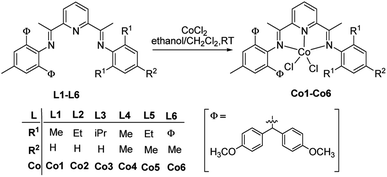
Open Access Article
Open Access Article4,4′-Dimethoxybenzhydryl substituent augments performance of bis(imino)pyridine cobalt-based catalysts in ethylene polymerization†
Shi-Fang Yuan*a,
Luyao Wangab,
Yi Yanab,
Tian Liuab,
Zygmunt Flisak *bc,
Yanping Ma
*bc,
Yanping Ma b and
Wen-Hua Sun
b and
Wen-Hua Sun *b
*b
aThe School of Chemistry and Chemical Engineering, Institute of Applied Chemistry, Shanxi University, Taiyuan 030006, China. E-mail: yuansf@sxu.edu.cn
bKey Laboratory of Engineering Plastics and Beijing National Laboratory for Molecular Sciences, Institute of Chemistry Chinese Academy of Sciences, Beijing 100190, China. E-mail: whsun@iccas.ac.cn
cFaculty of Chemistry, University of Opole, Oleska 48, 45-052 Opole, Poland
First published on 24th May 2022
Abstract
A series of cobalt complexes with bis(imino)pyridine derivatives featuring unsymmetrical substitution with bulky groups has been synthesized and characterized. The molecular structures of two representatives have been determined by the single-crystal X-ray diffraction study, revealing distorted tetrahedral geometry with different degrees of steric hindrance imparted by the two inequivalent aryl groups attached to the imine nitrogen atoms. On activation with either MAO or MMAO, these complexes display high activity toward ethylene polymerization, reaching 8.71 × 106 g of PE (mol of Co)−1 h−1 at 60 °C and produce polyethylene of high molecular weight (Mw = 5.27 × 105 g mol−1) and low dispersity. The presence of the methoxy-substituent noticeably enhances the activity of the cobalt catalyst and increases the molecular weight of the resultant polyethylene.
1. Introduction
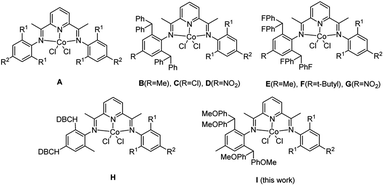
Seminal reports on Ni2+ and Pd2+ complexes with α-diimines1 as well as Fe2+ and Co2+ complexes with bis(imino)pyridines2,3 as highly active precatalysts toward ethylene polymerization have induced considerable interest in the late-transition-metal-based precatalysts within academia and industry. Recent reviews4–10 reflect significant progress in the development of such precatalysts as researchers are encouraged to design the novel catalytic systems instrumental in the synthesis of advanced polymeric materials. Our group has long been involved in the modification of bis(imino)pyridine-based systems.11 The structure of the catalysts can be adjusted in a wide range,12–23 thus affecting the properties of the polymeric products. Derivation of the original model (A, Scheme 1),2 which involves the modification of the aniline starting material,24–38 is an example of the catalyst fine tuning. The steric hindrance introduced to these anilines (B, Scheme 1)25 improves the catalytic activity, while the electron-withdrawing substituents (C and D, Scheme 1)27,28 increase the molecular weight of the resultant polyethylene. Encouraged with these observations, we carried out extensive tests in order to verify our hypothesis. As a result, new promising catalytic systems exhibiting desired thermal stability and generating potentially useful polymeric products were conceived (E, F and G, Scheme 1).33–35 Further modifications, such as substituting one of the aryl rings with the dibenzocycloheptyl groups (H, Scheme 1)36 improved the thermal stability of the catalyst. One of the other promising templates is the methoxy-substituted benzhydryl group,39–41 offering the high activity, appreciable lifetime and thermal stability of the iron-based catalytic systems,41 which lead to the industrially important polyethylene of high molecular weight and high density. However, the cobalt analogs have not been explored so far and we are convinced that they are worthy detailed investigation.In the current work, the bis(imino)pyridines bearing methoxy-substituted benzhydryl groups (I, Scheme 1) were synthesized together with the corresponding cobalt complexes. Significant thermal stability of the catalytic system was observed in the ethylene polymerization study and the polymer of high molecular weight was produced. We performed the optimization of the polymerization parameters, including ethylene pressure, type and amount of the cocatalyst, reaction temperature and run time. The investigation of the properties of the resulting polyethylene, such as molecular weight, dispersity, melting point and microstructure was also carried out.
2 Experimental segment
2.1 General considerations
The air- and moisture-sensitive compounds were manipulated using Schlenk reactors under inert atmosphere of the high-purity nitrogen. Solvents were pre-treated and distilled in nitrogen before application. Most of the common solvents were exposed to sodium wire, except for dichloromethane purified by activated molecular sieves (4 Å) and ethanol dried with calcium oxide. Akzo Nobel Corp produced methylaluminoxane (MAO, toluene solution 1.46 M) and modified methylaluminoxane (MMAO, 1.93 M solution in n-heptane). Ethylene of polymerization quality was sourced from Yanshan, the local branch of the petrochemical company SinoPec. Other chemicals were acquired from Beijing Reagents as well as Aldrich and Acros. Nuclear magnetic resonance experiments on organometallics were conducted on a Bruker DMX 400 MHz instrument with the internal standard of tetramethylsilane (TMS) at room temperature, while the polyethylene NMR spectra were recorded using the polymer solution in deuterated 1,1,2,2-tetrachloroethane in presence of TMS at 100 °C with a Bruker DMX 300 MHz instrument. The 2000 FT IR spectrometer of PerkinElmer System was employed to record the infrared spectra and the 1112-microanalyzer of Flash EA to perform the elemental analysis. The 1,2,4-trichlorobenzene solutions of polyethylene were measured at 150 °C by a PL-GPC 220 to determine the molecular weight and dispersity; the differential scanning calorimetry traces and the melting point of polyethylene were recorded within the second scan using the DSC Q2000 instrument in the range of up to 160 °C with the heating rate of 10 °C per minute.2.2 Preparation of [2-{{2,6-((p-MeOPh)2CH)2-4-MeC6H2}N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e001.gif) CMe}-6-(ArN
CMe}-6-(ArN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e001.gif) CMe)C5H3N]CoCl2
CMe)C5H3N]CoCl2
Co1 with R1 = Me and R2 = H (Ar as 2,6-Me2C6H3): under an inert atmosphere of nitrogen, a mixture of L1 (0.11 g, 0.14 mmol) in dichloromethane (15 mL) and CoCl2 (0.01 g, 0.14 mmol) in ethanol (5 mL) was stirred at ambient temperature for 24 h. The solution was concentrated into about 5 mL under vacuum, then 20 mL diethyl ether was added to precipitate the brown solid, which was filtrated, washed three times with 10 mL diethyl ether and finally dried to yield 0.06 g of the Co1 brown powder (83%). 1H NMR (400 MHz, CDCl3, TMS): δ 112.56 (s, 1H, Py-H), 110.85 (s, 1H, Py-H), 47.06 (s, 1H, Py-H), 20.85 (s, 3H, CH3), 9.83 (s, 2H, aryl-Hm), 9.27 (s, 2H, aryl-Hm), 6.42 (s, 4H, aryl-H), 3.31–2.73 (m, 24H, aryl-H, CH3), 2.09 (s, 2H, 2× CH(PhOMe)2), 1.18 (s, 3H, CH3), −9.28 (s, 1H, aryl-Hp), −17.93 (s, 3H, CH3), −23.94 (s, 6H, 2× N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3). FT-IR (cm−1): 2979 (w), 2287 (w), 2113 (w), 1893 (w), 1737 (m), 1610 (m), 1580 (m), 1507 (s), 1459 (m), 1373 (w), 1299 (w), 1251 (s), 1174 (s), 1032 (s), 865 (s), 834 (m), 814 (w), 771 (w), 654 (m). Anal. Calcd for C54H53Cl2CoN3O4 (937.87): C, 69.16; H, 5.70; N, 4.48%, found: C, 68.98; H, 5.47; N, 4.29%.
