Open Access Article
Open Access ArticleCryostorage of unstable N-acetylglucosaminyltransferase-V by synthetic zwitterions†
Tetsuya Hirata‡
 *a,
Takahiro Takekiyo‡*b,
Yukihiro Yoshimura*b,
Yuko Tokoroa,
Takeru Ishizakic,
Yasuhiko Kizukaa and
Kosuke Kuroda
*a,
Takahiro Takekiyo‡*b,
Yukihiro Yoshimura*b,
Yuko Tokoroa,
Takeru Ishizakic,
Yasuhiko Kizukaa and
Kosuke Kuroda *cd
*cd
aInstitute for Glyco-core Research (iGCORE), Gifu University, Gifu, Gifu 501-1193, Japan. E-mail: hirata01@gifu-u.ac.jp
bDepartment of Applied Chemistry, National Defense Academy, Yokosuka, Kanagawa 239-8686, Japan. E-mail: take214@nda.ac.jp; muki@nda.ac.jp
cFaculty of Biological Science and Technology, Institute of Science and Engineering, Kanazawa University, Kakuma-machi, Kanazawa, 920-1192, Japan. E-mail: kkuroda@staff.kanazawa-u.ac.jp
dNanoMaterials Research Institute, Kanazawa University, Kakuma-machi, Kanazawa, 920-1192, Japan
First published on 14th April 2022
Abstract
We report biocompatible materials for cryostorage of unstable proteins such as cancer-related enzyme, N-acetylglucosaminyltransferase-V (GnT-V). GnT-V activity and the amount of protein after freezing were better retained in synthetic zwitterion solutions than in the glycerol solution. This study highlights the potential utility of synthetic zwitterions as novel cryoprotectants.
Asparagine-linked glycosylation (N-glycosylation) is the most common glycosylation found on cell surfaces, and it plays critical roles in regulating protein folding, stability, and activity.1–3 Mature N-glycans have two to five N-acetylglucosamine (GlcNAc) branches in mammals.2,4 One of these branches, β1,6-GlcNAc, is synthesised by N-acetylglucosaminyltransferase-V (GnT-V)5 (Fig. S1†) and is deeply involved in the aggravation of cancer. For example, it has been shown that tumour growth and metastasis are highly inhibited in GnT-V deficient mice.6 Another study showed that colorectal cancer patients have a poor prognosis when their tumour cells highly express GnT-V.7 Mechanistically, the β1,6-GlcNAc branch promotes cell proliferation through oncogenic signalling from epidermal growth factor receptors.8 In addition, the β1,6-GlcNAc branch on N-cadherin enhances cell migration and invasion.9 These studies suggest that research on GnT-V contributes to cancer therapeutics. Recently, we and others solved the crystal structure of human GnT-V,10,11 allowing us to design novel inhibitors of GnT-V activity for use in cancer therapeutics. Furthermore, the activity of chemically synthesised erythropoietin protein was enhanced when bearing a β1,6-GlcNAc branched N-glycan because of stabilisation in the bloodstream of mice.12 This suggests that GnT-V is potentially applicable to the development of medicines.
However, the progress of research on GnT-V and its medical usage has been critically delayed because of the difficulty in long term storing purified GnT-V. Recombinant proteins, including GnT-V, are usually unstable and must be stored in deep freezers. The problem is that cryostorage in water or buffers generally denatures proteins, and thus a combination use of protein cryoprotectants is necessary.13 Although glycerol is the most common protein cryoprotectant,13,14 unfortunately, its protein stabilizing ability was insufficient for GnT-V (described below), and the development of novel cryoprotectants is necessary.
We focused on artificial zwitterions (ZIs) to develop a novel protein stabiliser applicable to cryostorage.15–17 Artificial ZIs are analogues of ionic liquids (ILs) but have positive and negative charges in the same ion structures. Fujita et al. and we have previously reported that ZI solutions do not denature proteins such as cytochrome c even at high concentrations.18–20 ZIs have organised bound water structures and enable the renaturation of proteins.18,19,21 We also have reported that the cell toxicity of artificial ZIs is lower than that of dimethyl sulfoxide, which is generally used for biological and medical research, and can be used for cryostorage of animal cells.22,23 Therefore, we hypothesised that ZIs have protein-stabilizing potential under cryostorage of unstable proteins, especially GnT-V.
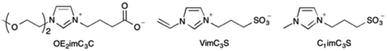
In this study, we selected the ZI, OE2imC3C (Fig. 1), which exhibits enzyme compatibility and the cryoprotective ability for cells.20,22,23 Two more artificial ZIs, VimC3S and C1imC3S,23 were also utilised to investigate the effect of different zwitterionic structures on the ability (Fig. 1), because additional ZIs can be synthesised at a lower cost than expensive OE2imC3C.24
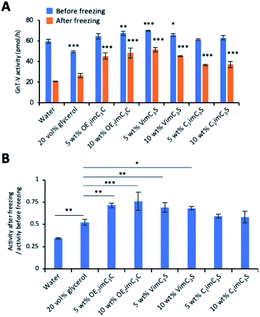
We first examined GnT-V activity in each cryoprotectant solution before freezing. GnT-V activity in a 20 vol% glycerol solution was slightly lower than that in water. This is not caused by the negative effect of glycerol on the GnT-V activity because the activity per protein did not decrease as shown below (see Fig. 4). However, activity in the OE2imC3C and C1imC3C solutions did not decrease, and a slight but non-significant increase was observed (Fig. 2A). Notably, the activity in the 5 and 10 wt% VimC3S solutions was significantly higher than that in water. An increase in enzymatic activity in another IL-containing solution has been reported,25 and this may be the case. Although the mechanism of this increase is unclear, and further analyses are needed to elucidate the precise mechanisms, we speculate that the ion interactions between ZIs and the surface charges of GnT-V possibly affect the structure of GnT-V. This might reduce fluctuations in the GnT-V structure, resulting in the stabilisation of GnT-V, strengthening the interaction with the glycan substrate with its catalytic pocket and the surrounding residues.26 This presumably supports the results that the other two ZIs (OE2imC3C and C1imC3S) also increase the activity. We believe that the increased activity is certain because the ZIs increase the viscosity of the solutions17 and therefore decrease in activity if they are to merely maintain the activity.
We then investigated GnT-V activity after freezing. GnT-V activity was decreased to approximately 30% by freezing in water (Fig. 2A and S2†), demonstrating that GnT-V is prone to inactivation by freeze stress. Even so, a typical cryoprotectant, 20 vol% glycerol, was not sufficiently effective for GnT-V because the residual activity was just approximately 50% after freezing (Fig. 2B). In contrast, GnT-V activity in OE2imC3C and VimC3S solutions was retained much higher even after freezing (residual activity: 68–76%, Fig. 2B). The cryoprotective ability resulted in 1.5-fold activity after freezing in the OE2imC3C and VimC3S solutions, compared to the glycerol solution (Fig. 2A).
The ZIs were concentrated at sub-zero temperatures as the water freezes. The concentrated ZIs were not frozen at −80 °C and might protect GnT-V from physical damage by ice crystals. For example, in the case of the 5 wt% OE2imC3C solution, 8 wt% (5 wt% of OE2imC3C and 3 wt% water) did not crystallise even at −100 °C (Fig. S3†). The unfrozen portion formed a glass transition at −85 °C, but it was unclear whether the unfrozen portion was glass or liquid. From these results, ZIs, especially OE2imC3C and VimC3S, are effective cryoprotectants for GnT-V. The cryoprotection efficiency of C1imC3S was lower than that of the other ZIs but still higher than that of glycerol.
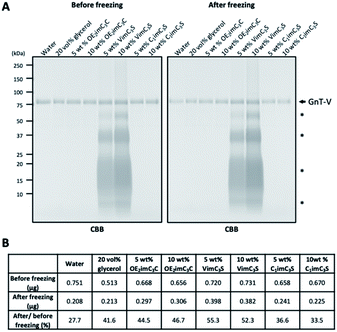
Freeze stress potentially affects the structure of GnT-V, resulting in aggregation, precipitation, and adsorption to e.g., plastic tubes. Therefore, we next investigated whether ZIs stabilised GnT-V. Consequently, the amount of GnT-V in the solutions before and after freezing was determined by electrophoresis with Coomassie brilliant blue (CBB) staining (Fig. 3A). The residual amount of GnT-V in water substantially decreased to 28% after freezing, and that in 20 vol% glycerol, it was 42% (Fig. 3B). The residual amount of GnT-V in the OE2imC3C and VimC3S solutions was similar to or even higher than that in the glycerol solution (45–55%). These results indicate that ZI stabilises GnT-V by preventing aggregation and/or adsorption to plastic tubes. C1imC3S was not as efficient from this viewpoint, supporting the relatively low cryoprotective effect (Fig. 2).
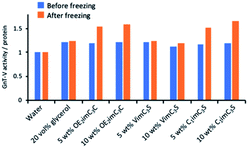 | ||
| Fig. 4 GnT-V activity per protein amount was calculated based on Fig. 2A and 3B. The values were normalised by those in water before and after freezing. | ||
ZI-derived bands were detected in the 5 and 10 wt% VimC3S solutions (Fig. 3A). The same bands were also detected when using the protein-free VimC3S solution (Fig. S4†), indicating that they were derived from the interaction between VimC3S and CBB. The polymerisation of VimC3S during electrophoresis or its pretreatment (heating at 95 °C for 5 min), based on the vinyl group, is possibly the reason, but further investigation is required. The amount of GnT-V might be overestimated by overlap with the VimC3S-derived band.
We calculated the ratio of residual GnT-V activity (shown in Fig. 2) to the residual protein amount (Fig. 3) after freezing. The value was 1.2-times higher in the 20 vol% glycerol solution than in water, which was at most 1.6-times higher in the OE2imC3C and C1imC3S solutions (Fig. 4). The values in the VimC3S solutions were not so high (∼1.2 times), presumably because the GnT-V amount might not be accurately quantified because of the VimC3S-derived band. We can find a clue to possible explanations on the significant increase in our study. We have previously reported the activity of chicken lysozymes in an IL of ethylammonium nitrate (EAN) aqueous solution.27 EAN partly denatures, but the lysozyme partly renatured after freezing and thawing because of the EAN–lysozyme interaction. We speculate that the same phenomenon occurred in this study: (1) the GnT-V protein we obtained was a mixture of natural and partly denatured ones, and ZIs renatured the structure of the latter only; and (2) ZI-stabilised the GnT-V protein and activated it.
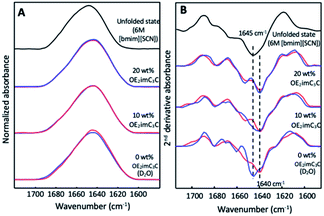
Finally, we further investigated the effect of ZIs on protein structures from a physical-chemical aspect by using representative OE2imC3C, which showed good effects on both GnT-V activity and protein stability (Fig. 2 and 3). However, unfortunately, a sufficient amount of GnT-V protein was not obtained because of low expression in cells and aggregation during culture and/or purification. Instead, we selected α-lactalbumin (α-Lac) as a model protein for the following reasons. The structural features of α-Lac are similar to those of GnT-V, as the α-helix and β-sheet contents are similar to those of GnT-V (the α-helix and β-sheet content: 45% and 9% for α-Lac; 43% and 17% for GnT-V, respectively). Moreover, both proteins have high contents of Leu and Lys residues, and the positions of these residues are similar, being that the Leu residues are located in the interior, whereas the Lys residues are on the surface (Fig. S5†). These features guarantee the structural similarity between GnT-V and α-Lac. Furthermore, α-Lac is a relatively unstable protein, as estimated from its free energy,28 although most commercially available proteins are stable for freezing, indicating that α-Lac is potentially vulnerable to freeze stress. The structural change in α-Lac was analysed using Fourier transform infrared (FTIR) spectroscopy (Fig. 5A). In the second derivative FTIR spectra of α-Lac in D2O before freezing, a sharp peak at 1640 cm−1 derived from α-helix29 was observed (Fig. 5B). It shifted to 1645 cm−1 after freezing, which corresponded to the signal of an unfolded state, confirming the unfolding of α-Lac by freezing in D2O. In sharp contrast, OE2imC3C maintained its peak at 1640 cm−1, even after freezing. The FTIR spectra of the 20 wt% OE2imC3C before and after freezing were almost identical. Therefore, OE2imC3C exhibited a cryoprotective effect, at least through the protection of the α-helix, resulting in a higher residual activity of GnT-V after freezing.
The ZIs achieved cryostorage of unstable GnT-V. Interestingly, the addition of ZIs slightly enhanced the activity, even before freezing. Moreover, the residual activity after freezing in ZI solutions was 1.6-times higher than that frozen in water. Given that it was 1.2-times in the 20 vol% glycerol solution, a general cryoprotecting solution, ZIs have the potential to be novel cryoprotectants. The ZIs, especially OE2imC3C and VimC3S, prevented protein aggregation and/or adsorption on plastic tubes at least by protecting the α-helix structure.
Generally, commercially unavailable proteins purified by researchers are unstable. Although utilization enzymes in the ZI solutions may not be an option now, it will be an option because activation and storage of vulnerable enzymes is critical for the life sciences and utilization of the ZI solution in vitro does not cause any problem. Therefore, we believe that ZIs will be key materials for accelerating the life sciences. The modification of ZI structures may facilitate the generation of ideal cryoprotectants of proteins. It is important to elucidate the generality of the cryoprotective effects of ZIs on protein stability in the future. Moreover, GnT-V activity was significantly increased in the ZI solutions before freezing, indicating their potential use as chemical chaperones, such as therapeutics for neurodegenerative diseases caused by protein misfolding.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank Editage (https://www.editage.com) for English language editing. This work was supported by an ACT-X grant (JPMJAX201B to T. H. and JPMJAX1915 to K. K., from the Japan Science and Technology Agency), A-STEP (to K.K. from the Japan Science and Technology Agency), the Leading Initiative for Excellent Young Researchers (to K. K., from the Ministry of Education, Culture, Sports, Science and Technology-Japan), and Kanazawa University SAKIGAKE project 2020 (to K. K.) and NIBB Collaborative Research Program (21-608).Notes and references
- A. Varki, Glycobiology, 2017, 27, 3–49 CrossRef CAS PubMed.
- K. W. Moremen, M. Tiemeyer and A. V. Nairn, Nat. Rev. Mol. Cell Biol., 2012, 13, 4482 CrossRef PubMed.
- Y. Zhao, Y. Sato, T. Isaji, T. Fukuda, A. Matsumoto, E. Miyoshi, J. Gu and N. Taniguchi, FEBS J., 2008, 275, 1939 CrossRef CAS PubMed.
- Y. Kizuka and N. Taniguchi, Biomolecules, 2016, 6, 25 CrossRef PubMed.
- M. Shoreibah, G. S. Perng, B. Adler, J. Weinstein, R. Basu, R. Cupples, D. Wen, J. K. Browne, P. Buckhaults, N. Fregien and M. Pierce, J. Biol. Chem., 1993, 268, 15381 CrossRef CAS.
- M. Granovsky, J. Fata, J. Pawling, W. J. Muller, R. Khokha and J. W. Dennis, Nat. Med., 2000, 6, 306 CrossRef CAS PubMed.
- K. Murata, E. Miyoshi, M. Kameyama, O. Ishikawa, T. Kabuto, Y. Sasaki, M. Hiratsuka, H. Ohigashi, S. Ishiguro, S. Ito, H. Honda, F. Takemura, N. Taniguchi and S. Imaoka, Clin. Cancer Res., 2000, 6, 1772 CAS.
- E. A. Partridge, C. Le Roy, G. M. Di Guglielmo, J. Pawling, P. Cheung, M. Granovsky, I. R. Nabi, J. L. Wrana and J. W. Dennis, Science, 2004, 306, 120 CrossRef CAS PubMed.
- H. B. Guo, H. Johnson, M. Randolph and M. Pierce, J. Biol. Chem., 2009, 284, 34986 CrossRef CAS PubMed.
- M. Nagae, Y. Kizuka, E. Mihara, Y. Kitago, S. Hanashima, Y. Ito, J. Takagi, N. Taniguchi and Y. Yamaguchi, Nat. Commun., 2018, 9, 3380 CrossRef PubMed.
- J. F. Darby, A. K. Gilio, B. Piniello, C. Roth, E. Blagova, R. E. Hubbard, C. Rovira, G. J. Davies and L. Wu, ACS Catal., 2020, 10, 8590 CrossRef CAS.
- Y. Maki, R. Okamoto, M. Izumi and Y. Kajihara, J. Am. Chem. Soc., 2020, 142, 20671 CrossRef CAS PubMed.
- T. J. Kamerzell, R. Esfandiary, S. B. Joshi, C. R. Middaugh and D. B. Volkin, Adv. Drug Deliv. Rev., 2011, 63, 1118 CrossRef CAS PubMed.
- V. Vagenende, M. G. S. Yap and B. L. Trout, Biochemistry, 2009, 48, 11084 CrossRef CAS PubMed.
- M. Yoshizawa, M. Hirao, K. Ito-Akita and H. Ohno, J. Mater. Chem., 2001, 11, 1057 RSC.
- K. Kuroda, H. Satria, K. Miyamura, Y. Tsuge, K. Ninomiya and K. Takahashi, J. Am. Chem. Soc., 2017, 139, 16052 CrossRef CAS PubMed.
- H. Satria, K. Kuroda, Y. Tsuge, K. Ninomiya and K. Takahashi, New J. Chem., 2018, 42, 13225 RSC.
- K. Fujita, Y. Nikawa and H. Ohno, Chem. Commun., 2013, 49, 3257 RSC.
- K. Kuroda, Y. Kohno and H. Ohno, Chem. Lett., 2017, 46, 870 CrossRef CAS.
- K. Kuroda, C. Kodo, K. Ninomiya and K. Takahashi, Aust. J. Chem., 2019, 72, 139 CrossRef CAS.
- J. B. Schlenoff, Langmuir, 2014, 30, 9625 CrossRef CAS PubMed.
- K. Kuroda, T. Komori, K. Ishibashi, T. Uto, I. Kobayashi, R. Kadokawa, Y. Kato, K. Ninomiya, K. Takahashi and E. Hirata, Commun. Chem., 2020, 3, 163 CrossRef CAS.
- Y. Kato, T. Uto, D. Tanaka, K. Ishibashi, A. Kobayashi, M. Hazawa, R. W. Wong, K. Ninomiya, K. Takahashi, E. Hirata and K. Kuroda, Commun. Chem., 2021, 5, 151 CrossRef.
- G. Sharma, Y. Kato, A. Hachisu, K. Ishibashi, K. Ninomiya, K. Takahashi, E. Hirata and K. Kuroda, Cellulose, 2022, 29, 3017 CrossRef CAS.
- H. Hebal, N. Boucherba, B. Binay and O. Turunen, Biocatal. Biotransform., 2021, 39, 242 CrossRef CAS.
- T. Hirata, M. Nagae, R. F. Osuka, S. K. Mishra, M. Yamada and Y. Kizuka, Biochim. Biophys. Acta, 2020, 1864, 129726 CrossRef CAS PubMed.
- Y. Yoshimura, T. Takekiyo and T. Mori, Chem. Phys. Lett., 2016, 664, 44 CrossRef CAS.
- F. Ahmad and C. C. Bigelow, J. Biol. Chem., 1982, 257, 12935 CrossRef CAS.
- W. Dzwolak, M. Kato, A. Shimizu and Y. Taniguchi, Biochim. Biophys. Acta, 1999, 1433, 45 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01575g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |