn | 0.410** | 0.296* | 0.151 | 0.463** | 0.033 | −0.139 | −0.010 | 0.122 | 0.135 | 0.022 | −0.012 | 0.806** | 1.000 | ||||||
| As | 0.134 | 0.136 | 0.109 | 0.315* | 0.125 | 0.602** | 0.043 | −0.409** | 0.251* | −0.290* | −0.380** | −0.172 | 0.015 | 1.000 | |||||
| Se | 0.206 | −0.072 | −0.300* | 0.117 | −0.239 | −0.205 | 0.141 | 0.071 | −0.215 | 0.012 | 0.014 | 0.357** | 0.475** | −0.146 | 1.000 | ||||
| Sr | 0.088 | 0.245* | 0.183 | 0.152 | 0.520** | 0.247* | −0.007 | −0.444** | 0.112 | −0.405** | −0.378** | −0.110 | −0.054 | 0.362** | −0.278* | 1.000 | |||
| Mo | 0.561** | −0.147 | 0.112 | 0.386** | 0.108 | 0.004 | −0.059 | −0.858** | 0.186 | −0.794** | −0.887** | 0.050 | 0.070 | 0.457** | 0.040 | 0.466** | 1.000 | ||
| Cd | −0.480** | 0.366** | −0.026 | −0.230 | −0.005 | −0.113 | 0.176 | 0.794** | 0.034 | 0.723** | 0.688** | 0.174 | 0.084 | −0.282* | −0.101 | −0.421** | −0.614** | 1.000 | |
| Pb | −0.060 | 0.024 | 0.286* | −0.206 | −0.057 | −0.560** | −0.159 | 0.355** | 0.124 | 0.298* | 0.264* | 0.112 | 0.050 | −0.332** | −0.009 | −0.202 | −0.252* | 0.331** | 1.000 |
It can be assumed that elements do not cycle independently in vegetation due to their involvement in the basic cellular and structural functions, similar biochemical pathways. Thus, concentrations of different elements must be correlated. Interactions observed within plants between elements have also indicated that these processes are quite complex and are simultaneously antagonistic and synergistic. Moreover, they are occasionally involved in the metabolism of over two elements.46 Few studies have delved into the relationships between the concentrations of elements within peanuts.43–45,47 The information yielded in this study will shed light on peanut nutrition breeding and the risk assessment of peanut quality and safety.
3.5 Linear discriminant analysis (LDA)
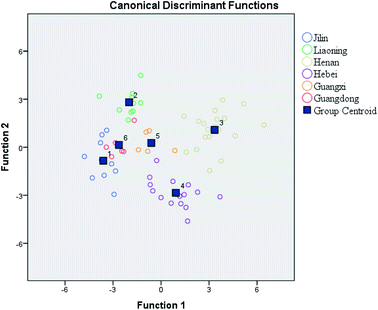
Linear discriminant analysis (LDA) was used to identify the most useful variables and to remove non-essential information for the discrimination of peanuts regions. LDA was carried out based on the 19 elements analyzed in the peanut samples. A cross-validation procedure was used to evaluate this model. Fig. 3 shows the differentiation of the peanut samples for six regions when the first two discriminant functions were represented. Five canonical discriminant functions explained 100% of the variance, and the first two functions explained 72.3% of the variance (function 1 explained 47.7% of the total variance, and function 2 explained 24.6% of the total variance). When all the variables were introduced in the LDA, the percentage of correct classification was 97.0% for all locations and the percentages obtained after cross-validation decreased to 78.8% (Table 5). It can be deduced that the content of mineral and trace elements coupled with LDA is a good instrument for establishing the geographical origin of peanuts and their cultivars.| Predicted group membership | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Jilin | Liaoning | Henan | Hebei | Guangxi | Guangdong | Total | |||
| Original | Count | Jilin | 10 | 0 | 0 | 0 | 0 | 0 | 10 |
| Liaoning | 0 | 10 | 0 | 0 | 0 | 0 | 10 | ||
| Henan | 0 | 0 | 19 | 0 | 0 | 0 | 19 | ||
| Hebei | 0 | 0 | 0 | 15 | 0 | 0 | 15 | ||
| Guangxi | 0 | 0 | 0 | 0 | 5 | 0 | 5 | ||
| Guangdong | 1 | 0 | 0 | 0 | 1 | 5 | 7 | ||
| % | 100 | 100 | 100 | 100 | 100 | 71.4 | 97.0 | ||
| Cross-validated | Count | Jilin | 10 | 0 | 0 | 0 | 0 | 0 | 10 |
| Liaoning | 1 | 8 | 0 | 0 | 1 | 0 | 10 | ||
| Henan | 0 | 2 | 14 | 2 | 0 | 1 | 19 | ||
| Hebei | 1 | 0 | 0 | 12 | 1 | 1 | 15 | ||
| Guangxi | 0 | 0 | 0 | 0 | 4 | 1 | 5 | ||
| Guangdong | 1 | 0 | 0 | 0 | 2 | 4 | 7 | ||
| % | 100 | 80 | 73.7 | 80 | 80 | 57.1 | 78.8 | ||
This study investigated the classification of geographical origins of peanuts by the multi-element analysis combined with multivariate data analysis. Some marked differences have been noted in some elements (Na, Cu, Zn, Mo, Mn, Ni, etc.) in different regions, which could be attributed to a soil background value, agricultural inputs, or other activities. Certain elements may serve as a preliminary classifier for determining geographical origin. None of the toxic elements has exceeded the limit value and can be temporarily deemed safe for human consumption. The research related to exposure assessment and risk assessment merits further academic attention. More attention should be allocated to specific elements in specific areas through the analysis of toxic elements in different areas. The establishment of an effective method to identify peanut-producing areas can protect geographical indication products and improve the early warning ability of peanut-producing areas with high risks, thereby reducing the risk of human intake of toxic substances from peanuts.