CCH3). FT-IR (cm−1): 2979 (w), 2287 (w), 2113 (w), 1893 (w), 1737 (m), 1610 (m), 1580 (m), 1507 (s), 1459 (m), 1373 (w), 1299 (w), 1251 (s), 1174 (s), 1032 (s), 865 (s), 834 (m), 814 (w), 771 (w), 654 (m). Anal. Calcd for C54H53Cl2CoN3O4 (937.87): C, 69.16; H, 5.70; N, 4.48%, found: C, 68.98; H, 5.47; N, 4.29%.
Co2 with R1 = Et and R2 = H (Ar as 2,6-Et2C6H3): employing the similar procedure to Co1, 0.07 g of the Co2 brown powder were isolated (89%). 1H NMR (400 MHz, CDCl3, TMS): δ 112.33 (s, 1H, Py-H), 111.05 (s, 1H, Py-H), 46.63 (s, 1H, Py-H), 21.11 (s, 3H, CH3), 10.63 (s, 2H, aryl-Hm), 9.39 (s, 2H, aryl-Hm), 6.63 (s, 4H, aryl-H), 3.40–2.88 (m, 24H, aryl-H, CH3), 2.19 (s, 2H, 2× CH(PhOMe)2), 1.23 (s, 3H, CH3), −9.44 (s, 1H, aryl-Hp), −18.12 (s, 3H, CH3), −19.24 (s, 6H, 2× N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), −34.93 (s, 2H, CH2), −38.30 (s, 2H, CH2). FT-IR (cm−1): 2968 (w), 2275 (w), 2113 (w), 1894 (w), 1609 (m), 1582 (m), 1508 (s), 1459 (m), 1373 (w), 1299 (w), 1253 (s), 1177 (s), 1110 (w), 1034 (s), 870 (w), 833 (m), 811 (w), 769 (w), 658 (m). Anal. Calcd for C56H57Cl2CoN3O4 (965.92): C, 69.63; H, 5.95; N, 4.35%, found: C, 69.88; H, 6.17; N, 4.16%.
CCH3), −34.93 (s, 2H, CH2), −38.30 (s, 2H, CH2). FT-IR (cm−1): 2968 (w), 2275 (w), 2113 (w), 1894 (w), 1609 (m), 1582 (m), 1508 (s), 1459 (m), 1373 (w), 1299 (w), 1253 (s), 1177 (s), 1110 (w), 1034 (s), 870 (w), 833 (m), 811 (w), 769 (w), 658 (m). Anal. Calcd for C56H57Cl2CoN3O4 (965.92): C, 69.63; H, 5.95; N, 4.35%, found: C, 69.88; H, 6.17; N, 4.16%.
Co3 with R1 = iPr and R2 = H (Ar as 2,6-iPr2C6H3): similarly to Co1, 0.12 g of the Co3 brown powder were collected (80%). 1H NMR (400 MHz, CDCl3, TMS): δ 113.57 (s, 1H, Py-H), 110.91 (s, 1H,Py-H), 48.15 (s, 1H, Py-H), 20.71 (s, 3H, CH3), 9.90 (s, 2H, aryl-Hm), 9.11 (s, 2H, aryl-Hm), 6.60 (s, 4H, aryl-H), 3.48–2.53 (m, 24H, aryl-H, CH3), 1.73 (s, 2H, 2× CH(PhOMe)2), 1.41 (s, 2H, 2× CHMe2), 1.26 (s, 3H, CH3), −9.19 (s, 1H, aryl-Hp), −17.61 (s, 6H, 2× CH3), −18.11 (s, 6H, CH3, 2× N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3). FT-IR (cm−1): 2958 (w), 2255 (w), 2113 (w), 1924 (w), 1609 (m), 1580 (m), 1507 (s), 1459 (m), 1370 (w), 1299 (w), 1247 (s), 1174 (s), 1108 (w), 1033 (s), 834 (m), 811 (w), 773 (w). Anal. Calcd for C58H61Cl2CoN3O4 (993.98): C, 70.09; H, 6.19; N, 4.23%, found: C, 69.93; H, 6.02; N, 4.45%.
CCH3). FT-IR (cm−1): 2958 (w), 2255 (w), 2113 (w), 1924 (w), 1609 (m), 1580 (m), 1507 (s), 1459 (m), 1370 (w), 1299 (w), 1247 (s), 1174 (s), 1108 (w), 1033 (s), 834 (m), 811 (w), 773 (w). Anal. Calcd for C58H61Cl2CoN3O4 (993.98): C, 70.09; H, 6.19; N, 4.23%, found: C, 69.93; H, 6.02; N, 4.45%.
Co4 with R1 = R2 = Me (Ar as 2,4,6-Me3C6H2): 0.05 g of the Co4 brown powder were formed (79%). 1H NMR (400 MHz, CD2Cl2, TMS): δ 112.99 (s, 1H, Py-H), 110.99 (s, 1H, Py-H), 45.35 (s, 1H, Py-H), 20.75 (s, 3H, CH3), 20.48 (s, 3H, CH3), 9.72 (s, 2H, aryl-Hm), 9.34 (s, 2H, aryl-Hm), 6.41 (s, 4H, aryl-H), 3.38–2.66 (m, 24H, aryl-H, CH3), 1.97 (s, 2H, 2× CH(PhOMe)2), 1.24 (s, 3H, CH3), −18.26 (s, 3H, CH3), −24.83 (s, 6H, 2× N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3). FT-IR (cm−1): 2971 (w), 2267 (w), 2113 (w), 1913 (w), 1610 (m), 1581 (m), 1507 (s), 1457 (m), 1374 (w), 1299 (w), 1247 (s), 1174 (s), 1112 (w), 1032 (s), 834 (m), 813 (w), 736 (w). Anal. Calcd for C55H55Cl2CoN3O4 (951.90): C, 69.40; H, 5.82; N, 4.41%, found: C, 69.73; H, 5.67; N, 4.23%.
CCH3). FT-IR (cm−1): 2971 (w), 2267 (w), 2113 (w), 1913 (w), 1610 (m), 1581 (m), 1507 (s), 1457 (m), 1374 (w), 1299 (w), 1247 (s), 1174 (s), 1112 (w), 1032 (s), 834 (m), 813 (w), 736 (w). Anal. Calcd for C55H55Cl2CoN3O4 (951.90): C, 69.40; H, 5.82; N, 4.41%, found: C, 69.73; H, 5.67; N, 4.23%.
Co5 with R1 = Et and R2 = Me (Ar as 2,6-Et2-4-MeC6H2): 0.11 g of the Co5 brown powder were obtained in (86%). 1H NMR (400 MHz, CDCl3, TMS): δ 111.32 (s, 1H, Py-H), 110.90 (s, 1H, Py-H), 46.69 (s, 1H, Py-H), 20.93 (s, 3H, CH3), 20.64 (s, 3H, CH3), 10.41 (s, 2H, aryl-Hm), 9.35 (s, 2H, aryl-Hm), 6.54 (s, 4H, aryl-H), 3.81–2.55 (m, 24H, aryl-H, CH3), 1.81 (s, 2H, 2× CH(PhOMe)2), 1.17 (s, 3H, CH3), −17.83 (s, 3H, CH3), −19.53 (s, 6H, 2× N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3), −34.53 (s, 2H, CH2), −35.99 (s, 2H, CH2). FT-IR (cm−1): 2971 (w), 2259 (w), 2113 (w), 1919 (w), 1610 (m), 1581 (m), 1507 (s), 1459 (m), 1374 (w), 1299 (w), 1250 (s), 1172 (s), 1114 (w), 1032 (s), 862 (m), 834 (m), 813 (w). Anal. Calcd for C57H59Cl2CoN3O4 (979.95): C, 69.86; H, 6.07; N, 4.29%, found: C, 70.09; H, 5.95; N, 4.17%.
CCH3), −34.53 (s, 2H, CH2), −35.99 (s, 2H, CH2). FT-IR (cm−1): 2971 (w), 2259 (w), 2113 (w), 1919 (w), 1610 (m), 1581 (m), 1507 (s), 1459 (m), 1374 (w), 1299 (w), 1250 (s), 1172 (s), 1114 (w), 1032 (s), 862 (m), 834 (m), 813 (w). Anal. Calcd for C57H59Cl2CoN3O4 (979.95): C, 69.86; H, 6.07; N, 4.29%, found: C, 70.09; H, 5.95; N, 4.17%.
Co6 with R1 = ϕ and R2 = Me (Ar as 2,6-ϕ2-4-MeC6H2): 0.08 g of the Co6 brown powder were obtained (81%). 1H NMR (400 MHz, CDCl3, TMS): δ 114.07 (s, 2H, Py-H), 111.28 (s, 1H, Py-H), 21.05 (s, 6H, CH3), 9.21–9.16 (m, 16H, aryl-H), 8.56 (d, J = 8.4 Hz, 8H, aryl-H), 7.42 (d, J = 9.2 Hz, 12H, aryl-H), 3.84–2.63 (m, 48H, aryl-H, CH3), 1.92 (s, 4H, 4× CH(PhOMe)2), −19.09 (s, 6H, CH3, 2× N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCH3). FT-IR (cm−1): 2965 (w), 2834 (w), 1609 (νC
CCH3). FT-IR (cm−1): 2965 (w), 2834 (w), 1609 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, m), 1581 (νC
N, m), 1581 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, m), 1509 (s), 1459 (m), 1374 (w), 1298 (m), 1249 (s), 1177 (m), 1110 (m), 1032 (m), 830 (m), 776 (m), 734 (m), 688 (w). Anal. Calcd for C83H79Cl2CoN3O8 (1376.39): C, 72.43; H, 5.79; N, 3.05%, found: C, 72.75; H, 5.46; N, 2.96%.
N, m), 1509 (s), 1459 (m), 1374 (w), 1298 (m), 1249 (s), 1177 (m), 1110 (m), 1032 (m), 830 (m), 776 (m), 734 (m), 688 (w). Anal. Calcd for C83H79Cl2CoN3O8 (1376.39): C, 72.43; H, 5.79; N, 3.05%, found: C, 72.75; H, 5.46; N, 2.96%.
2.3 X-ray single-crystal analysis
Crystal structures of Co2 and Co5 were determined by the X-ray diffraction analysis. The crystals were acquired by dispersing a small amount of diethyl ether onto the dichloromethane solution of the corresponding complex at ambient temperature. The single-crystal diffraction study was performed using a XtaLAB AFC10 (RCD3) fixed-chi single diffractometer with Mo-Kα radiation (λ = 0.71073 Å) monochromated with graphite at −100(2) °C. The positions of all collected reflections were globally refined to determine the cell parameters. The intensities were corrected for empirical absorption as well as Lorentz polarization effects.43 The nonhydrogen atoms were computed by direct methods and adjusted by full-matrix least squares on F2 using the SHELXL-97 package, along with the hydrogen atoms localized by calculations.42 The data related to determinations and refinements of molecular structures are collected in Table 1.| Co2 | Co5 | |
|---|---|---|
| CCDC no. | 2124397 | 2124398 |
| Empirical formula | C113H115Cl6Co2N6O8 | C57H59Cl2CoN3O5 |
| Formula weight | 2015.66 | 995.90 |
| Temperature, K | 173.01(3) | 173.00(3) |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | P21/n |
| a, Å | 12.4375(2) | 9.5954(3) |
| b, Å | 21.6032(3) | 35.3574(12) |
| c, Å | 40.2761(6) | 16.2139(5) |
| β, ° | 97.5830(10) | 100.772(3) |
| Volume, Å3 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727.1(3) 727.1(3) |
5403.9(3) |
| Z | 4 | 4 |
| ρcalc, g cm−3 | 1.248 | 1.224 |
| μ, mm−1 | 0.516 | 0.465 |
| F (000) | 4220.0 | 2092.0 |
| Crystal size, mm3 | 0.288 × 0.232 × 0.101 | 0.421 × 0.111 × 0.075 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2Θ range for data collection, ° | 6.82 to 54.998 | 6.886–55 |
| Index ranges | −16 ≤ h ≤ 14, −28 ≤ k ≤ 23, −49 ≤ l ≤ 52 | −11 ≤ h ≤ 12, −45 ≤ k ≤ 45, −21 ≤ l ≤ 19 |
| Reflections collected | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 625 625 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 485 |
| R (int) | 0.0198 | 0.0602 |
| Data/restraints/parameters | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 586/0/1234 586/0/1234 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 395/0/614 395/0/614 |
| Goodness-of-fit on F2 | 1.022 | 1.060 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0375 wR2 = 0.0914 | R1 = 0.0445 wR2 = 0.1129 |
| Final R indexes [all data] | R1 = 0.0427 wR2 = 0.0940 | R1 = 0.0642 wR2 = 0.1214 |
| Largest diff. peak/hole, (e Å−3) | 0.98/−1.00 | 0.69/−0.59 |
3 Result and discussion
3.1 Synthesis and characterization
The ligands (L1–L6) were obtained according to the literature procedure41 from the unsymmetrically substituted anilines containing the 4,4′-dimethoxybenzhydryl group, prepared via Friedel–Crafts alkylation of p-toluidine catalyzed by zinc chloride.44 The yellow solids L1–L5 were purified by the column chromatography with silica gel using the eluent of ethyl acetate and petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33). Meanwhile, the symmetrical bis(imino)pyridine L6 (Ar = 2,6-((p-MeOPh)2CH)2-4-MeC6H2) was synthesized by the acid-catalyzed reaction in one step as a light-green powder and purified by the column chromatography of basic-alumina using the eluent of ethyl acetate and petroleum ether (1
33). Meanwhile, the symmetrical bis(imino)pyridine L6 (Ar = 2,6-((p-MeOPh)2CH)2-4-MeC6H2) was synthesized by the acid-catalyzed reaction in one step as a light-green powder and purified by the column chromatography of basic-alumina using the eluent of ethyl acetate and petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). The ligands reacted at room temperature for 24 hours with the molar equivalent of cobalt(II) chloride dissolved in the dichloromethane/ethanol mixture to produce the corresponding cobalt complexes (Co1–Co6, Scheme 1) with the yields between 79 and 89%. Subsequently, they were analyzed with 1H NMR and FT-IR spectroscopy and the carbon, hydrogen and nitrogen content was determined. Additionally, the molecular structure of Co2 and Co5 species has been confirmed by the single-crystal X-ray diffraction study.
100). The ligands reacted at room temperature for 24 hours with the molar equivalent of cobalt(II) chloride dissolved in the dichloromethane/ethanol mixture to produce the corresponding cobalt complexes (Co1–Co6, Scheme 1) with the yields between 79 and 89%. Subsequently, they were analyzed with 1H NMR and FT-IR spectroscopy and the carbon, hydrogen and nitrogen content was determined. Additionally, the molecular structure of Co2 and Co5 species has been confirmed by the single-crystal X-ray diffraction study.
The structures are illustrated individually in Fig. 1 and 2; Table 2 shows the selected interatomic distances and angles. The two structures are similar despite the difference in the substitution pattern within the ligands (viz. Ar = 2,6-Et2C6H3 in Co2 and Ar = 2,6-Et2-4-MeC6H2 in Co5). The coordination around the cobalt core consists of two chlorine atoms and three sp2-hybridized nitrogen atoms of the bis(arylimino)pyridine; in this way a distorted-square pyramid with the apex of Cl and the square base of N1, N2, N3 and another Cl around cobalt ion is formed. Such five-coordinate geometry is documented for many complexes of bis(imino)pyridine cobalt(II) halides.25,30,33,37,45 The cobalt atom sits 2.734 Å above the basal plane in Co2 and 2.791 Å in Co5. Certain variations in the cobalt–nitrogen bond lengths are evident with the Co(1)–N(2)pyridine equal 2.0466(13) Å in Co2, and 2.0473(15) Å in Co5, respectively. These interatomic distances are markedly shorter comparing with the corresponding Co(1)–Nimine bonds, whose lengths are equal 2.1740(13) and 2.2212(13) Å in Co2; 2.2184(15) and 2.2074(16) Å in Co5, respectively. The change is likely caused by stronger binding to the Npyridine atom and the constriction of a tridentate ligand. Notwithstanding the differences in the steric properties of the inequivalent aryl groups, only modest dissimilarities in the Co–Nimine distances are apparent. The N-aryl groups are approximately perpendicular to the N,N,N-cobalt coordination plane with the dihedral angles of 75.04° and 83.30° in Co2; 73.36° and 74.40° in Co5, respectively. Therefore the steric properties of the ortho-substituents play the important role in protecting the active species.
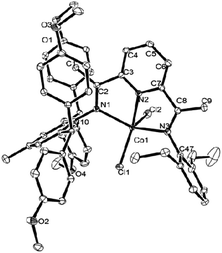 | ||
| Fig. 1 ORTEP drawing of Co2 with the thermal ellipsoids set at the 30% probability level and the omission of hydrogen atoms. | ||
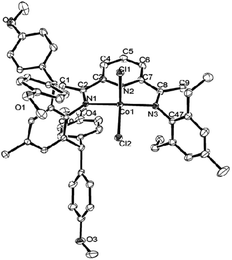 | ||
| Fig. 2 ORTEP drawing of Co5 with the thermal ellipsoids set at the 30% probability level and the omission of hydrogen atoms. | ||
| Co2 | Co5 | |
|---|---|---|
| Interatomic distances | ||
| Co(1)–N(1) | 2.1740(13) | 2.2184(15) |
| Co(1)–N(2) | 2.0466(13) | 2.0473(15) |
| Co(1)–N(3) | 2.2212(13) | 2.2074(16) |
| Co(1)–Cl(1) | 2.2432(4) | 2.2887(6) |
| Co(1)–Cl(2) | 2.2921(4) | 2.2512(5) |
| N(1)–C(2) | 1.284(19) | 1.290(2) |
| N(2)–C(7) | 1.332(2) | 1.334(2) |
| N(3)–C(8) | 1.286(2) | 1.285(2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Angles | ||
| N(1)–Co(1)–N(2) | 74.51(5) | 74.36(6) |
| N(1)–Co(1)–N(3) | 141.01(5) | 140.92(6) |
| N(2)–Co(1)–N(3) | 73.87(5) | 73.88(6) |
| N(1)–Co(1)–Cl(2) | 99.99(3) | 98.67(4) |
| N(2)–Co(1)–Cl(2) | 90.81(4) | 152.57(5) |
| N(3)–Co(1)–Cl(2) | 102.67(3) | 99.08(4) |
| N(1)–Co(1)–Cl(1) | 99.76(4) | 102.04(4) |
| N(2)–Co(1)–Cl(1) | 156.46(4) | 94.25(5) |
| N(3)–Co(1)–Cl(1) | 100.15(4) | 102.45(4) |
| Cl(1)–Co(1)–Cl(2) | 112.72(19) | 113.18(2) |
The 1H NMR spectra of Co1–Co6 display highly shifted peaks due to the paramagnetism of the complexes. Nonetheless, some degree of assignment was possible by consideration of the proximity of the proton environments to the Co(II) ion and the comparison with the data published elsewhere.25,27,28 By taking Co2 (Fig. 3) as an example, the peaks for the inequivalent meta-pyridyl protons (F, F′) can be seen at 112.33av ppm and 111.05av ppm, respectively. This reflects the unsymmetrical nature of the bis(imino)pyridine ligand. Moreover, the upfield signal of para-pyridyl proton (G) appears at 46.63av ppm. The para-methyl signal (E) in Co5, absent in Co2, is visible at 20.64 ppm (Fig. 4).
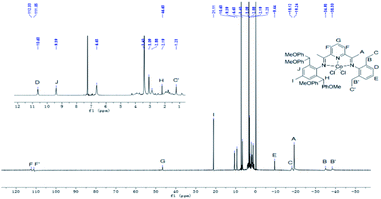 | ||
| Fig. 3 1H NMR spectrum of Co2 with an expansion of the 2–10 ppm region; recorded in CDCl3 at ambient temperature. | ||
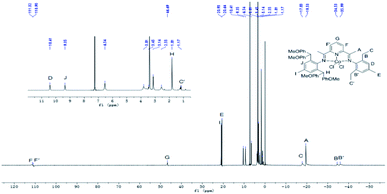 | ||
| Fig. 4 1H NMR spectrum of Co5 with an expansion of the 2–10 ppm region; recorded in CDCl3 at ambient temperature. | ||
The FT-IR spectra of Co1–Co6 reveal the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Nimine stretching vibrations in the range of 1580–1610 cm−1. These values are lower about 30 cm−1 in comparison with the free ligands (L1–L6). Such shifts in wavenumber confirm the effective coordination of the imine groups to cobalt.25,27,33 In addition, microanalytical data were measured for both ligands and complexes.
Nimine stretching vibrations in the range of 1580–1610 cm−1. These values are lower about 30 cm−1 in comparison with the free ligands (L1–L6). Such shifts in wavenumber confirm the effective coordination of the imine groups to cobalt.25,27,33 In addition, microanalytical data were measured for both ligands and complexes.
3.2 Typical procedure for ethylene polymerization
3.2.3.1 Optimization of reaction conditions with Co1–Co6/MAO. The polymerization parameters, such as the Al/Co molar ratio, reaction temperature and time were first optimized for the Co4 precatalyst. The results of polymerization tests with MAO at constant ethylene pressure are given in Table 3.
| Entry | Precatalyst | Al/Co | T, °C | t, min | Yield, g | Activityb | Mwc | Mw/Mc | Tmd, °C |
|---|---|---|---|---|---|---|---|---|---|
| a Conditions: 2.0 μmol of Co4; 100 mL toluene, 10 atm ethylene (except for items 15 and 16).b 106 g (PE) mol−1 (Co) h−1.c Determined by GPC; Mw: 105 g mol−1.d Determined by DSC.e 5 atm of ethylene.f 1 atm of ethylene. | |||||||||
| 1 | Co4 | 1750 | 30 | 30 | 1.01 | 1.01 | 1.87 | 1.9 | 136.3 |
| 2 | Co4 | 2000 | 30 | 30 | 1.78 | 1.78 | 3.39 | 1.7 | 136.0 |
| 3 | Co4 | 2250 | 30 | 30 | 6.46 | 6.46 | 1.84 | 2.3 | 135.7 |
| 4 | Co4 | 2500 | 30 | 30 | 6.75 | 6.75 | 4.37 | 1.9 | 135.5 |
| 5 | Co4 | 2750 | 30 | 30 | 6.38 | 6.38 | 3.16 | 2.2 | 135.8 |
| 6 | Co4 | 3000 | 30 | 30 | 4.19 | 4.19 | 3.36 | 2.7 | 135.5 |
| 7 | Co4 | 2500 | 40 | 30 | 7.27 | 7.27 | 2.24 | 2.9 | 135.3 |
| 8 | Co4 | 2500 | 50 | 30 | 8.63 | 8.63 | 2.25 | 3.1 | 134.8 |
| 9 | Co4 | 2500 | 60 | 30 | 8.71 | 8.71 | 2.12 | 3.3 | 135.5 |
| 10 | Co4 | 2500 | 70 | 30 | 3.33 | 3.33 | 0.64 | 1.7 | 135.1 |
| 11 | Co4 | 2500 | 60 | 5 | 3.67 | 22.02 | 1.66 | 3.4 | 135.4 |
| 12 | Co4 | 2500 | 60 | 15 | 6.03 | 12.06 | 2.01 | 2.9 | 135.0 |
| 13 | Co4 | 2500 | 60 | 45 | 8.78 | 5.85 | 4.08 | 11.3 | 134.9 |
| 14 | Co4 | 2500 | 60 | 60 | 9.06 | 4.53 | 5.27 | 8.4 | 135.2 |
| 15e | Co4 | 2500 | 60 | 30 | 2.71 | 2.71 | 1.39 | 2.8 | 135.5 |
| 16f | Co4 | 2500 | 60 | 30 | 1.03 | 1.03 | 0.59 | 2.5 | 133.8 |
| 17 | Co1 | 2500 | 60 | 30 | 7.13 | 7.13 | 0.44 | 1.7 | 135.6 |
| 18 | Co2 | 2500 | 60 | 30 | 6.37 | 6.37 | 3.38 | 4.7 | 135.4 |
| 19 | Co3 | 2500 | 60 | 30 | 4.61 | 4.61 | 3.08 | 1.7 | 135.4 |
| 20 | Co5 | 2500 | 60 | 30 | 7.48 | 7.48 | 2.98 | 3.5 | 135.4 |
| 21 | Co6 | 2500 | 60 | 30 | 2.01 | 2.01 | 4.36 | 2.5 | 135.8 |
The Al/Co molar ratio was systematically varied with the temperature and pressure kept constant at 30 °C and 10 atm, respectively. The catalytic activity gradually increased as the molar ratio of Al/Co was raised from 1750 to 3000 (entries 1–6, Table 3). The optimal activity of 6.75 × 106 g of PE (mol of Co)−1 h−1 is observed at the Al/Co molar ratio of 2500 (entry 4, Table 3). The molecular weight of polyethylene, contained in the 1.87–4.37 × 105 g mol−1 range, decreases as the Al/Co molar ratio increases (Fig. 5). This is due to higher rate of chain transfer from cobalt to aluminium for the large concentrations of alkyl aluminium reagent.20,21 The molecular weight distribution of the obtained polyethylene is relatively narrow, in the range of 1.7–2.7. Previously, similar values have also been reported.18,46–53
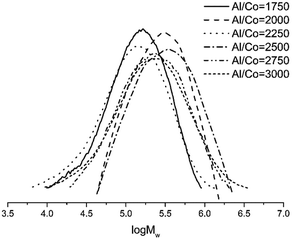 | ||
| Fig. 5 GPC curves of polyethylene (entries 1–6, Table 3). | ||
With the Al/Co molar ratio of 2500, the temperature was varied between 30 and 70 °C (entries 4 and 7–10, Table 3). The maximum catalytic activity of 8.71 × 106 g of PE (mol of Co)−1 h−1 was attained at 60 °C (entry 9, Table 3). Beyond 60 °C, the activity slightly decreased due to the partial deactivation of the active species at higher temperature.31,35,54 Nevertheless, even at 70 °C the Co4/MAO system maintained a remarkable value of 3.33 × 106 g of PE (mol of Co)−1 h−1 (entry 10, Table 3). It was also found that the polyethylene molecular weight decreases from 4.37 to 0.64 × 105 g mol−1 with the temperature, which indicates higher possibility of the chain transfer and reaction termination (entries 4 and 7–10, Table 3). This trend is revealed in Fig. 6.
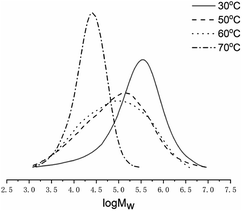 | ||
| Fig. 6 GPC curves of polyethylene (entries 4 and 7–10, Table 3). | ||
With the Al/Co molar ratio and the temperature of 60 °C, the ethylene polymerization by Co4/MAO was conducted over different reaction times from 5 to 60 min (entries 9 and 11–14, Table 3). For the reaction time of 5 min (entry 11, Table 3), the observed activity of 22.0 × 106 g of PE (mol of Co)−1 h−1 was the highest value, likely indicating the rapid formation of the active species without the prolonged induction. Although the activity exhibits gradual decline with time, Co4/MAO still maintains the favourable value of 4.53 × 106 g of PE (mol of Co)−1 h−1 for 60 min, which suggests that there is still sufficient amount of active species. It was found that the molecular weight of polyethylene shows an increasing trend with the reaction time, which is depicted in Fig. 7.
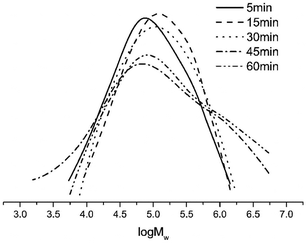 | ||
| Fig. 7 GPC curves of polyethylene (entries 9 and 11–14, Table 3). | ||
Regarding ethylene pressure, both the activity of Co4/MAO as well as the microstructure of the polymers obtained are greatly affected by its variations. In the experiment, the three different values of pressure, i.e., 1, 5 and 10 atm were selected and the polymerization was conducted at Al/Co = 2500 and T = 60 °C (entries 16, 15 and 9 in Table 3). Without surprise, higher ethylene pressure results in the polyethylene of higher molecular weight. Moreover, the higher activity is also achieved, which is attributed to the increased monomer concentration.35,55
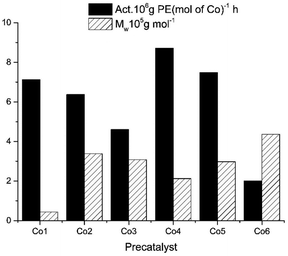
With the optimal conditions established for Co4/MAO (the Al/Co molar ratio of 2500, 60 °C and 10 atm), Co1–Co3, Co5 and Co6 were further investigated (entries 17–21, Table 3). The precatalysts displayed excellent activities ranging from 2.01 to 8.71 × 106 g of PE (mol of Co)−1 h−1. When these results are put together with those for Co4, the activities decrease in the following order: Co4 [2,4,6-tri(Me)] > Co5 [2,6-di(Et)-4-Me] > Co1 [2,6-di(Me)] > Co2 [2,6-di(Et)] > Co3 [2,6-di(i-Pr)] > Co6 [2,6-((p-MeOPh)2CH)2-4-MeC6H3]. As shown in Fig. 8, the catalytic activity of Co4 with a methyl group at the para position of the phenyl ring attached to the imine nitrogen atom is higher than that of Co1 with a hydrogen atom at the para position, indicating that the methyl electron-donating group has a positive influence on the catalytic activity. Since Co6 has the largest steric hindrance, it also displays the lowest activity. Regarding the molecular weight of the polymer, it ranges from 0.44 to 4.36 × 105 g mol−1, with Co6 [2,6-((p-MeOPh)2CH)2-4-MeC6H3] showing the highest value (entry 21, Table 3). This indicates that the large steric hindrance in the Co6/MAO system suppresses the termination reactions, which results in polyethylene of relatively high molecular weight.
3.2.3.2 Optimization of reaction conditions with Co1–Co6/MMAO. With the ethylene pressure established at 10 atm in most of the cases, the other conditions of polymerization were again optimized for Co4/MMAO. These are the Al/Co molar ratio, reaction temperature and reaction time. The results of polymerization tests are collected in Table 4. It was found that under comparable conditions, the Co4/MAO system typically displays higher activity in ethylene polymerization compared with Co4/MMAO.
| Entry | Precatalyst | Al/Co | T, °C | t, min | Yield, g | Activityb | Mwc | Mw/Mnc | Tmd, °C |
|---|---|---|---|---|---|---|---|---|---|
| a Conditions: 2.0 μmol of Co4; 100 mL toluene, 10 atm ethylene (except for items 15 and 16).b 106 g (PE) mol−1 (Co) h−1.c Determined by GPC; Mw: 105 g mol−1.d Determined by DSC.e 5 atm of ethylene.f 1 atm of ethylene. | |||||||||
| 1 | Co4 | 1500 | 30 | 30 | 4.24 | 4.24 | 3.27 | 2.5 | 135.6 |
| 2 | Co4 | 1750 | 30 | 30 | 4.96 | 4.96 | 2.73 | 3.0 | 135.6 |
| 3 | Co4 | 2000 | 30 | 30 | 5.43 | 5.43 | 2.79 | 2.7 | 135.6 |
| 4 | Co4 | 2250 | 30 | 30 | 4.35 | 4.35 | 2.86 | 3.4 | 135.6 |
| 5 | Co4 | 2500 | 30 | 30 | 3.82 | 3.82 | 2.43 | 2.8 | 135.2 |
| 6 | Co4 | 2000 | 20 | 30 | 4.23 | 4.23 | 2.55 | 2.9 | 135.0 |
| 7 | Co4 | 2000 | 40 | 30 | 4.52 | 4.52 | 1.61 | 3.3 | 134.6 |
| 8 | Co4 | 2000 | 50 | 30 | 4.01 | 4.01 | 1.01 | 2.4 | 134.8 |
| 9 | Co4 | 2000 | 60 | 30 | 3.92 | 3.92 | 0.55 | 2.3 | 134.9 |
| 10 | Co4 | 2000 | 70 | 30 | 3.23 | 3.23 | 0.47 | 2.0 | 134.7 |
| 11 | Co4 | 2000 | 30 | 5 | 3.19 | 19.14 | 1.57 | 2.0 | 135.3 |
| 12 | Co4 | 2000 | 30 | 15 | 3.96 | 7.43 | 1.70 | 2.1 | 135.8 |
| 13 | Co4 | 2000 | 30 | 45 | 7.04 | 4.69 | 3.25 | 4.6 | 136.2 |
| 14 | Co4 | 2000 | 30 | 60 | 8.03 | 4.02 | 3.95 | 3.8 | 136.3 |
| 15e | Co4 | 2000 | 30 | 30 | 2.78 | 2.78 | 1.89 | 1.8 | 135.9 |
| 16f | Co4 | 2000 | 30 | 30 | 1.28 | 1.28 | 1.44 | 2.4 | 134.8 |
| 17 | Co1 | 2000 | 30 | 30 | 4.31 | 4.31 | 0.64 | 3.0 | 134.9 |
| 18 | Co2 | 2000 | 30 | 30 | 3.88 | 3.88 | 3.30 | 2.6 | 135.3 |
| 19 | Co3 | 2000 | 30 | 30 | 4.61 | 4.61 | 3.67 | 3.8 | 135.5 |
| 20 | Co5 | 2000 | 30 | 30 | 3.36 | 3.36 | 3.89 | 2.1 | 135.7 |
| 21 | Co6 | 2000 | 30 | 30 | 1.12 | 1.12 | 4.49 | 3.6 | 135.8 |
First, the aluminium to cobalt molar ratio was varied from 1500 to 2500 and the reaction was carried out at 30 °C (entries 1–5, Table 4). The highest activity for Co4/MMAO, equal 5.43 × 106 g of PE (mol of Co)−1 h−1, was achieved at Al/Co = 2000 and the polymer of high molecular weight reaching 2.79 × 105 g mol−1 was produced. Polyethylene obtained with different values of Al/Co molar ratio showed slight variation in dispersity and no clear trend regarding the effect of the amount of cocatalyst on the molecular weight of the polyethylene could be determined, as illustrated in Fig. 9.
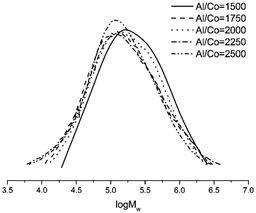 | ||
| Fig. 9 GPC curves of polyethylene (entries 1–5, Table 4). | ||
With the Al/Co molar ratio set at 2000, the polymerization temperature was varied between 20 and 70 °C (entries 3 and 6–10, Table 4). The activity of 5.43 × 106 g of PE (mol of Co)−1 h−1 (entry 3, Table 4) was achieved at 30 °C. With elevating the reaction temperature, the activity gradually decreased to a minimum at 70 °C (entry 10, Table 4). Clearly, at higher temperature the polyethylene of lower molecular weight is generated due to increase in termination reaction rates. As an example, the molecular weight of the polymer obtained at 30 °C is equal 2.79 × 105 g mol−1, while the polymer of molecular weight equal 0.47 × 105 g mol−1 is formed at 70 °C (entries 3 and 10, Table 4); see also Fig. 10.
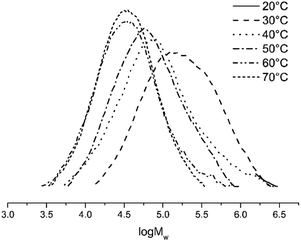 | ||
| Fig. 10 GPC curves of polyethylene (entries 3 and 6–10, Table 4). | ||
To evaluate the influence of the reaction time on the performance of the Co4/MMAO system, ethylene polymerization was conducted for 5, 15, 30, 45, and 60 min at 30 °C and the Al/Co molar ratio of 2000 (entries 3 and 11–14, Table 4). The maximum activity reaching 19.14 × 106 g of PE (mol of Co)−1 h−1 was observed at 5 min. Beyond this point, the activity continuously drops to reach the minimum of 4.02 × 106 g of PE (mol of Co)−1 h−1 at 60 min due to slow deactivation of the active species. Conversely, the polyethylene molecular weight slowly increases over time (Fig. 11).
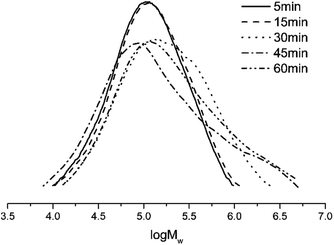 | ||
| Fig. 11 GPC curves of polyethylene (entries 3 and 11–14, Table 4). | ||
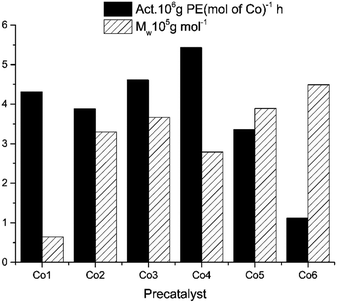
Polymerization tests performed under 5 and 1 atm of ethylene (entries 15 and 16, Table 4) indicate that low ethylene pressure leads to decline in the activity. Therefore the optimum reaction conditions for Co4/MMAO were established at the Al/Co molar ratio of 2000, temperature of 30 °C and ethylene pressure of 10 atm. Under the above optimal conditions, the study of the MMAO catalytic system was conducted for the Co1–Co3, Co5 and Co6 (entries 17–21, Table 4) and high activities ranging from 1.12 to 5.43 × 106 g of PE (mol of Co)−1 h−1 were found. The order of activity for the MMAO as a cocatalyst is now as follows: Co4 [2,4,6-tri(Me)] > Co3 [2,6-di(i-Pr)] > Co1 [2,6-di(Me)] > Co2 [2,6-di(Et)] > Co5 [2,6-di(Et)-4-Me] > Co6 [2,6-((p-MeOPh)2CH)2-4-MeC6H3] – see Fig. 12. Like in the case of MAO, Co4 exhibits the highest activity and the molecular weight of the polymer ranges from 0.64 to 4.49 × 105 g mol−1. The Co6 complex produces polyethylene of the highest molecular weight, which is equal 6.29 × 105 g mol−1. This fact indicates that the steric hindrance in Co6 also decreases the termination rate.20,33
To enable comparison with the previously synthesized unsymmetrical bis(arylimino)acenaphthylene-cobalt catalysts,2,25,27,28,33–36 the molecular weights and catalytic activities for B [–CH(Ph)2] and E [–CH(p-FPh)2] (Chart 1) are illustrated together with those corresponding to the current I system [–CH(p-MeOPh)2] in Fig. 13. It should be stressed that the properties of the precatalysts were evaluated in comparable conditions with MAO as a cocatalyst. Among these precatalysts, I [–CH(p-MeOPh)2] shows the highest activity, therefore it can be concluded that the introduction of the methoxy group is beneficial. In comparison, the precatalyst bearing benzhydryl group B [–CH(Ph)2] exhibits much lower activity and the E [–CH(p-FPh)2] produces polyethylene of the lowest molecular weight – see Fig. 13.
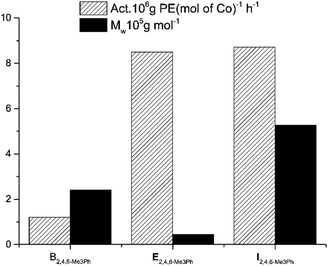 | ||
| Fig. 13 Comparison of the catalytic performance of the current system with the previously reported analogues (B, E and I, see Chart 1). | ||
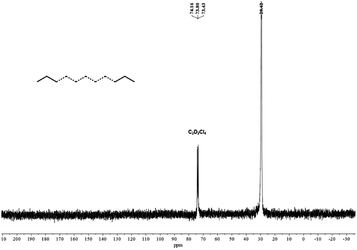 | ||
| Fig. 14 13C NMR spectrum of the polyethylene produced by Co4/MAO (in C2D2Cl4 at 100 °C; entry 9, Table 3). | ||
4 Conclusions
The series of cobalt-based ethylene polymerization precatalysts ligated by bis(imino)pyridine, whose one imine nitrogen atom is substituted with the 2,6-bis(4,4′-dimethoxybenzhydryl)-4-methyl phenyl group and the group attached to the other nitrogen atom is varied in terms of its steric and electronic profile, has been synthesized and characterized. On activation with MMAO or MAO, the complexes show high activity (up to 8.71 × 106 g of PE (mol of Co)−1 h−1) in the ethylene polymerization process and the resultant polyethylene exhibits the molecular weight of up to 5.27 × 105 g mol−1. In comparison with the literature, the introduction of the methoxy group enhances the catalytic activity of the corresponding complexes and increases the molecular weight of polyethylene obtained. This finding provides important guidance and direction for the design and development of catalysts in the future.Author contributions
Shi-Fang Yuan: project administration: conceptual design; writing – original draft. Luyao Wang: investigation: synthesis and characterizations of compounds. Yi Yan: investigation: characterization of polyethylene and polymeric property. Tian Liu: investigation: ethylene polymerization. Zygmunt Flisak: investigation: revised draft. Yanping Ma: investigation: ethylene polymerization and characterizations of polyethylenes. Wen-Hua Sun: project administration: conceptual design; manuscript.Conflicts of interest
All authors declared that they have no conflict of interest.Acknowledgements
The National Natural Science Foundation of China (No. 21871275) supported this work.Notes and references
- L. K. Johnson, C. M. Killian and M. Brookhart, J. Am. Chem. Soc., 1995, 117, 6414–6415 CrossRef CAS.
- B. L. Small, M. Brookhart and A. M. A. Bennett, J. Am. Chem. Soc., 1998, 120, 4049–4050 CrossRef CAS.
- G. J. P. Britovsek, V. C. Gibson, S. J. McTavish, G. A. Solan, A. J. P. White, D. J. Williams, B. S. Kimberley and P. J. Maddox, Chem. Commun., 1998, 849–850 RSC.
- V. C. Gibson, C. Redshaw and G. A. Solan, Chem. Rev., 2007, 107, 1745–1776 CrossRef CAS PubMed.
- C. Bianchini, G. Giambastiani, L. Luconi and A. Meli, Coord. Chem. Rev., 2010, 254, 431–455 CrossRef CAS.
- W. Zhang, W.-H. Sun and C. Redshaw, Dalton Trans., 2013, 42, 8988–8997 RSC.
- J. Ma, C. Feng, S. Wang, K. Q. Zhao, W.-H. Sun, C. Redshaw and G. A. Solan, Inorg. Chem. Front., 2014, 1, 14–34 RSC.
- Z. Flisak and W.-H. Sun, ACS Catal., 2015, 5, 4713–4724 CrossRef CAS.
- B. L. Small, Acc. Chem. Res., 2015, 48, 2599–2611 CrossRef CAS PubMed.
- F. Huang, Q. Xing, T. Liang, Z. Flisak, B. Ye, X. Hu, W. Yang and W.-H. Sun, Dalton Trans., 2014, 43, 16818–16829 RSC.
- Z. Wang, G. A. Solan, W. Zhang and W.-H. Sun, Coord. Chem. Rev., 2018, 363, 92–108 CrossRef CAS.
- W. Zhang, W. Chai, W.-H. Sun, X. Hu, C. Redshaw and X. Hao, Organometallics, 2012, 31, 5039–5048 CrossRef CAS.
- W.-H. Sun, S. Kong, W. Chai, T. Shiono, C. Redshaw, X. Hu, C. Guo and X. Hao, Appl. Catal., A, 2012, 447–448, 67–73 CrossRef CAS.
- J. Ba, S. Du, E. Yue, X. Hu, Z. Flisak and W.-H. Sun, RSC Adv., 2015, 5, 32720–32729 RSC.
- S. F. Yuan, Z. Fan, Y. Yan, Y. Ma, M. Han, T. Liang and W.-H. Sun, RSC Adv., 2020, 10, 43400–43411 RSC.
- Y. Zhang, H. Suo, F. Huang, T. Liang, X. Hu and W.-H. Sun, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 830–842 CrossRef CAS.
- F. Huang, W. Zhang, E. Yue, T. Liang, X. Hu and W.-H. Sun, Dalton Trans., 2016, 45, 657–666 RSC.
- F. Huang, W. Zhang, Y. Sun, X. Hu, G. A. Solan and W.-H. Sun, New J. Chem., 2016, 40, 8012–8023 RSC.
- V. K. Appukuttan, Y. Liu, B. C. Son, C. S. Ha, H. Suh and I. Kim, Organometallics, 2011, 30, 2285–2294 CrossRef CAS.
- S. Du, X. Wang, W. Zhang, Z. Flisak, Y. Sun and W.-H. Sun, Polym. Chem., 2016, 7, 4188–4197 RSC.
- S. Du, W. Zhang, E. Yue, F. Huang, T. Liang and W.-H. Sun, Eur. J. Inorg. Chem., 2016, 1748–1755 CrossRef CAS.
- Z. Wang, G. A. Solan, Q. Mahmood, Q. Liu, Y. Ma, X. Hao and W.-H. Sun, Organometallics, 2018, 37, 380–389 CrossRef CAS.
- M. Khoshsefat, Y. Ma and W.-H. Sun, Coord. Chem. Rev., 2021, 434, 213788 CrossRef CAS.
- J. Yu, H. Liu, W. Zhang, X. Hao and W.-H. Sun, Chem. Commun., 2011, 47, 3257–3259 RSC.
- J. Yu, W. Huang, L. Wang, C. Redshaw and W.-H. Sun, Dalton Trans., 2011, 40, 10209–10214 RSC.
- X. Cao, F. He, W. Zhao, Z. Cai, X. Hao, T. Shiono, C. Redshaw and W.-H. Sun, Polymer, 2012, 53, 1870–1880 CrossRef CAS.
- F. He, W. Zhao, X.-P. Cao, T. Liang, C. Redshaw and W.-H. Sun, J. Organomet. Chem., 2012, 713, 209–216 CrossRef CAS.
- Q. Mahmood, Y. P. Ma, X. Hao and W.-H. Sun, Appl. Organomet. Chem., 2019, 33, e4857 CrossRef.
- W. Zhao, J. Yu, S. Song, W. Yang, H. Liu, X. Hao, C. Redshaw and W.-H. Sun, Polymer, 2012, 53, 130–137 CrossRef CAS.
- J. Lai, W. Zhao, W. Yang, C. Redshaw, T. Liang, Y. Liu and W.-H. Sun, Polym. Chem., 2012, 3, 787–793 RSC.
- S. Wang, B. Li, T. Liang, C. Redshaw, Y. Li and W.-H. Sun, Dalton Trans., 2013, 42, 9188–9197 RSC.
- W.-H. Sun, W. Zhao, J. Yu, W. Zhang, X. Hao and C. Redshaw, Macromol. Chem. Phys., 2012, 213, 1266–1273 CrossRef CAS.
- S. Wang, W. Zhao, X. Hao, B. Li, C. Redshaw, Y. Li and W.-H. Sun, J. Organomet. Chem., 2013, 731, 78–84 CrossRef CAS.
- Q. Y. Zhang, Y. P. Ma, H. Y. Suo, G. A. Solan, T. L. Liang and W.-H. Sun, Appl. Organomet. Chem., 2019, 33, e5134 Search PubMed.
- R. D. Zhang, Y. P. Ma, M. Y. Han, G. A. Solan, Y. P. Ma, Y. Sun and W.-H. Sun, Appl. Organomet. Chem., 2019, 33, e5157 Search PubMed.
- M. Zada, L. W. Guo, Y. P. Ma, W. J. Zhang, Z. Flisak, Y. Sun and W.-H. Sun, Molecules, 2019, 24, 2007 CrossRef CAS PubMed.
- W. Zhang, S. Wang, S. Du, C. Y. Guo, X. Hao and W.-H. Sun, Macromol. Chem. Phys., 2014, 215, 1797–1809 CrossRef CAS.
- W. Zhao, E. Yue, X. Wang, W. Yang, Y. Chen, X. Hao, X. Cao and W.-H. Sun, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 988–996 CrossRef CAS.
- T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795 CrossRef PubMed.
- S. F. Yuan, Z. Fan, M. Han, Y. Yan, Z. Flisak, Y. Ma, T. Liang and W.-H. Sun, Eur. J. Inorg. Chem., 2021, 16, 1571–1580 CrossRef.
- T. Liu, Y.-P. Ma, G. A. Solan, T. Liang and W.-H. Sun, Appl. Organomet. Chem., 2021, 35, e6259 CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 Search PubMed.
- W.-H. Sun, W. Zhao, J. Yu, W. Zhang, X. Hao and C. Redshaw, Macromol. Chem. Phys., 2012, 213, 1266–1273 CrossRef CAS.
- S. Du, S. Kong, Q. Shi, J. Mao, C. Guo, J. Yi, T. Liang and W.-H. Sun, Organometallics, 2015, 34, 582–590 CrossRef CAS.
- M. Han, Q. Zhang, I. I. Oleynik, H. Suo, G. A. Solan, I. V. Oleynik, Y. Ma, T. Liang and W.-H. Sun, Dalton Trans., 2020, 49, 4774–4784 RSC.
- H. Suo, I. I. Oleynik, C. Bariashir, I. V. Oleynik, Z. Wang, G. A. Solan, Y. Ma, T. Liang and W.-H. Sun, Polymer, 2018, 149, 45–54 CrossRef CAS.
- Y. Yan, S. F. Yuan, M. Liu, G. A. Solan, Y. P. Ma, T. L. Liang and W.-H. Sun, Chin. J. Polym. Sci., 2022, 40, 266–279 CrossRef CAS.
- J. Guo, Z. Wang, W. Zhang, I. I. Oleynik, A. Vignesh, I. V. Oleynik, X. Hu, Y. Sun and W.-H. Sun, Molecules, 2019, 24, 1176 CrossRef PubMed.
- C. Bariashir, Z. Wang, H. Suo, M. Zada, G. A. Solan, Y. Ma, T. Liang and W.-H. Sun, Eur. Polym. J., 2019, 110, 240–251 CrossRef CAS.
- Y. Huang, R. Zhang, T. Liang, X. Hu, G. A. Solan and W.-H. Sun, Organometallics, 2019, 38, 1143–1150 CrossRef CAS.
- M. Zada, A. Vignesh, H. Suo, Y. Ma, H. Liu and W.-H. Sun, Mol. Catal., 2020, 492, 110981 CrossRef CAS.
- M. Han, Q. Zhang, I. I. Oleynik, H. Suo, I. V. Oleynik, G. Solan, Y. Ma, T. Liang and W.-H. Sun, Catalysts, 2020, 10, 1002 CrossRef CAS.
- S. Zhang, W.-H. Sun, T. Xiao and X. Hao, Organometallics, 2010, 29, 1168–1173 CrossRef.
- Q. Mahmood, E. Yue, J. Guo, W. Zhang, Y. Ma, X. Hao and W.-H. Sun, Polymer, 2018, 159, 124–137 CrossRef CAS.
Footnote |
| † CCDC 2124397 (Co2), 2124398 (Co4). For crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2ra01547a |
| This journal is © The Royal Society of Chemistry 2022 |