Open Access Article
Open Access ArticleRobust photocatalytic activity of two-dimensional h-BN/Bi2O3 heterostructure quantum sheets†
Jianwei Zhou *ab,
Fangfang Duoa,
Chubei Wanga,
Liangliang Chua,
Mingliang Zhanga and
Donglei Yanb
*ab,
Fangfang Duoa,
Chubei Wanga,
Liangliang Chua,
Mingliang Zhanga and
Donglei Yanb
aHenan Photoelectrocatalytic Material and Micro-Nano Application Technology Academician Workstation, Xinxiang University, Xinxiang, Henan, China. E-mail: jwchow@163.com; Fax: +86-373-3682028; Tel: +86-373-3682028
bCollege of Chemistry and Material Engineering, Xinxiang University, Xinxiang 453003, PR China
First published on 4th May 2022
Abstract
Herein, defect intrinsic hexagonal boron nitride (h-BN) quantum sheets (QS) and bismuth oxide (Bi2O3) QS were prepared from bulk materials by ball milling and solvent stripping, respectively. The h-BN/Bi2O3 heterostructure was fabricated via a facile self-assembly method. The structure and performance of samples were systematically characterized. As expected, the layered h-BN QS is tightly coated on the surface of Bi2O3 QS in a face-to-face stacking structure and interconnected by strong interface interactions. The introduction of h-BN QS can significantly enhance the separation efficiency of the photogenerated carriers of h-BN/Bi2O3. The experimental results show that the photocatalytic activity of h-BN/Bi2O3 is markedly improved. The first-order reaction rate constant of the 3wt%-BN/Bi2O3 sample is 3.2 × 10−2 min−1, about 4.5 times that of Bi2O3 QS. By means of the active species capture test, it is found that the main oxidation species are holes (h+), followed by hydroxyl radicals (˙OH). Based on the surface charge transfer characteristics, the photogenerated carrier transfer and separation efficiency can be improved by coupling h-BN and a Bi2O3 semiconductor to the Schottky heterojunction, and the strong interaction between heterogeneous interfaces also enhances the surface catalytic reaction efficiency, which improves dramatically the photocatalytic performance.
1. Introduction
The problems associated with pollution and energy production have arisen along with the rapid development of industry and society, and have become the two main global challenges facing us today. The sustainable supply of clean and safe water is an essential guarantee of human life and health. In recent years, photocatalytic advanced oxidation technology has been considered a promising method for the degradation of organic pollutants due to the production of efficient active free radicals. Therefore, catalysts with high visible-light response and their applications have been attracted extensive attention.1 A series of novel photocatalysts have been prepared and applied to the photo-induced chemical transformation reactions.2–6 The photogenerated carriers can migrate to the catalyst surface and initiate chemical reactions in the process of photocatalysis, and have been used in the fields of pollutant degradation, water decomposition, air purification and disinfection.7–9 However, the main challenges in the application of traditional semiconductor photocatalysts include insufficient utilization of sunlight, and high carrier recombination rate.10,11 Therefore, it is urgent to develop novel catalytic materials with high light collection efficiency, high charge transfer efficiency and high chemical stability over the whole solar spectrum to accelerate the reaction.Hexagonal boron nitride (h-BN) nanosheet structure has the characteristics of wide band gap, non-toxicity, high thermal conductivity and high specific surface area, which is used as holes transfer promoter in the process of semiconductor photocatalytic reaction, and can add attractive characteristics to photocatalysts.12–15 In recent years, after combining with h-BN, the photocatalytic performance of semiconductors will be improved. Thus, many encouraging achievements have been achieved in h-BN based photocatalytic composites.16–22 The enhanced catalytic performance is usually attributed to the promotion of photogenerated carriers transfer and migration rate, the inhibition of e−–h+ pairs recombination, the expansion of light response range and the increase of active sites of surface adsorbed reactants.23,24 However, there is still a lack of strong theoretical analysis and experimental evidence. Indeed, the structure of BN plays an important role in BN/semiconductor heterostructures, but its inherent semiconductor characteristics and band gap adjustment are often ignored.25,26
Two-dimensional (2D) material has been widely investigated owning to their unique properties in electronics, optics, thermotics, catalysis and so on.27 As the lateral size of 2D nanomaterial down to sub-20 nm regime, two-dimensional quantum sheets (2D QS) have appeared.28 2D QS has recently attracted more and more attention due to the integration of the inherent characteristics, quantum confinement and prominent edge effects of 2D materials.29 The beauty of two-dimensional materials is that the material behavior is dominated via the interfaces. Therefore, interface interaction is the main consideration in the design of heterostructures, which directly determines the performance of the final device.30 Bi2O3 semiconductor (Eg = ∼2.7 eV) have a non-layered crystal structure and 3D intrinsic isotropic chemical bonds. Considering the creation of two-dimensional geometric Bi2O3 QS from bulk Bi2O3 materials may offer significant impact on catalyst design. Firstly, Bi2O3 QS may have large structural deformation and produce a large number of surface hanging bonds, which can achieve highly chemically active sites and enhance catalytic properties. Second, the structure and electronic properties of Bi2O3 QS can be adjusted by structure and surface engineering to further tuned the catalytic performance. Third, the exposed surface atoms with low coordination numbers can induce rapid interfacial carriers transfer and promote the chemisorption of reactants. In addition, defects will have additional effects on the surface electronic structure and charge transfer characteristics of semiconductors.31 Meanwhile, the significant catalytic activity of Bi2O3 films is related to the existence of different oxidation states of Bi and the high oxygen mobility on the surface.32 Many recent advances in the heterostructure of 2D materials have opened new routes and possibilities in catalysis field, such as TNS/WS2,33 Fe2O3/g-C3N4,34 h-BN/BiOCl35 and so on. In general, van der Waals junctions have weak bonding force and may not be suitable for building energy conversion devices.36–38 According to literature reports, relatively tightly bonded 2D–2D heterojunctions have been successfully prepared,39,40 but the preparation process is generally complicated and difficult to extend its application.
In this study, employing low-cost raw bulk precursors, a novel 2D h-BN/Bi2O3 heterostructure has been prepared by ex situ self-assembly of Bi2O3 QS and h-BN QS. The effects of h-BN QS incorporating into the structure, surface chemistry and catalytic activity were systematically investigated. In addition, the unique photocatalytic performance and reactive mechanism are discussed, which will contribute to the design and fabrication of the high performance catalysts, establish reliable structure–activity relationships and promote the application of BN-based materials in environmental purification and energy conversion.
2. Experimental section
2.1 Materials
Hexagonal boron nitride (h-BN) and bismuth trioxide (Bi2O3) were purchased from Sinopharm Chemical Reagent Co., Ltd. N-Methyl-2-pyrrolidone (NMP) was purchased from Shanghai Macklin Biochemical Co., Ltd. Acetate ether (C5H10O3), ethanol (C2H5OH), sodium chloride (NaCl), were purchased from Beijing Chemical Works. All of reagents were used as received. Deionized water was used in the experiments.2.2 Preparation of h-BN and Bi2O3 quantum sheets
Herein, h-BN QS and Bi2O3 QS were prepared by sequential combination of salt assisted ball milling and ultrasonic assisted solvent exfoliation of corresponding bulk materials. Briefly, a certain quality of sample and NaCl was mixed and put into agate ball milling tank, and ball milled at 350 rpm for 12 h (Planetary ball mill, QM-1SP4, Nanjing University Instrument Factory). Then, the powder mixture was washed with water until there was no precipitation in the filtrate when AgNO3 is added. The dried filter cake was then dispersed in NMP solution for sonication (KQ-250DE, Kunshan Ultrasonic Instruments Co., Ltd) at 250 W for 4 h. When a certain amount of acetate ether was added, the solid phase could be completely precipitated from the dispersion.And then, the dispersion was centrifuged (L550, Xiangyi Centrifuge Instrument Co., Ltd) at 4000 rpm for 20 min. After vacuum drying, the quantum sheet powders were collected.
2.3 Construction of h-BN/Bi2O3 heterostructures
The h-BN/Bi2O3 composites were synthesized via a facile electrostatic self-assembly approach. Driven by electrostatic attraction, components with opposite surface charges can be easily assembled into heterojunctions.41 Briefly, under magnetic agitation, a certain amount of Bi2O3 QS was dispersed in absolute ethanol. Subsequently, different amounts of h-BN QS were added into the above suspension stirring for 3 h. Then, a homogenous suspension was obtained after sonicating for 3 h. The resultant suspension was centrifuged and dried at 60 °C overnight. The obtained h-BN/Bi2O3 composites with different h-BN weight ratios were denominated as 1%-BN/Bi2O3, 3%-BN/Bi2O3 and 5%-BN/Bi2O3, respectively.2.4 Characterization
X-ray diffractometer (XRD, Bruker D8 Advance) was used to characterize the crystal structures. DRS spectra and IR spectra were identified on a Hitachi UV-3010 spectrophotometer and a Thermo iS50 FT-IR instrument, respectively. Photoluminescence (PL) spectra were recorded on a Hitachi F-4500 spectrofluorimeter, and the time-resolved photoluminescence (TRPL) decay spectra were recorded on a FLS 980 fluorescence spectrometer (Edinburgh Instruments) with a 320 nm laser as the light source. X-ray photoelectron spectroscopy (XPS, PHI Quantera SXM) was performed with Mg Kα radiation and the binding energies were normalized to the C 1s at 284.5 eV. The specific surface areas of the samples were determined by the Microtrac BEL instrument. The photoelectric performance was measured using an electrochemical system (CHI-660B, China). The morphology and microstructure images were obtained by scanning electron microscopy (SEM, FEI QUANTAFEG 250) and transmission electron microscopy (TEM, FEI Talos F200X), respectively.2.5 Photocatalytic activity evaluation and active species capturing experiments
The photocatalytic activity of the obtained samples was measured by degrading RhB in XPA photoreaction apparatus, 500 W long-arc xenon lamp (the maximum emission at about 470 nm, the light intensity about 150 mW cm−2) with a 420 nm cutoff filter as visible-light source. The temperature of the reaction solution is maintained at approximately 25 °C with continuous cooling. In detail, 25 mg catalyst was dispersed in 50 mL of RhB solution (c = 1 × 10−5 mol L−1) in a quartz tube reactor for ultrasound treating 10 minutes to form a homogeneous suspension solution. Prior to irradiation, the suspension was stirred for 30 minutes away from light, in order to achieve adsorption–desorption equilibrium. Then, the photocatalytic reaction was carried out with a sampling interval of 20 minutes, and the liquid samples were centrifuged at 8000 rpm for 10 minutes and the supernatant analyzed. The absorbance of solution was measured by a spectrophotometer at λmax = 554 nm. The blank test without catalyst was carried out under the same experimental conditions.To detect the roles of reactive species during the photocatalytic process, the photodegradation performance of RhB has also been tested under the visible-light (λ > 420 nm) irradiation, employing 1.0 mmol L−1 edetate disodium (EDTA-2Na), p-benzoquinone (BQ) and tert-butanol (TBA) as scavengers for holes (h+), superoxide radicals (˙O2−) and hydroxyl radicals (˙OH), respectively.
3. Results and discussion
3.1 Characterization
Fig. 1 shows the XRD patterns of the obtained samples. As shown in Fig. 1a and b, h-BN sample shows a main diffraction peak around 26.8°, attributable to the (002) crystal lanes of the graphitic phase h-BN. The diffraction peaks of Bi2O3 samples with the main peaks at 2θ = 27.4°, 33.2° and 46.3°, corresponding to the (120), (121) and (041) lattice plane, can be attributed to monoclinic α-Bi2O3 (JCPDS No. 41-1449). After post-treatment with ball-milling and sonication, all QS samples featured XRD peaks are similar to those of raw material, but slightly broadened the main peak. As can be seen from Fig. 1c and d, compared with bare Bi2O3 QS, the main peaks of h-BN/Bi2O3 composites decreased and shifted slightly with increasing the h-BN QS loading, which could indicates the formation of new interface between h-BN QS and Bi2O3 QS. On the other hand, the sign of h-BN QS diffraction peak was hardly found in the h-BN/Bi2O3 composites, which could be related to its low content and existence mode in the heterostructures.The SEM images are presented in Fig. 2. As shown in Fig. 2a and b, the plate-like h-BN and h-BN QS are stacked together, with petal-like features observed on the surface. However, compared with raw h-BN, the thickness of h-BN QS become thinner and the size is relatively uniform. For Bi2O3 QS (Fig. 2d), the SEM image shows well-dispersed nanoplates, and the morphology is obviously different from that of original bulk Bi2O3 (Fig. 2c). After the introduction of h-BN QS, the surface of h-BN/Bi2O3 composites (Fig. 2e and f) becomes rough, owing to the layered h-BN QS coating on the surface of the Bi2O3 QS. The heterostructure is stacked via face-to-face and assembled into a laminated structure to form a large contact interface, which indicates that there is tight contact interface between h-BN QS and Bi2O3 QS, and potential factors such as defects and local surface structure changes may also play an important role in composites.
 | ||
| Fig. 2 SEM images of (a) raw h-BN, (b) h-BN QS, (c) raw Bi2O3, (d)Bi2O3 QS and (e and f) h-BN/Bi2O3. | ||
The microstructures of samples were further investigated by TEM analysis. In the insets of Fig. 3a and b, the h-BN QS display nanoplate morphology and the Bi2O3 QS exhibit uniform and hexagonal nanoplate structure, respectively. Interestingly, as shown in Fig. 3c, the h-BN/Bi2O3 composite shows a compact contact interface with an approximate wedge-shaped edges structure. Furthermore, from Fig. 3d and f, the lattice fringe phase with interplanar spacing of 0.290 nm and 0.340 nm are observed in the HRTEM images, which correspond to the crystallographic (211) plane of Bi2O3 QS and (002) plane of h-BN QS, respectively. Accordingly, the Bi2O3 QS with exposed facets is illustrated in Fig. 3d (in red circles). Given two different lattice fringes were clearly observed from Fig. 3d and f, it is believed that there exists a strong interaction between h-BN QS and Bi2O3 QS, implying that the two components form a heterostructure in close contact with each other instead of a simple physical mixture. In Fig. 3g, the EDX spectrum indicates the presence of Bi, O, B, and N elements in the composite. These analysis results also confirmed that h-BN/Bi2O3 heterostructure was successfully prepared in the experiment.
 | ||
| Fig. 3 TEM images of h-BN (a), Bi2O3 (b) and h-BN/Bi2O3 (c). HRTEM images (d–f) and EDX elemental mapping (g) of h-BN/Bi2O3. | ||
The Fourier transform infrared (FT-IR) spectra of h-BN, Bi2O3 and h-BN/Bi2O3 with different h-BN contents were exhibited in the Fig. 4. For bare Bi2O3 QS, the weak and broad band centered at 3500 cm−1 is due to stretching vibrations of O–H chemical bond from physically adsorbed water. While the absorption peak at 520 cm−1 is attributed to the Bi–O bands of BiO6 octahedral in Bi2O3 structure,42 the peaks at 1390 cm−1 and 803 cm−1 are all belonged to the Bi–O bonds stretching vibration.43 In the IR spectrum of h-BN, there are the intense absorption peaks in the 1380 cm−1 and 780 cm−1 corresponding to the stretching vibration and deformation vibration of N–B–N bond. In addition, the –OH groups on the surface of h-BN are also contributing to the electron transfer in the photocatalysis.44 Component structure information corresponding to both h-BN and Bi2O3 are presented in the IR spectra of h-BN/Bi2O3 composites. Moreover, it is noted that the B–N bond stretching vibration peaks of h-BN/Bi2O3 composites display slight shift, implying the strong interaction between the h-BN QS and Bi2O3 QS.
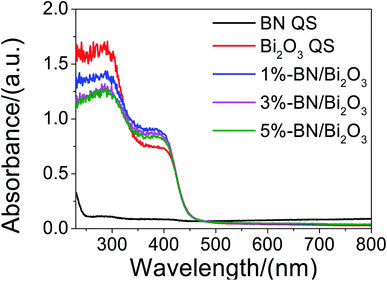
In order to explore the effect of h-BN modification on the light absorption of Bi2O3, the ultraviolet-visible optical absorption spectra of the samples in the wavelength range of 230–800 nm were displayed in Fig. 5. It is found that the absorption edge of h-BN/Bi2O3 samples changes slightly with the increase of h-BN content, enhancing the visible-light absorption at the wavelength below 420 nm, which is attributed to the narrowing of the band gap of the composites with the h-BN introduced to the Bi2O3. Compared with bare Bi2O3, the slight wider absorption edge and the narrower band gap indicate that h-BN/Bi2O3 has greater carrier concentration, which can be conducive to photocatalytic reactions.
The elements and surface states of the obtained samples were acquired to further confirm by X-ray photoelectron spectroscopy (XPS) characterization. The binding energy (BE) of the measured elements was corrected according to C 1s (284.5 eV). As expected, the survey scan XPS spectrum (Fig. 6a) showed that all chemical elements of B, N, Bi and O in h-BN/Bi2O3 composite were detected. As shown from the high-resolution spectrum of Bi 4f (Fig. 6b), the BEs of 159.2 eV (Bi 4f7/2) and 164.5 (Bi 4f5/2) are attributed to Bi3+. Similarly, the peak value at 530.1 eV in Fig. 6c can be designated as O 1s and is characteristic of Bi–O binding energy in Bi2O3. As can be seen from Fig. 6d and e, the signals of B 1s and N 1s indicated the existence of BN in h-BN/Bi2O3 composite. Meanwhile, compared with bare h-BN QS, the BEs of B and N core electrons of h-BN/Bi2O3 displays a positive shift ∼0.2 eV, suggesting the migration of photogenerated carriers in the interface of h-BN/Bi2O3 heterostructure. These XPS results demonstrate that the structure of h-BN/Bi2O3 is a hybrid structure with strong interaction between two components interface, and this interaction is beneficial for enhancing the photocatalytic performance. XPS analysis confirms the coexistence of h-BN and Bi2O3 in h-BN/Bi2O3 sample and further demonstrating h-BN/Bi2O3 heterostructure has been successfully prepared by liquid phase self-assembly method. Further, comparing the peaks located at h-BN QS, Bi2O3 QS and h-BN/Bi2O3 indicate that the BE of B 1s, N 1s and Bi 4f both increased 0.2 eV, 0.2 eV and 0.1 eV, respectively, except for the peak of O 1s spectrum. This higher binding energy indicated that more h+ were concentrated on the h-BN surface via contact with Bi2O3. In contrast to Bi2O3 BE, a slight increase was indicated in Bi 4f spectra for 0.1 eV. This suggests that the electron density around the element and defect in Bi2O3 was a decrease caused by the pile with h-BN.45 These increases in BEs corresponding to electrons transferred from Bi2O3 to h-BN might be generated by the internal electron field at the interface between Bi2O3 and h-BN.46 All the above results suggested that the Schottky heterojunction was formed between Bi2O3 QS and h-BN QS by strong interaction rather than the simple physical mixture.
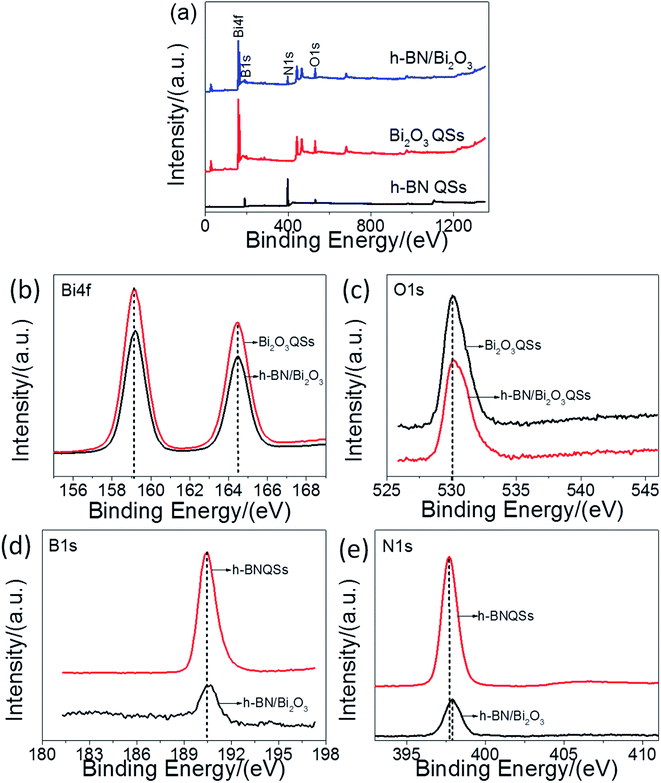 | ||
| Fig. 6 (a) XPS survey spectra, high-resolution XPS spectra of (b) Bi 4f, (c) O 1s, (d) B 1s, and (e) N 1s for Bi2O3 QS, h-BN QS and the 3%-BN/Bi2O3 composite. | ||
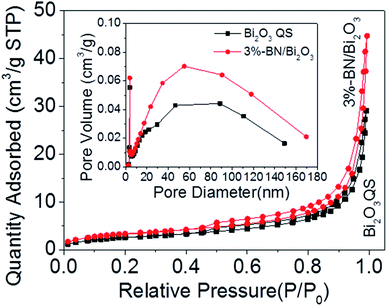
The specific surface areas of Bi2O3 QS, h-BN QS and h-BN/Bi2O3 composite were characterized by nitrogen adsorption–desorption isotherms in the Fig. 7. The BET surface areas of raw Bi2O3, Bi2O3 QS, raw h-BN, h-BN QS and 3wt%-BN/Bi2O3 were measured to be 0.14 m2 g−1, 9.79 m2 g−1, 12.93 m2 g−1, 41.33 m2 g−1 and 12.53 m2 g−1, respectively. From the BET data, the surface area of the 3wt%-BN/Bi2O3 sample is about 1.3 times as large as Bi2O3 QS, suggesting that the enlarged surface area can provide more active sites for photocatalytic reaction. It is generally believed that the adsorption performance of a photocatalyst towards the target pollutant is one of the main factors to affect the reaction kinetics. Therefore, it is considered that the surface area of h-BN/Bi2O3 may be an important index affecting the catalytic efficiency. As can be seen from the pore size distribution curves (inset), it reflects the effect of h-BN QS on the pore size and volume of heterostructures. It is well known that higher specific surface area can provide rich active reaction sites and promote the adsorption of more pollutant molecules on its surface. Moreover, it can be seen from the insertion pore size distribution diagram that the catalyst is rich in mesopores. The presence of large mesopores in a porous photocatalyst favors multilight scattering/reflection, resulting in enhanced harvesting of the exciting light and thus improved photocatalytic activity.47,48 In addition, large mesopores facilitate mass transport, resulting in improved performance.49,50 Therefore, the h-BN/Bi2O3 is expected to possess a better photocatalytic activity than Bi2O3.
Electron paramagnetic resonance (EPR) spectroscopy can provide information about the valence state, concentration, local environment and interaction properties of transition metal ions. Moreover, the EPR spectrum can provide more evidence for the existence of defect states in the crystal structure and unpaired electrons in materials. In Fig. 8a, the stronger signal strength indicates of raw h-BN that the characteristic peak of defect, which is caused by the occupation and absorption of rich electrons at the defect site by adsorbed oxygen molecules. During the process of preparation quantum sheets by ball milling, the crystal lattices of sample undergo severe crystal lattice dislocations, plastic deformation, producing stresses and strains. Thus a large number of stripping- and point-defects are produced. On the contrary, the Bi2O3 QS uniquely exhibits a symmetrical EPR signal, manifesting the electron trapping at oxygen vacancies in the Fig. 8b, it shows that the corresponding Bi2O3 QS has a large number of oxygen vacancy defects, further validated by the obviously observed lattice disorder in Fig. 3. On the contrary, the defects of h-BN mainly exist in raw bulk materials. As can be seen from the EPR signal of h-BN/Bi2O3 composite is extremely weak in Fig. 8c and d. Based on EPR analysis, it is speculated that oxygen vacancies mainly exist on the surface of Bi2O3 QS, h-BN/Bi2O3 mainly presents face-to-face stacking layered structure. These analysis results also confirmed the formation of a hybrid heterostructure in the contact interface between Bi2O3 and h-BN, which can greatly promote the separation of photogenerated e−–h+ pairs.
Photoluminescence (PL) spectra were used to explore the transfer and separation efficiency of carriers. The effective separation of photogenerated e−–h+ pairs is the key role in the photocatalytic process. The PL spectra of the Bi2O3 QS and BN/Bi2O3 were shown in Fig. 9a. The Bi2O3 QS sample displayed the emission peak centered at around 468 nm under the excitation wavelength of 320 nm. The PL intensity of the BN QS modified Bi2O3 QS become weaker than that of the pure Bi2O3 QS, implying the presence of h-BN QS in this system could decrease the recombination rate of photogenerated e−–h+ pairs. In addition, lower PL spectra quenching occurs on the 3%-BN/Bi2O3 sample relative to Bi2O3 QS, suggesting the suppressed carriers recombination and improved carriers separation efficiency, possibly owning to the formation of h-BN/Bi2O3 heterostructure with strong interaction.
 | ||
| Fig. 9 (a) PL spectra of Bi2O3 QS and BN/Bi2O3 composites (inset of the h-BN QS), (b) time-resolved photoluminescence decay spectra of Bi2O3 QS and 3wt%-BN/Bi2O3. | ||
Moreover, time-resolved photoluminescence (TRPL) spectroscopy was employed to further explore the photo-excited carrier dynamics. As shown in Fig. 9b, the average fluorescence lifetime of 3%-BN/Bi2O3 composite is calculated to be 2.36 ns, which is longer than that of Bi2O3 (1.66 ns), indicating the prolonged lifetime of photogenerated carriers in the composite due to the presence of the Schottky heterojunction, which prevent the recombination of photogenerated e−–h+ pairs in Bi2O3. Thus, it is that the 3%-BN/Bi2O3 has both longer fast decay lifetime (τ1) and slow decay lifetime (τ2) than that Bi2O3 QS (inset in Fig. 7b).
It is well known that photocurrent comes from the diffusion of photoelectrons to the back contact. In order to investigate the transition of photogenerated electrons before and after h-BN modification, photoelectrochemical measurement was performed. Fig. 10 displays the photocurrent response of h-BN/Bi2O3 and Bi2O3 under visible-light irradiation. It can be observed that the 3%-BN/Bi2O3 shows remarkably increased photocurrent intensity than 5%-BN/Bi2O3 and the single component Bi2O3, implying that photogenerated carrier separation efficiency is enhanced and more photoexcited carriers are generated. This result is consistent with the catalytic activity.
The photocatalytic activities of the as-prepared samples were evaluated by photocatalytic degradation RhB solution under visible-light irradiation and the concentration changes are provided in Fig. 11. As shown in Fig. 11a, the blank test demonstrated that RhB was stable under visible-light irradiation for 120 minutes. The results showed that the raw Bi2O3 exhibited very poor photocatalytic activity, with only 5% RhB degradation, after visible-light irradiation for 120 minutes. In comparison with bare Bi2O3 QS, the h-BN/Bi2O3 composites exhibit superior photocatalytic activities and the h-BN QS content plays a key role. The experimental results show that RhB (c = 1 × 10−5 mol L−1) can be completely degraded by 3%-BN/Bi2O3 catalyst within 120 minutes under visible-light irradiation, while the sole Bi2O3 QS can only decompose 60% of RhB during the same interval. With the content of h-BN increasing, it leads to the decrease of photocatalytic activity. This was attributed to that while a massive overdose of h-BN covered on the surface of Bi2O3 would shield light absorb and the reaction active sites. Meanwhile, the kinetic properties of photocatalytic reaction were further studied. The degradation reaction rate is subject to the pseudo-first order kinetic equation (−ln(C/C0) = kt), there is a nice linear correlation for the experimental data. As shown in Fig. 11b, 3%-BN/Bi2O3 sample has the best photocatalytic activity, which rate constant value (k = 0.03191 min−1) is about 4.5 times that of Bi2O3 QS (k = 0.00702 min−1). The enhancement of photocatalytic activity of h-BN/Bi2O3 may also be due to energy band well matching in the heterostructure.
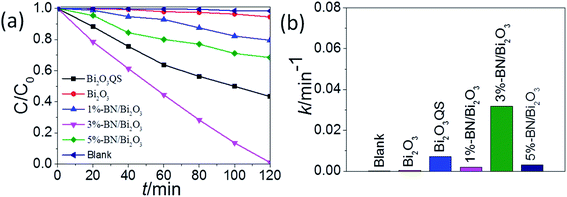 | ||
| Fig. 11 (a) Variation curve of RhB concentration with irradiation time. (b) The first-order kinetics of photocatalytic reaction under visible-light irradiation (λ > 420 nm). | ||
In order to understanding the catalysis mechanism, the reactive species of photocatalysis are very important. As shown in Fig. 12a, the photocatalytic activity of h-BN/Bi2O3 was inhibited by EDTA-2Na and TBA, and to a lesser extent by BQ, indicating the significant roles of h+ and ˙OH and suggesting a minor role of ˙O2− radical in the degradation process. It should be noted that the influence of ˙O2− radical on the photocatalytic activity of h-BN/Bi2O3 is less significant than that of Bi2O3/g-C3N4,51 which shows the overall influence on the two components of the composite. In h-BN/Bi2O3 photocatalytic system, the oxidizing h+ and ˙OH radicals worked as the main active groups, contributed to the photocatalytic oxidation ability.
 | ||
| Fig. 12 (a) Effects of reactive species scavengers on photocatalytic performance of BN/Bi2O3. (b) Cycling runs of BN/Bi2O3 under visible-light irradiation (λ > 420 nm). | ||
In addition to photocatalytic activity, the durability of catalyst is also very important for its practical application. The stability of the obtained h-BN/Bi2O3 photocatalyst was also evaluated via the circulating run experiment of RhB degradation. As shown in Fig. 12b, the crystal structure of h-BN/Bi2O3 catalyst remains unchanged and there is no photo corrosion after 3 cycling runs in 6 h, and its photocatalytic performance did not show a distinct attenuation, which indicate that the h-BN/Bi2O3 catalysts possess good reusability for practical applications.
As a binary h-BN/Bi2O3 composite with the characteristics of Schottky heterojunction, it shows excellent catalytic properties under visible-light irradiation. A solid-state Schottky junction mechanism can be proposed to explain the effective photocatalytic response of h-BN/Bi2O3. Under the light excitation, the interface can react with electrons as an intermediate, and indirectly lead to the transmission of electrons. Regarding the activity enhancing mechanism through the incorporation of h-BN QS and Bi2O3 QS nanostructures, the photocatalytic mechanism of h-BN/Bi2O3 is tentatively proposed and schematically illustrated in Fig. 13. In h-BN/Bi2O3 systems, the h-BN QS could be the photogenerated carrier transfer and separation promoter. Under visible light irradiation, Bi2O3 can be easily excited to yield photon-generated carriers, that is, photoinduced electrons from the valence band are easily transferred to the corresponding conduction band, leaving h+ in the valence band of Bi2O3. The h+ on the VB of Bi2O3 (2.69 eV vs. NHE) is capable to generate ˙OH 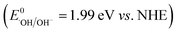 , while the CB of Bi2O3 barely meets the requirement for O2 reduction
, while the CB of Bi2O3 barely meets the requirement for O2 reduction 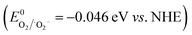 , suggesting that ˙O2− can be generated in this system. The light excitation electrons generated form Bi2O3 transfer to h-BN, while photogenerated holes on h-BN transfer to Bi2O3, enhancing the e−–h+ pairs effective separation via the heterojunction interface. Therefore, the photogenerated carriers are spatially separated and their lifetime can be significant improved for h-BN/Bi2O3 composites which bring about superior photocatalytic activity and stability. According to the above mentioned process, the efficient photocatalytic degradation progress of RhB can smoothly proceed.
, suggesting that ˙O2− can be generated in this system. The light excitation electrons generated form Bi2O3 transfer to h-BN, while photogenerated holes on h-BN transfer to Bi2O3, enhancing the e−–h+ pairs effective separation via the heterojunction interface. Therefore, the photogenerated carriers are spatially separated and their lifetime can be significant improved for h-BN/Bi2O3 composites which bring about superior photocatalytic activity and stability. According to the above mentioned process, the efficient photocatalytic degradation progress of RhB can smoothly proceed.
The possible processes can be described as follows:
| Bi2O3 + hν → Bi2O3 (h+ + e−) | (1) |
| Bi2O3 (h+ + e−) + BN → Bi2O3 (h+) + BN (e−) | (2) |
| BN (e−) + O2 → BN + ˙O2− | (3) |
| ˙O2− + H2O → HO2˙ + OH− | (4) |
| HO2˙ + H2O → H2O2 + ˙OH | (5) |
| H2O2 + hν → 2˙OH | (6) |
| h+ + ˙OH + RhB → Intermediate → CO2 + H2O | (7) |
This enhanced photocatalytic performance of h-BN/Bi2O3 heterostructure is attributed to the increased efficiency of photoinduced generated carries transfer, suppressed photogenerated e−–h+ recombination probability and produced more active species with the introduction of h-BN QS in the Bi2O3 QS semiconductor.
4. Conclusions
Herein, a facile method of fabricating h-BN/Bi2O3 heterostructure from Bi2O3 QS and h-BN QS is reported. Quantum sheet has both novel bulk characteristics and two-dimensional characteristics, resulting in abundant unique phenomena. Creating 2D geometry quantum sheets from the raw bulk h-BN and Bi2O3 can significantly advance the high-performance photocatalysts design, which can induce high surface chemical activity, rapid interfacial charge transfer and promote the adsorption of reactants, so as to improve the photocatalytic performance. 2D h-BN/Bi2O3 heterostructure self-assembled by the surface stacking of Bi2O3 QS and h-BN QS has intimate interface with strong interaction, which is conducive to charge transfer and separation in the photocatalytic process. In addition, potential factors such as surface defects and local surface structure changes may also play an important role in the heterostructure. These experimental facts show that the advantages of h-BN/Bi2O3 composite composed of QSs exhibit significant photocatalytic activity, which is expected to become a promising alternative for wastewater purification.Author contributions
Zhou Jianwei: conceptualization, data curation, formal analysis, writing – original draft. Duo Fangfang: writing – review & editing. Wang Chubei: investigation, writing – review & editing. Chu Liangliang: investigation, writing – review & editing. Zhang Mingliang: writing – review & editing. Yan Donglei: writing – review & editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is supported by the Project of Science and Technology of the Henan Province (192102310496).References
- X. Li, J. Yu, S. Wageh, A. A. Al-Ghamdi and J. Xie, Graphene in photocatalysis: a review, Small, 2016, 12, 6640–6696 CrossRef CAS PubMed.
- X. J. Chen, Q. Chen, W. J. Jiang, Z. Wei and Y. F. Zhu, Separation-free TiO2-graphene hydrogel with 3D network structure for efficient photoelectrocatalytic mineralization, Appl. Catal., B, 2017, 211, 106–113 CrossRef CAS.
- C. Lai, M. M. Wang, G. M. Zeng, Y. G. Liu, D. L. Huang, C. Zhang, R. Z. Wang, P. Xu, M. Cheng and C. Huang, Synthesis of surface molecular imprinted TiO2/graphene photocatalyst and its highly efficient photocatalytic degradation of target pollutant under visible light irradiation, Appl. Surf. Sci., 2016, 390, 368–376 CrossRef CAS.
- Q. Zheng, D. P. Durkin, J. E. Elenewski, Y. Sun, N. A. Banek, L. Hua, H. Chen, M. J. Wagner, W. Zhang and D. Shuai, Visible-light-responsive graphitic carbon nitride: Rational design and photocatalytic applications for water treatment, Environ. Sci. Technol., 2016, 50, 12938–12948 CrossRef CAS PubMed.
- F. T. Yi, J. Q. Ma, C. W. Lin, H. N. Zhang, Y. X. Qian, H. X. Jin and K. F. Zhang, Electronic and thermal transfer actuating memory catalysis for organic removal by a plasmonic photocatalyst, Chem. Eng. J., 2022, 427, 132028–132038 CrossRef CAS.
- F. T. Yi, H. H. Gan, H. F. Jin, W. Y. Zhao, K. F. Zhang, H. X. Jin, H. N. Zhang, Y. X. Qian and J. Q. Ma, Sulfur- and chlorine-co-doped g-C3N4 nanosheets with enhanced active species generation for boosting visible-light photodegradation activity, Sep. Purif. Technol., 2020, 233, 115997–116009 CrossRef CAS.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, Graphene-based semiconductor photocatalysts, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- G. F. Liao, C. X. Li, X. Z. Li and B. Z. Fang, Emerging polymeric carbon nitride Z-scheme systems for photocatalysis, Cell Rep. Phys. Sci., 2021, 2, 100355–100390 CrossRef CAS.
- G. F. Liao, C. X. Li, B. Z. Fang and H. M. Yang, Emerging frontiers of Z-schemephotocatalytic systems, Trends Chem., 2022, 4(2), 111–127 CrossRef CAS.
- H. M. Gao, D. Y. Zhang, M. N. Yang and S. Dong, Photocatalytic behavior of fluorinated rutile TiO2 (110) surface: Understanding from the band model, Sol. RRL, 2017, 1, 1700183–1700188 CrossRef.
- T. Hisatomi, J. Kubota and K. Domen, Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting, Chem. Soc. Rev., 2014, 43, 7520–7536 RSC.
- C. Y. Zhou, C. Lai, C. Zhang, G. M. Zeng, D. L. Huang, M. Cheng, L. Hu, W. P. Xiong, M. Chen, J. J. Wang, Y. Yang and L. B. Jiang, Semiconductor/boron nitride composites: Synthesis, properties, and photocatalysis applications, Appl. Catal., B, 2018, 238, 6–18 CrossRef CAS.
- X. Li, J. Zhang, S. J. Zhang, S. S. Xu, X. G. Wu, J. C. Chang and Z. L. He, Hexagonal boron nitride composite photocatalysts for hydrogen production, J. Alloys Compd., 2020, 864, 158153–158191 CrossRef.
- K. L. Zhang, Y. L. Feng, F. Wang, Z. C. Yang and J. Wang, Two dimensional hexagonal boron nitride (2D-hBN): synthesis, properties and applications, J. Mater. Chem. C, 2017, 5, 11992–12022 RSC.
- Q. Weng, X. Wang, X. Wang, Y. Bando and D. Golberg, Functionalized hexagonal boron nitride nanomaterials: emerging properties and applications, Chem. Soc. Rev., 2016, 45, 3989–4012 RSC.
- Z. He, C. Kim, L. Lin, T. H. Jeon, S. Lin, X. Wang and W. Choi, Formation of heterostructures via direct growth CN on h-BN porous nanosheets for metal-free photocatalysis, Nano Energy, 2017, 42, 58–68 CrossRef CAS.
- Z. G. Chen, X. L. Chen, J. Di, Y. L. Liu, S. Yin, J. X. Xia and H. M. Li, Graphene-like boron nitride modified bismuth phosphate materials for boosting photocatalytic degradation of enrofloxacin, J. Colloid Interface Sci., 2017, 492, 51–60 CrossRef CAS PubMed.
- C. S. Zhu, J. T. Zheng, L. Y. Fang, P. Hu, Y. K. Liu, X. Q. Cao and M. B. Wu, Advanced visible-light driven photocatalyst with enhanced chargeseparation fabricated by facile deposition of Ag3PO4 nanoparticles on graphene-like h-BN nanosheets, J. Mol. Catal. A: Chem., 2016, 424, 135–144 CrossRef CAS.
- Q. H. Weng, Y. Ide, X. B. Wang, X. Wang, C. Zhang, X. F. Jiang, Y. M. Xue, P. C. Dai, K. Komaguchi, Y. Bando and D. Golberg, Design of BN poroussheets with richly exposed (002) plane edges and their application as TiO2 visible light sensitizer, Nano Energy, 2015, 16, 19–27 CrossRef CAS.
- S. S. Ding, D. J. Mao, S. G. Yang, F. Wang, L. J. Meng, M. S. Han, H. He, C. Sun and B. Xu, Graphene-analogue h-BN coupled Bi-rich Bi4O5Br2 layered microspheres for enhanced visible-light photocatalytic activity and mechanism insight, Appl. Catal., B, 2017, 210, 386–399 CrossRef CAS.
- S. Raheman, R. S. Mane, H. M. Wilson and N. Jha, CdSe quantum dot/white graphene hexagonal porous boron nitride sheet (h-PBNs) heterostructure photocatalyst for solar driven H2 production, J. Mater. Chem. C, 2021, 9, 9331–9338 RSC.
- D. Tu, H. W. Liao and Q. L. Deng, Synthesis of BN/g-C3N4 as visible-light-driven photocatalysts for degradation of different organic pollutants, ChemistrySelect, 2018, 3, 7170–7177 CrossRef CAS.
- N. Wang, G. Yang, H. X. Wang, R. Sun and C. P. Wong, Visible light-responsive photocatalytic activity of boron nitride incorporated composites, Front. Chem., 2018, 6, 440–452 CrossRef CAS PubMed.
- J. K. Ren and P. Innocenzi, 2D Boron nitride heterostructures: Recent advances and future challenges, Small Struct., 2021, 8, 2100068–2100083 CrossRef.
- C. J. Huang, C. Chen, M. W. Zhang, L. H. Lin, X. X. Ye, S. Lin, M. Antonietti and X. C. Wang, Carbon-doped BN nanosheets for metal-free photoredox catalysis, Nat. Commun., 2015, 6, 7698–7704 CrossRef PubMed.
- Y. H. Cao, R. Y. Zhang, T. L. Zhou, S. M. Jin, J. D. Huang, L. Q. Ye, Z. Huang, F. Wang and Y. Zhou, B-O bond in ultrathin boron nitride nanosheets to promote photocatalytic carbon dioxide conversion, ACS Appl. Mater. Interfaces, 2020, 12, 9935–9950 CrossRef CAS PubMed.
- D. Deng, K. S. Novoselov, Q. Fu, N. Zheng, Z. Tiang and X. Bao, Catalysis with two-dimensional materials and their heterostructures, Nat. Nanotechnol., 2016, 11, 218–230 CrossRef CAS PubMed.
- X. Wang, G. Sun, N. Li and P. Chen, Quantum dots derived from two-dimensional materials and their applications for catalysis and energy, Chem. Soc. Rev., 2016, 45, 2239–2262 RSC.
- C. Han, Y. Zhang, P. Gao, S. Chen, X. Liu, Y. Mi, J. Zhang, Y. Ma, W. Jiang and J. Chang, High-yield production of MoS2 and WS2 quantum sheets from their bulk materials, Nano Lett., 2017, 17, 7767–7772 CrossRef CAS PubMed.
- J. T. Yuan, W. B. Chen and J. Lou, Two dimensional heterostructure: perfect platform for exploring interface interaction, Sci. Bull., 2017, 62, 381–382 CrossRef CAS.
- Y. Z. Wang, Z. Y. Zhang, Y. C. Mao and X. D. Wang, Two-dimensional non-layered materials for electrocatalysis, Energy Environ. Sci., 2020, 13, 3993–4016 RSC.
- G. Bandoli, D. Barreca, E. Brescacin, G. A. Rizzi and E. Tondello, Pure and mixed phase Bi2O3 thin films obtained by metal organic chemical vapor deposition, Chem. Vap. Deposition, 1996, 2, 238–241 CrossRef CAS.
- Y. C. Wu, Z. M. Liu, Y. R. Li, J. T. Chen, X. X. Zhu and P. Na, Construction of 2D-2D TiO2 nanosheet/layered WS2 heterojunctions with enhanced visible-light-responsive photocatalytic activity, Chin. J. Catal., 2019, 40, 60–69 CrossRef CAS.
- Q. L. Xu, B. C. Zhu, C. J. Jiang, C. Bei and J. Yu, Constructing 2D/2D Fe2O3/g-C3N4 direct Z-scheme photocatalysts with enhanced H2 generation performance, Sol. RRL, 2018, 2, 1800006–1800016 CrossRef.
- W. H. He, Y. W. Wang, C. M. Fan, Y. Wang, X. Zhang, J. Liu and R. Li, Enhanced charge separation and increased oxygen vacancies of h-BN/OV-BiOCl for improved visible light photocatalytic performance, RSC Adv., 2019, 9, 14286–14295 RSC.
- Z. Y. Zhang, J. D. Huang and M. Y. Zhang, Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity, Appl. Catal., B, 2015, 163, 298–305 CrossRef CAS.
- F. K. Meng, J. T. Li, S. K. Cushing, M. J. Zhi and N. Q. Wu, Solar hydrogen generation by nanoscale p-n junction of p-type molybdenum disulfide/n-type nitrogen-doped reduced graphene oxide, J. Am. Chem. Soc., 2013, 135, 10286–10289 CrossRef CAS PubMed.
- X. Q. An, J. C. Yu and J. W. Tang, Biomolecule-assisted fabrication of copper doped SnS2 nanosheet-reduced graphene oxide junctions with enhanced visible-light photocatalytic activity, J. Mater. Chem. A, 2014, 2, 1000–1005 RSC.
- J. Li, G. M. Zhan, Y. Yu and L. Zhang, Superior visible light hydrogen evolution of Janus bilayer junctions via atomic level charge flow steering, Nat. Commun., 2016, 7, 1–9 Search PubMed.
- Z. Xing, J. Hu, M. Ma, H. Lin, Y. M. An, Z. H. Liu, Y. Zhang, J. Y. Li and S. H. Yang, From one to two: In-situ construction of an ultrathin 2D-2D closely-bonded heterojunction from a single-phase monolayer nanosheet, J. Am. Chem. Soc., 2019, 141, 19715–19727 CrossRef CAS PubMed.
- X. L. Yang, F. F. Qian, G. J. Zou, M. L. Li, J. R. Lu, Yi M. Li and M. T. Bao, Facile fabrication of acidified g-C3N4/g-C3N4 hybrids with enhanced photocatalysis performance under visible light irradiation, Appl. Catal., B, 2016, 193, 22–35 CrossRef CAS.
- V. Dimitrov, Y. Dimitriev and A. Montenero, IR spectra and structure of V2O5-GeO2-Bi2O3 glasses, J. Non-Cryst. Solids, 1994, 180, 51–57 CrossRef CAS.
- L. Liu, J. Jiang, S. Jin, Z. Xia and M. Tang, Hydrothermal synthesis of β-bismuth oxide nanowires from particles, CrystEngComm, 2011, 13, 2529–2532 RSC.
- D. J. Lee, B. Lee, K. H. Park, H. J. Ryu, S. Jeon and S. H. Hong, Scalable exfoliation process for highly soluble boron nitride nanoplatelets by hydroxide-assisted ball milling, Nano Lett., 2015, 15, 1238–1244 CrossRef CAS PubMed.
- F. T. Yi, J. Q. Ma, C. W. Lin, L. Y. Wang, H. N. Zhang, Y. X. Qian and K. F. Zhang, Insights into the enhanced adsorption/photocatalysis mechanism of a Bi4O5Br2/g-C3N4 nanosheet, J. Alloys Compd., 2020, 821, 153557–153569 CrossRef CAS.
- F. T. Yi, J. Liu, G. P. Liang, X. Xiao and H. F. Wang, Insight into the enhanced degradation mechanism of g-C3N4/g-C3N5 heterostructures through photocatalytic molecular oxygen activation in Van der Waals junction and excitation, J. Alloys Compd., 2022, 905, 164064–164076 CrossRef CAS.
- B. Z. Fang, A. Bonakdarpour, K. Reilly, Y. L. Xing, F. Taghipour and D. P. Wilkinson, Large-scale synthesis of TiO2 microspheres with hierarchical nanostructure for highly efficient photodriven reduction of CO2 to CH4, ACS Appl. Mater. Interfaces, 2014, 6, 15488–15498 CrossRef CAS PubMed.
- B. Z. Fang, Y. L. Xing, A. Bonakdarpour, S. C. Zhang and D. P. Wilkinson, Hierarchical CuO-TiO2 hollow microspheres for highly efficient photodriven reduction of CO2 to CH4, ACS Sustainable Chem. Eng., 2015, 3(10), 2381–2388 CrossRef CAS.
- Y. L. Xing, B. Z. Fang, A. Bonakdarpour, S. C. Zhang and D. P. Wilkinson, Facile fabrication of mesoporous carbon nanofibers with unique hierarchical nanoarchitecture for electrochemical hydrogen storage, Int. J. Hydrogen Energy, 2014, 39(15), 7859–7867 CrossRef CAS.
- Y. Zhang, X. X. Wang, F. Q. Luo, Y. Tan, L. X. Zeng, B. Z. Fang and A. H. Liu, Rock salt type NiCo2O3 supported on ordered mesoporous carbon as a highly efficient electrocatalyst for oxygen evolution reaction, Appl. Catal., B, 2019, 256, 117852 CrossRef CAS.
- R. G. He, J. Q. Zhou, H. J. Fu, S. Y. Zhang and C. J. Jiang, Room-temperature in situ fabrication of Bi2O3/g-C3N4 direct Z-scheme photocatalyst with enhanced photocatalytic activity, Appl. Surf. Sci., 2018, 430, 273–282 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra02115c |
| This journal is © The Royal Society of Chemistry 2022 |