For further research in this field, more samples of each place of origin should be analyzed to allow evaluation by multivariate data analysis. Rare earth elements such as ytterbium (Yb), lutetium (Lu), lanthanum (La), yttrium (Y), etc. could try to be determined which then will help to establish a more robust discrimination model that will allow the verification of the geographical origin of unknown samples. In addition to geographical region, the differences of element contents in peanut may be affected by other factors including peanut varieties, harvest season, agronomic practices, etc. Therefore, further research on the respective effect extent of each factor to element variation in peanuts should be taken into consideration.26
4. Conclusions
We analyzed 19 elements in peanuts from six provinces in China using ICP-MS. The applied analytical techniques were validated by quality assurance parameters in which the limits of detection, precision and accuracy confirmed that the analytical methods used are efficient. There are differences in element content in peanuts between different regions. K, Mg and Ca were the most abundant elements with concentrations >100 mg kg−1. The average concentrations of toxic trace elements such as Pb, Cd, As, and Cr were very low and within the safe limits. A radar plot based on chosen elements was used to better show the elemental differences between different provinces. Correlation analysis showed that there was a strong correlation between individual elements in peanut samples. LDA model based on concentrations of 19 elements allowed 97.0% of correct prediction. This study provides a reliable and efficient method to identify the geographical origin to enhance regional capabilities for quality assurance and control.Conflicts of interest
There are no conflicts to declare.References
- S. S. Arya, A. R. Salve and S. Chauhan, J. Food Sci. Technol., 2016, 53, 31–41 CrossRef CAS PubMed.
- Y. Li, R. Zhang, X. Qin, Y. Liao, K. H. M. Siddique and D. M. Prasad, Plant Breed., 2018, 137, 746–756 CrossRef CAS.
- J. Y. Asibuo, R. Akromah, O. Safo-Kantanka, H. K. Adu-Dapaah, S. Ohemeng-Dapaah and A. Agyeman, Afr. J. Biotechnol., 2008, 7, 2203–2208 CAS.
- A. G. de Oliveira Sousa, D. C. Fernandes, A. M. Alves, J. B. de Freitas and M. M. V. Naves, Food Res. Int., 2011, 44, 2319–2325 CrossRef.
- H. S. Manzoor, I. H. Bukhari, M. Riaz, N. Rasool, U. Sattar, G. Rehman and Q. Ul Ain, Int. J. Chem. Biochem. Sci., 2013, 3, 74–82 Search PubMed.
- V. F. Laurie, V. Evelyn, T. Jaime, J. E. S. Sarkis and A. M. Hortellani, Cienc. Invest. Agrar., 2010, 37, 77–85 Search PubMed.
- H. Sun and Y. Lu, Anal. Chim. Acta, 2002, 457, 305–310 CrossRef CAS.
- H. Sun, Z. Liu, W. Wu, L. Li and H. Shi, Anal. Bioanal. Chem., 2005, 382, 1060–1065 CrossRef CAS PubMed.
- J. Naozuka, E. Carvalho Vieira, A. N. Nascimento and P. V. Oliveira, Food Chem., 2011, 124, 1667–1672 CrossRef CAS.
- G. T. K. Danzer, Fresenius’ J. Anal. Chem., 1997, 357, 553–557 CrossRef.
- P. G. Jesus and M. Anzano, Microchem. J., 2000, 64, 141–145 CrossRef.
- B. Yang, C. Zhang, X. Zhang, G. Wang, L. Li, H. Geng, Y. Liu and C. Nie, Food Control, 2020, 118, 107372 CrossRef CAS.
- K. Phan-Thien, G. Wright and N. Lee, Food Chem., 2012, 34, 453–460 CrossRef.
- E. P. Nardi, F. S. Evangelista, L. Tormen, T. D. Saint'Pierre, A. J. Curtius, S. S. d. Souza and F. Barbosa, Food Chem., 2009, 112, 727–732 CrossRef CAS.
- A. Ataro, R. I. McCrindle, B. M. Botha, C. M. E. McCrindle and P. P. Ndibewu, Food Chem., 2008, 111, 243–248 CrossRef CAS.
- M. Chudzinska and D. Baralkiewicz, Food Chem. Toxicol., 2011, 49, 2741–2749 CrossRef CAS PubMed.
- F. Wang, H. Zhao, C. Yu, J. Tang, W. Wu and Q. Yang, J. Sci. Food Agric., 2020, 100, 1294–1300 CrossRef CAS PubMed.
- G. Ma, Y. Zhang, J. Zhang, G. Wang, L. Chen, M. Zhang, T. Liu, X. Liu and C. Lu, Food Control, 2016, 59, 714–720 CrossRef CAS.
- C. Benincasa, J. Lewis, E. Perri, G. Sindona and A. Tagarelli, Anal. Chim. Acta, 2007, 585, 366–370 CrossRef CAS PubMed.
- C. Cabrera-Vique, P. R. Bouzas and M. J. Oliveras-López, Food Chem., 2012, 134, 434–439 CrossRef CAS.
- B. Bronzi, C. Brilli, G. M. Beone, M. C. Fontanella, D. Ballabio, R. Todeschini, V. Consonni, F. Grisoni, F. Parri and M. Buscema, Food Chem., 2020, 315 Search PubMed.
- H. Zhao, B. Guo, Y. Wei and B. Zhang, Food Chem., 2013, 138, 1902–1907 CrossRef CAS PubMed.
- J. S. Kim, I. M. Hwang, G. H. Lee, Y. M. Park, J. Y. Choi, N. Jamila, N. Khan and K. S. Kim, Meat Sci., 2017, 123, 13–20 CrossRef CAS PubMed.
- L. Wang, Q. Yang and H. Zhao, Food Control, 2021, 124, 107879 CrossRef CAS.
- C. Zhang, J. Selvaraj, Q. Yang and Y. Liu, Toxins, 2017, 9, 40 CrossRef PubMed.
- H. Zhao, F. Wang and Q. Yang, J. Sci. Food Agric., 2020, 100, 4040–4048 CrossRef CAS PubMed.
- G. Vlahov, P. D. Re and N. Simone, J. Agric. Food Chem., 2003, 51, 5612–5615 CrossRef CAS PubMed.
- D. C. Fechner, M. J. Hidalgo, J. D. R Díaz, R. A. Gil and R. G. Pellerano, Food Biosci., 2020, 33, 100483 CrossRef CAS.
- F. M. S. Alaviani, S. Faranak Miraftabi, M. Hossein Salehisormghi and M. Qomi, J. Basic Appl. Histochem., 2012, 2, 50–54 Search PubMed.
- I. H. H. Al-Hilfy and S. A. A. Al-Salmani, Iraqi J. Agric. Sci., 2015, 46, 704–713 Search PubMed.
- A. M. Helmy and M. F. Ramadan, J. Agric. Res., 2013, 40, 877–889 Search PubMed.
- S. Chen, Z. Liang, R. Webster, G. Zhang, Y. Zhou, H. Teng, B. Hu, D. Arrouays and Z. Shi, Sci. Total Environ., 2019, 655, 273–283 CrossRef CAS PubMed.
- G. Lawrence, M. David and W. Shortle, Nature, 1995, 378, 162–165 CrossRef CAS.
- B. S. K. a. S. R. Shankar, Communications in Plant Sciences, 2013, 3, 25–29 Search PubMed.
- X. Dai, Y. Bai, J. Jiang, X. Chen, H. Zhou, N. Yin, L. Chen, X. Ding and P. Li, J. Agric. Food Chem., 2016, 64, 7849–7855 CrossRef CAS PubMed.
- Y. Teng, J. Wu, S. Lu, Y. Wang, X. Jiao and L. Song, Environ. Int., 2014, 69, 177–199 CrossRef CAS PubMed.
- Y. Wang, doctoral dissertation, Nanjing Agricultural University, Nanjing, 2013.
- U. Tinggi, Food Addit. Contam., 1998, 15, 789–792 CrossRef CAS PubMed.
- S. A. Baluka, D. Schrunk, P. Imerman, J. N. Kateregga, E. Camana, C. Wang, W. K. Rumbeiha and F. Yildiz, Cogent Food Agric., 2017, 3, 1313925 CrossRef.
- F. Jing, X. Chen, Z. Yang and B. Guo, Environ. Earth Sci., 2018, 77, 1–9 CrossRef.
- M. Cresser, K. Killham and T. Edwards, Soil Chem. Appl., 1993, 1–9 Search PubMed.
- I. Geana, A. Iordache, R. Ionete, A. Marinescu, A. Ranca and M. Culea, Food Chem., 2013, 138, 1125–1134 CrossRef CAS PubMed.
- T. Jaoual and D. A. Cox, J. Plant Nutr., 1998, 21, 353–386 CrossRef.
- W. Branch and T. Gaines, Peanut Sci., 1983, 10, 5–8 CrossRef CAS.
- S. Jiang, J. Wu, Y. Feng, X. Yang and C. Shi, J. Agric. Food Chem., 2007, 55, 9608–9613 CrossRef CAS PubMed.
- B. Markert, Fresenius' Z. Anal. Chem., 1987, 329, 462–465 CrossRef CAS.
- B. Kafaoğlu, A. Fisher, S. Hill and D. Kara, Food Addit. Contam., Part A, 2014, 31, 1529–1538 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |