Open Access Article
Open Access ArticleRetracted Article: The anti-Alzheimer potential of Tamarindus indica: an in vivo investigation supported by in vitro and in silico approaches†
Abeer H. Elmaidomy‡
a,
Usama Ramadan Abdelmohsen‡ *bc,
Faisal Alsenani
*bc,
Faisal Alsenani d,
Hanan F. Alye,
Shams Gamal Eldin Shamse,
Eman A. Younise,
Kawkab A. Ahmedf,
Ahmed M. Sayed
d,
Hanan F. Alye,
Shams Gamal Eldin Shamse,
Eman A. Younise,
Kawkab A. Ahmedf,
Ahmed M. Sayed g,
Asmaa I. Owisah,
Naglaa Afifia and
Dalia El Amira
g,
Asmaa I. Owisah,
Naglaa Afifia and
Dalia El Amira
aDepartment of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University, Beni-Suef 62514, Egypt
bDepartment of Pharmacognosy, Faculty of Pharmacy, Minia University, Minia 61519, Egypt. E-mail: usama.ramadan@mu.edu.eg
cDepartment of Pharmacognosy, Faculty of Pharmacy, Deraya University, 7 Universities Zone, New Minia 61111, Egypt
dDepartment of Pharmacognosy, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia
eTherapeutic Chemistry Department, National Research Centre (NRC), El-Bouth St., P.O. 12622 Cairo, Egypt
fDepartment of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt
gDepartment of Pharmacognosy, Faculty of Pharmacy, Nahda University, Beni-Suef, 62513, Egypt
hDepartment of Pharmacognosy, Faculty of Pharmacy, Heliopolis University for Sustainable Development, Cairo, Egypt
First published on 19th April 2022
Abstract
Tamarindus indica Linn. (Tamarind, F. Fabaceae) is one of the most widely consumed fruits in the world. A crude extract and different fractions of T. indica (using n-hexane, dichloromethane, ethyl acetate, and n-butanol) were evaluated in vitro with respect to their DPPH scavenging and AchE inhibition activities. The results showed that the dichloromethane and ethyl acetate fractions showed the highest antioxidant activities, with 84.78 and 86.96% DPPH scavenging at 0.10 μg mL−1. The n-hexane, dichloromethane, and ethyl acetate fractions inhibited AchE activity in a dose-dependent manner, and the n-hexane fraction showed the highest inhibition at 20 μg mL−1. The results were confirmed by using n-hexane, dichloromethane, and ethyl acetate fractions in vivo to regress the neurodegenerative features of Alzheimer's dementia in an aluminum-intoxicated rat model. Phytochemical investigations of those three fractions afforded two new diphenyl ether derivative compounds 1–2, along with five known ones (3–7). The structures of the isolated compounds were confirmed via 1D and 2D NMR and HRESIMS analyses. The isolated compounds were subjected to extensive in silico-based investigations to putatively highlight the most probable compounds responsible for the anti-Alzheimer activity of T. indica. Inverse docking studies followed by molecular dynamics simulation (MDS) and binding free energy (ΔG) investigations suggested that both compounds 1 and 2 could be promising AchE inhibitors. The results presented in this study may provide potential dietary supplements for the management of Alzheimer's disease.
1. Introduction
Alzheimer's disease (AD) is the most common neurodegenerative disorder affecting the elderly with cumulative neurocognitive decline and memory impairment (dementia) caused by multiple pathogenic factors, involving amyloid-β plaques, neurofibrillary tangles (NFTs), cholinergic dysfunction and oxidative stress.1 Accounting for about 60–80% of dementia cases in the elderly populations, AD has become one of the major global health challenges of the century.2 During ageing, humans and animals show a decline in motor and cognitive functions that could be attributed to increased susceptibility to the cumulative effects of oxidative stress and inflammation.3 The glutamatergic pyramidal neurons in the brain are highly vulnerable to deterioration in both age-associated and AD-induced cognitive impairment.4 To date, only symptomatic remedies can be applied for AD, all seeking to counterbalance the neurotransmitter disorder. Three cholinesterase inhibitors are presently applicable and have been accepted for the treatment of mild to moderate AD. The current symptomatic treatment of mild-to-moderate AD patients is based on drugs such as donepezil, rivastigmine, galantamine and memantine, which help in alleviating the clinical symptoms of AD, but are associated with side effects and show little potential for AD treatment.5 A significant remedial choice useable for moderate to severe AD is memantine, an N-methyl-D-aspartate receptor noncompetitive antagonist. Methods efficient at holding or at least adequately adapting the course of AD, submitted as ‘disease-modifying’ cures, are still under comprehensive investigation. To stop the advance of the disorder, they must impede the pathogenic steps related to clinical manifestations, covering the removal of extracellular amyloid β plaques and the development of intracellular neurofibrillary tangles, oxidative damage, inflammation, cholesterol metabolism, and iron deregulation.6On the other hand, AD is a typical example of a complex multifactorial disease; which means that the ‘one change, one disease, one drug’ strategy is no longer applicable.7 There is a high demand for a search for new drugs of natural origin, directed at protection from this neurodegenerative disease, or even at its prevention, slowing down and/or halting the progression of the disease and deterioration in its early stages, which may reduce the side effects of clinically used drugs and hence increase healthy ageing.8,9
Research on plants with antioxidative potential has become a topic of increasing interest considering the important roles of antioxidative compounds in the treatment and prevention of pathologies linked to oxidative stress that is generated by free radicals.10 Indeed, antioxidants help to neutralize free radicals which can damage cellular membranes and interact with the genetic material of cells.10 Natural products provide great opportunities to weaken the progress and symptoms of AD.11,12 Of particular interest are those plants that contain flavonoids, lignans, polyphenols, tannins, sterols, triterpenes, and alkaloids and that exhibit antioxidant, anti-inflammatory, anticholinesterase, and anti-amyloidogenic actions, such as Curcuma longa, Bacopa monnieri, Convolvulus pluricaulis, Centella asiatica, Ginkgo biloba, Zingiber officinale, and Allium sativum, or plant-derived natural products such as quercetin, epigallocatechin-3-gallate, berberine, resveratrol, huperzine A, rosmarinic acid, and luteolin.11,12
Tamarind is a plant native to Eastern Africa, including parts of Madagascar. The tree grows wild throughout Sudan.13,14 Its fruits were well known to the ancient Egyptians and to the Greeks in the fourth century. Now tamarind trees have been introduced into most tropical areas around the world, particularly India, south-east Asia, tropical America, the Pacific Islands, and the Caribbean.13,14 The fruit pulp, seeds, flowers, and leaves are edible. The fruit pulp has multifarious food uses. It is eaten fresh or raw or as a condiment or a spice in various sauces and food dishes. Tamarind is available in specialty food stores worldwide in pod form or as a paste or concentrate.13,14 Tamarind exhibits a wide range of pharmacological activities, such as antioxidant,15 hypoglycaemic,16 antihyperlipidemic,17 antimicrobial,18 immunomodulatory,19 antivenom,20 antiplatelet,21 anti-inflammatory,22 antiemetic,23 antinociceptive,24 spasmolytic,25 anti-aging,26 UVB-protection and corneal wound healing,27 antitumor,28 and proteinase inhibitory29 activities.
Aluminium (Al) represents an important risk factor for several age-associated neurodegenerative disorders,30 including AD.31 Aluminium chloride (AlCl3) is a neurotoxicant that accumulates in the brain and negatively affects ionic, cholinergic, and dopaminergic neurotransmission.32 The present study aims to highlight the possible beneficial and therapeutic effects of different fractions of tamarind (n-hexane, dichloromethane, and ethyl acetate) in the regression of the neurodegenerative features of Alzheimer's dementia in an Al-intoxicated rat model.
2. Materials and methods
2.1 Plant material, chemicals, and reagents
T. indica pulp was purchased from a market (SH-Shahd, 6224007730164, India). T. indica was kindly identified by Dr Abd El-Halim A. Mohammed (Horticultural Research Institute, Department of Flora and Phytotaxonomy Research, Dokki, Cairo, Egypt). A voucher specimen (2021-BuPD 77) was deposited at the Department of Pharmacognosy, Faculty of Pharmacy, Beni-Suef University, Egypt.The solvents used in this work, including n-hexane (n-hex., boiling point b.p. 60–80 °C), dichloromethane (DCM), ethyl acetate (EtOAC), n-butanol (n-but.), ethanol and methanol (MeOH), were purchased from El-Nasr Company for Pharmaceuticals and Chemicals (Egypt). Deuterated solvents used for spectroscopic analyses were purchased from Sigma-Aldrich (Saint Louis, Missouri, USA), including methanol-d4 (CD3OD-d4) and dimethyl sulfoxide (DMSO-d6). Column chromatography (CC) was performed using silica gel 60 (63–200 μm, E. Merck, Sigma-Aldrich) in a Sephadex LH-20 (0.25–0.1 mm, GE Healthcare, Sigma-Aldrich). While silica gel GF254 for thin-layer chromatography (TLC) (El-Nasr Company for Pharmaceuticals and Chemicals, Egypt) was employed for vacuum liquid chromatography (VLC). Thin-layer chromatography (TLC) was carried out using pre-coated silica gel 60 GF254 plates (E. Merck, Darmstadt, Germany; 20 × 20 cm, 0.25 mm in thickness). Spots were visualized by spraying with para-anisaldehyde (PAA) reagent (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 absolute EtOH
0.5 absolute EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sulfuric acid
sulfuric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G.A.A.
G.A.A.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) para-anisaldehyde), followed by heating at 110 °C.33 For the biological study, donepezil, reagents, and all kits were purchased from the Sigma Chemical Company (USA), whereas aluminum chloride (AlCl3) was purchased from CDH, India.
para-anisaldehyde), followed by heating at 110 °C.33 For the biological study, donepezil, reagents, and all kits were purchased from the Sigma Chemical Company (USA), whereas aluminum chloride (AlCl3) was purchased from CDH, India.
2.2 Spectral analyses
Proton 1H and Distortionless Enhancement by Polarization Transfer-Q (DEPT-Q) 13C NMR spectra were recorded at 400 and 100 MHz, respectively. Tetramethylsilane (TMS) was used as an internal standard in methanol-d4 (CD3OD-d4) and dimethyl sulfoxide (DMSO-d6), using the residual solvent peaks (δH = 3.34, 4.78 and δC = 49.9; and δH = 2.50 and δC = 39.5) as references. Measurements were performed in a Bruker Advance III 400 MHz with a BBFO smart probe and a Bruker 400 MHz EON nitrogen-free magnet (Bruker AG, Billerica, MA, USA). Carbon multiplicities were determined using a DEPT-Q experiment. The ultraviolet radiation (UV) spectrum in methanol was obtained using a Shimadzu UV 2401PC spectrophotometer (Shimadzu Corporation – UV-2401PC/UV-2501PC, Kyoto, Japan). Infrared (IR) spectra were measured using a Jasco FTIR 300E infrared spectrophotometer. HRESIMS data were obtained using an Acquity ultra performance liquid chromatography system coupled to a Synapt G2 HDMS quadrupole time-of-flight hybrid mass spectrometer (Waters, Milford, MA, USA).2.3 Extraction and fractionation of Tamarindus indica pulp
T. indica pulp (6 kg) was extracted by maceration,34,35 using 70% ethanol (15 L, 2×, seven days each) at room temperature, and concentrated under vacuum at 45 °C using a rotary evaporator (Buchi Rotavapor R-300, Cole-Parmer, Vernon Hills, IL, USA) to afford 1.5 kg of crude extract. The dry extract was suspended in 500 mL of distilled water (H2O), and successively portioned with solvents of different polarities (n-hex., DCM, EtOAc, and n-but.).34,35 The organic phase in each step was separately evaporated under reduced pressure to afford the corresponding fractions I (20.0 g), II (23.0 g), III (20.0 g) and IV (200.0 g), respectively, while the remaining mother liquor was then concentrated down to give the aqueous fraction (V). All resulting fractions were kept at 4 °C for biological and phytochemical investigations.2.4 In vitro DPPH radical scavenging activity assays of Tamarindus indica
The radical scavenging activity of T. indica crude extract and different fractions (n-hexane, dichloromethane, and ethyl acetate), were tested using a stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay.36,37 In brief, a 1 mL solution of the tested extract at different concentrations (0.01, 0.05, and 0.1 μg mL−1 in absolute ethanol) was mixed with 2 mL of freshly prepared DPPH solution (20 μg mL−1 in absolute ethanol), followed by incubation at room temperature in the dark for 30 min. The absorbance was measured at λ517 (nm) using a UV-Vis Jenway 6003 spectrophotometer. Ascorbic acid was used as a positive control, and absolute ethanol was used as a blank. The DPPH radical scavenging activity was calculated according to the following equation:2.5 In vitro estimation of the cholinesterase activity of Tamarindus indica
The cholinesterase activity of T. indica crude extract and different fractions (n-hexane, dichloromethane, and ethyl acetate), were estimated according to the manufacturer's instructions.38 In brief, a 0.20 mL solution of the tested extract at different concentrations (10, and 20 μg mL−1 in absolute ethanol) was mixed with 3 mL of distilled water. Then 3 mL of phosphate solution was added. The pH was measured using a pH meter (pH-1). After that, 0.12 mL of 7.5%, acetylcholine iodide solution was added. The mixture was then transferred to a water bath at 37 °C, and incubated for 30 min. Then, the pH value of the mixture (pH-2) was measured. Then, the difference between pH-1 and pH-2, i.e., the change in pH within 30 min was calculated. The difference indicates the level of cholinesterase activity in the sample, i.e., ΔpH/30 min = pH-1 − pH-2/(pH of blank*).2.6 Anti-Alzheimer activity
The provided microtiter plate was pre-coated with ACh. During the reaction, ACh in the sample or standard competes with a fixed amount of ACh on the solid phase support for sites on the biotinylated detection antibody specific to ACh. Excess conjugate and unbound sample or standard were washed from the plate, and HRP-streptavidin (SABC) was added to each microplate well and incubated. Then a TMB substrate solution was added to each well. The enzyme–substrate reaction was terminated by the addition of a sulfuric acid solution and the color change was measured at 450 nm. The ACh concentration was then determined by comparing the O.D. of the samples to the standard curve. The brain/serum ACh level was measured by ELISA.
The concentrations of brain adrenaline (A), noradrenaline (NA), dopamine (DA), and serotonin (5-hydroxytryptamine; 5-HT) were determined using the high performance liquid chromatography with electrochemical detection (HPLC-ED) technique according to Giday et al. 2009.52 Tau protein and amyloid-β concentrations were assayed using ELISA kits according to the manufacturer's instructions.52 A primary antibody was coated on a plate, and samples and standard were added into the wells for the first reaction. After the reaction, HRP-conjugated secondary antibodies were added into the wells for a second reaction. After washing away the unbound secondary antibodies, tetra methyl benzidine (TMB) was added to the wells and the color developed. For tau protein, a “sandwich” enzyme-linked immunosorbent assay (ELISA) was used, where the target protein (antigen) is bound in a “sandwich” format by the primary capture antibodies coated onto each well-bottom and the secondary detection antibodies are added subsequently by the investigator. The capture antibodies coated on the bottom of each well are specific for a particular epitope on MAPτ (microtubule associated protein tau/tau protein) while the user-added detection antibodies bind to epitopes on the captured target protein. A series of washing steps must be performed to ensure the elimination of non-specific binding between proteins and other proteins or to the solid phase. After incubation and “sandwiching” of the target antigen, a peroxidase enzyme is conjugated to the constant heavy chain of the secondary antibody (either covalently or via avidin/streptavidin–biotin interactions), allowing for a colorimetric reaction to ensue upon substrate addition. When the substrate TMB (3,3′,5,5′-tetramethylbenzidine) is added, the reaction catalyzed by peroxidase yields a blue color that is representative of the antigen concentration. Upon sufficient color development, the reaction can be terminated through the addition of a stop solution (2 N sulfuric acid) when the color of the solution will turn yellow. The absorbance of each well can then be read with a spectrophotometer, allowing for the generation of a standard curve and subsequent determination of protein concentration.
TAC was determined in serum by the colorimetric assay method of Koracevic et al. 2001.53 ABTS (2,2′-azino-bis[3-ethylbenzthiazoline-6-sulfonic acid]) was incubated with metmyoglobin and H2O2 to produce green radical cation ABTS+. Antioxidants suppressed the color production, thereby reducing the intensity of the color that was proportional to the concentration of antioxidants, measured at 600 nm with a microplate reader. Trolox served as a standard or control antioxidant.
All animal groups were subjected to a determination of non-enzymatic glutathione (GSH) reduction according to the method of Beutler et al., 1963,54 where the method was based on the reduction of 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) with glutathione (GSH) to produce a yellow compound. The reduced chromogen is directly proportional to GSH concentration and its absorbance can be measured at 405 nm. For malondialdehyde (MDA),55 thiobarbituric acid (TBA) reacts with malondialdhyde (MDA) in an acidic medium at a temperature of 95 °C for 30 min to form thiobarbituric acid reactive product. The absorbance of the resultant pink product can be measured at 534 nm. For the enzymatic antioxidant superoxide dismutase (SOD),56 a superoxide dismutase activity assay uses a xanthine/xanthine oxidase (XOD) system to generate superoxide anions and a chromagen to produce a water-soluble formazan dye upon reduction by superoxide anions. The superoxide dismutase activity is determined as the inhibition or reduction of chromagen. Catalase (CAT), in brain tissues was determined using standard diagnostic kits according to the manufacturer's instructions.57 Catalase reacts with a known quantity of H2O2. The reaction is stopped after exactly one minute with a catalase inhibitor. In the presence of peroxidase (HRP) the remaining H2O2 reacts with 3,5-dichloro-2-hydroxybenzene sulfonic acid (DHBS) and 4-aminophenazone (AAP) to form a chromophore with a color intensity inversely proportional to the amount of catalase in the original sample.
2.7 Histopathological studies
Brain specimens were collected from all rats in a group and then fixed in 10% neutral buffered formalin. Paraffin sections of 5 μm thickness were prepared and stained with hematoxylin and eosin (H&E) for histopathological examination with a light microscope.58 An experienced pathologist performed the histological analysis without knowing the treatments. Neuropathological damage in the cerebral cortex and hippocampus were graded from 0 to 4 as follows: 0 indicated no changes; 1 indicated <10% of the area was affected; 2 indicated 20–30% of the area was affected; 3 indicated 40–60% of the area was affected and 4 indicated >60% of the area was affected.592.8 Immunohistochemistry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) (Dako, N-series ready-to-use primary antibody). The immune staining was amplified and completed by horseradish peroxidase complex (Dako, REAL™ EnVision™/HRP, Mouse ENV). Sections were developed and visualized using 3,3-diaminobenzidine (Dako, REAL™ DAB + chromogen). The substrate system produced a crisp brown product, at the site of the target antigen. Sections were counterstained with haematoxylin, then dehydrated in alcohol, cleaned in xylene and cover slipped for microscopic examination.60 Quantification of GFAP was estimated by measuring the area % expression from 5 randomly chosen fields in each section and averaged using image analysis software (ImageJ, version 1.46a, NIH, Bethesda, MD, USA).
500) (Dako, N-series ready-to-use primary antibody). The immune staining was amplified and completed by horseradish peroxidase complex (Dako, REAL™ EnVision™/HRP, Mouse ENV). Sections were developed and visualized using 3,3-diaminobenzidine (Dako, REAL™ DAB + chromogen). The substrate system produced a crisp brown product, at the site of the target antigen. Sections were counterstained with haematoxylin, then dehydrated in alcohol, cleaned in xylene and cover slipped for microscopic examination.60 Quantification of GFAP was estimated by measuring the area % expression from 5 randomly chosen fields in each section and averaged using image analysis software (ImageJ, version 1.46a, NIH, Bethesda, MD, USA).2.9 Isolation and purification of compounds
Fraction I (5 g) was subjected to normal VLC fractionation using a silica gel GF254 column (6 × 30 cm, 100 g). Elution was performed using n-hex.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc gradient mixtures in order of increasing polarity (0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 60, 80 and 100%, 250 mL of each). The effluents from the column were collected in fractions (150 mL each); and each collected fraction was concentrated and monitored by TLC using the system n-hex.
EtOAc gradient mixtures in order of increasing polarity (0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 60, 80 and 100%, 250 mL of each). The effluents from the column were collected in fractions (150 mL each); and each collected fraction was concentrated and monitored by TLC using the system n-hex.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 8
EtOAc 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and PAA reagent. Similar fractions were grouped and concentrated under reduced pressure to provide two sub-fractions (I1–I2). Subfractions I1 and I2 (0.50 g) were further fractionated separately on silica gel 60 (100 × 1 cm, 50 g). Elution was performed using n-hex.
2 and PAA reagent. Similar fractions were grouped and concentrated under reduced pressure to provide two sub-fractions (I1–I2). Subfractions I1 and I2 (0.50 g) were further fractionated separately on silica gel 60 (100 × 1 cm, 50 g). Elution was performed using n-hex.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc gradient mixtures in the order of increasing polarity (0, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10%, 1 L of each), to afford compounds 6 (10 mg), and 7 (12 mg), respectively.
EtOAc gradient mixtures in the order of increasing polarity (0, 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10%, 1 L of each), to afford compounds 6 (10 mg), and 7 (12 mg), respectively.
Fraction II (3 g) was subjected to normal VLC fractionation on a silica gel column (6 × 30 cm, 50 g). Elution was performed using DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH gradient mixtures in the order of increasing polarity (0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 60, 80 and 100%, 1 L of each). The effluents were collected in fractions (100 mL of each); each fraction was concentrated and monitored by TLC using the system DCM
MeOH gradient mixtures in the order of increasing polarity (0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 60, 80 and 100%, 1 L of each). The effluents were collected in fractions (100 mL of each); each fraction was concentrated and monitored by TLC using the system DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH 9
MeOH 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and PAA reagent. Similar fractions were grouped and concentrated under reduced pressure to provide two sub-fractions (II1–II2). Subfractions II1 and II2 (1.0 g) were further separately further purified on a Sephadex LH20 column (0.25–0.1 mm, 100 × 0.5 cm, 100 gm) which was eluted with MeOH to afford compounds 3 (30 mg) and 4 (50 mg).
1 and PAA reagent. Similar fractions were grouped and concentrated under reduced pressure to provide two sub-fractions (II1–II2). Subfractions II1 and II2 (1.0 g) were further separately further purified on a Sephadex LH20 column (0.25–0.1 mm, 100 × 0.5 cm, 100 gm) which was eluted with MeOH to afford compounds 3 (30 mg) and 4 (50 mg).
Fraction III (6.0 g) was subjected to normal VLC fractionation on a silica gel column (6 × 30 cm, 50 g). Elution was performed using DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH gradient mixtures in the order of increasing polarity (0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 60, 80 and 100%, 1 L of each). The effluents were collected in fractions (100 mL of each); each fraction was concentrated and monitored by TLC using the system DCM
MeOH gradient mixtures in the order of increasing polarity (0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 60, 80 and 100%, 1 L of each). The effluents were collected in fractions (100 mL of each); each fraction was concentrated and monitored by TLC using the system DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH 8
MeOH 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and PAA reagent. Similar fractions were grouped and concentrated under reduced pressure to provide three sub-fractions (III1–III3), and each fraction was separately further purified on a Sephadex LH20 column (0.25–0.1 mm, 100 × 0.5 cm, 100 gm) which was eluted with MeOH to afford compounds 1 (30 mg), 2 (50 mg), and 5 (70 mg), respectively.
2 and PAA reagent. Similar fractions were grouped and concentrated under reduced pressure to provide three sub-fractions (III1–III3), and each fraction was separately further purified on a Sephadex LH20 column (0.25–0.1 mm, 100 × 0.5 cm, 100 gm) which was eluted with MeOH to afford compounds 1 (30 mg), 2 (50 mg), and 5 (70 mg), respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 280 (5.5), 270 (6.0), 300 (4.5) nm]; IR νmax (KBr) 3600, 3500, 3400, 3100, 1700, 1300, 1300, 1000, 850, 700 cm−1; NMR data; see Table 1; HRESIMS m/z 277.0712 [M + H]+ (calc. for C14H13O6, 277.0712).
ε) 280 (5.5), 270 (6.0), 300 (4.5) nm]; IR νmax (KBr) 3600, 3500, 3400, 3100, 1700, 1300, 1300, 1000, 850, 700 cm−1; NMR data; see Table 1; HRESIMS m/z 277.0712 [M + H]+ (calc. for C14H13O6, 277.0712).
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| a qC, quaternary; CH, methine; CH3, methyl; δ, ppm. | ||||
| 1 | 172.8, qC | 172.8, qC | ||
| 2 | 122.6, qC | 122.6, qC | ||
| 3 | 115.3, CH | 6.88, d (2.4) | 115.3, CH | 6.89, d (2.4) |
| 4 | 146.2, qC | 149.7, qC | ||
| 5 | 163.1, qC | 149.7, qC | ||
| 6 | 113.6, CH | 7.13, d (8.0) | 116.5, CH | 6.88, d (8.0) |
| 7 | 119.0, CH | 7.33, dd (2.4, 8.0) | 126.9, CH | 7.83, dd (2.4, 8.0) |
| 1′ | 149.7, qC | 157.8, qC | ||
| 2′ | 104.9, CH | 6.61, dd (2.4, 3.1) | 113.6, CH | 7.13, dd (2.4, 3.1) |
| 3′ | 157.8, qC | 163.1, qC | ||
| 4′ | 102.8, CH | 6.93, dd (2.4, 3.1) | 102.8, CH | 6.93, dd (2.4, 3.1) |
| 5′ | 157.8, qC | 157.8, qC | ||
| 6′ | 104.9, CH | 6.61, dd (2.4, 3.1) | 104.9, CH | 6.61, dd (2.4, 3.1) |
| –OCH3 | 52.1, CH3 | 3.81, s | 52.1, CH3 | 3.81, s |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 280 (5.5), 270 (6.0), 300 (4.5) nm]; IR νmax (KBr) 3600, 3500, 3400, 3100, 1700, 1300, 1300, 1000, 850, 700 cm−1; NMR data; see Table 1; HRESIMS m/z 277.0712 [M + H]+ (calc. for C14H13O6, 277.0712).
ε) 280 (5.5), 270 (6.0), 300 (4.5) nm]; IR νmax (KBr) 3600, 3500, 3400, 3100, 1700, 1300, 1300, 1000, 850, 700 cm−1; NMR data; see Table 1; HRESIMS m/z 277.0712 [M + H]+ (calc. for C14H13O6, 277.0712).2.10 Molecular modeling
2.11 Statistical analysis
All data sets were expressed as mean ± SD. The data were analyzed statistically for normal distribution using one-way analysis of variance (ANOVA) software and Co-state for Windows, version 8. Values of different letters are statistically significant at p < 0.05.3. Results and discussion
3.1 In vitro DPPH radical scavenging activity assay of Tamarindus indica
The radical scavenging activity of T. indica crude extract and different fractions (n-hexane, dichloromethane, ethyl acetate, and n-butanol) were tested using the stable radical DPPH assay.36 The results showed that dichloromethane and ethyl acetate showed the significantly highest percentages of scavenging activity for DPPH in a dose-dependent manner compared to ascorbic acid (Table 2). While the crude extract showed significantly low DPPH scavenging activity percentages relative to ascorbic acid (Table 2).| Inhibition (%) | 0.01 μg mL−1 | 0.05 μg mL−1 | 0.10 μg mL−1 |
|---|---|---|---|
| a Data are expressed as mean ± SD (n = 3). Groups with similar letters are not significantly different, while those with different letters are significantly different at p ≤ 0.05. | |||
| Crude extract | 19.57 ± 0.81f | 43.48 ± 2.20d | 56.52 ± 2.90e |
| n-Hexane | 15.20 ± 1.80f | 13.04 ± 1.90g | 14.35 ± 1.00g |
| Dichloromethane | 4.35 ± 0.80h | 47.83 ± 3.78c | 84.78 ± 6.89a |
| Ethyl acetate | 32.61 ± 2.80d | 82.61 ± 5.90a | 86.96 ± 5.45b |
| n-Butanol | 33.61 ± 2.80d | 75.61 ± 5.90a | 70.61 ± 5.90a |
| Ascorbic acid | 81.00 ± 5.51a | 89.0 0 ± 5.80b | 91.00 ± 6.80b |
3.2 In vitro estimation of the cholinesterase activity of Tamarindus indica
The cholinesterase activity of T. indica crude extract and different fractions (n-hexane, dichloromethane, ethyl acetate, and n-butanol), were estimated.38 The results showed that all fractions including crude extract, n-hexane, dichloromethane, ethyl acetate and n-butanol, inhibited AchE activity in a dose-dependent manner (Table 3), where n-hexane showed the highest significant inhibition activity of AchE at 20 μg mL−1, followed by dichloromethane, ethyl acetate and finally n-butanol compared to the donepezil standard drug.| 10 μg mL−1 | 20 μg mL−1 | |
|---|---|---|
| a Data are expressed as mean ± SD (n = 3). Groups with similar letters are not significantly different, while those with different letters are significantly different at p ≤ 0.05. | ||
| Crude extract | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 989 ± 121.80d 989 ± 121.80d |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 ± 133.00c 710 ± 133.00c |
| n-Hexane | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 559 ± 150.00e 559 ± 150.00e |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654 ± 169.00f 654 ± 169.00f |
| Dichloromethane | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 ± 133.00b 840 ± 133.00b |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 279 ± 100.00g 279 ± 100.00g |
| Ethyl acetate | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 135.00d 000 ± 135.00d |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ± 1222.00a 800 ± 1222.00a |
| n-Butanol | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 135.00d 000 ± 135.00d |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 279 ± 100.00a 279 ± 100.00a |
| Donepezil | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 ± 119.11a 420 ± 119.11a |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869 ± 119.11a 869 ± 119.11a |
3.3 Anti-Alzheimer activity
Depending on in vitro DPPH radical scavenging and cholinesterase activities assays of T. indica crude extract and different fractions (n-hexane, dichloromethane, ethyl acetate, and n-butanol), n-hexane, dichloromethane, and ethyl acetate fractions were used for further in vivo investigations to estimate the anti-Alzheimer activity.3.4 Acute toxicity study
Serial concentrations of different fractions (n-hexane, dichloromethane, and ethyl acetate) were used for the determination of acute toxicity, from 500, 1000, 2000, 3000, 4000, and 5000 mg per kg b. wt, for 4 rats in each group (a total 24 rats for all groups). No mortality and no toxicity signs were observed up to 5000 mg per kg b. wt over 48 h. The selected dose used was 500 mg per kg b. wt.443.5 Potential effects of Tamarindus indica extracts in a T-maze test
Various T. indica fractions (n-hexane, dichloromethane, and ethyl acetate) were screened for their ability as anti-Alzheimer treatments at 500 mg per kg b. wt using a T-maze test. The T-maze test results demonstrated a significant increase in time (seconds) taken by rats to reach the food in the T-maze for the AlCl3-neurotoxicant rats (AD-group), denoting deteriorated neurocognitive function, with a percentage increase of 169.29% (Table 4). Whereas AD-groups treated with either of the different T. indica fractions (n-hexane or dichloromethane) showed a significant decrease in the time taken by the rats to reach food in the T-maze, in comparison to the AD-induced group, indicating improved cognitive abilities, with the percentage improvement reaching 116.14 and 101.10% (6 weeks of treatment), respectively. Additionally, the T. indica treated group treated with the ethyl acetate fraction showed the most significant decrease in time to achieve the task, compared to the AD-induced group, with the percentage improvement reaching 155.30% (6 weeks of treatment) (Table 4). Donepezil was used as a positive control.| Baseline | Induction (two months) | Treatment (6 weeks) | |
|---|---|---|---|
| a Data are expressed in seconds as mean ± SD (n = 8). Groups with similar letters are not significantly different, while those with different letters are significantly different at p ≤ 0.05. | |||
| Control | 16.00 ± 1.00a | 17.00 ± 1.00a | 16.60 ± 0.34a |
| AlCl3-AD | 19.40 ± 0.67b | 45.78 ± 0.67c | — |
| % Change | +21.25 | +169.29 | — |
| n-Hexane | — | — | 26.50 ± 1.65d |
| % Improvement | 116.14 | ||
| Dichloromethane | — | — | 29.00 ± 1.71d |
| % Improvement | 101.10 | ||
| Ethyl acetate | — | — | 20.00 ± 1.22e |
| % Improvement | 155.30 | ||
| Donepezil drug | — | — | 25.08 ± 1.60d |
| % Improvement | 124.69 | ||
3.6 Potential effects of Tamarindus indica extracts in a beam balance test
Beam balance test results showed that AlCl3 caused a significant deterioration in brain cognitive functions for AlCl3-neurotoxicant rats (AD-group) (Table 5) with a percentage decrease of 80%. However, treatment of rats with donepezil or different fractions of Tamarindus indica (n-hexane, dichloromethane, and ethyl acetate) resulted in an improvement in behavioral status, which was represented by improved motor coordination and improved cognition, with percentage improvements reaching 68.18, 63.64, 70.36%, respectively, compared to those treated with the standard drug donepezil which recorded 72.73% (6 weeks of treatment).| Baseline | Induction (two months) | Treatment (6 weeks) | |
|---|---|---|---|
| a Data are expressed in seconds as mean ± SD (n = 8). Groups with similar letters are not significantly different, while those with different letters are significantly different at p ≤ 0.05. | |||
| Control | 11.00 ± 0.10a | 10.00 ± 0.23a | 11.00 ± 0.31a |
| AlCl3-AD | 9.10 ± 0.17b | 2.00 ± 0.17c | — |
| % Change | −17.27 | −80.00 | |
| n-Hexane | — | — | 9.50 ± 0.05e |
| % Improvement | 68.18 | ||
| Dichloromethane | — | — | 9.00 ± 0.70d |
| % Improvement | 63.64 | ||
| Ethyl acetate | — | — | 9.74 ± 0.25e |
| % Improvement | 70.36 | ||
| Donepezil drug | — | — | 10.00 ± 0.10e |
| % Improvement | 72.73 | ||
3.7 Potential effects of Tamarindus indica extracts on oxidative stress markers and antioxidants
In AlCl3-neurotoxicant rats (AD-group), MDA increased while GSH, SOD, CAT and TAC levels significantly decreased (Table 6). Treatment of rats with either donepezil or different fractions of T. indica (n-hexane, dichloromethane, and ethyl acetate) showed their potent antioxidant activities through increasing the levels of the antioxidant defense system GSH, CAT SOD, TAC as well as reducing MDA in brain tissues (Table 6). The highest percentage improvements in GSH and MDA levels were in the group treated with T. indica ethyl acetate extract, recorded as 49.09 and 113.90%, respectively. While SOD, CAT and TAC exhibited remarkable improvement percentages, with those for T. indica n-hexane reaching 52.77, 62.90 and 48.00%, respectively.| Parameter | Control | AlCl3-AD | n-Hexane | Dichloromethane | Ethyl acetate | Donepezil drug |
|---|---|---|---|---|---|---|
| a Data are expressed as mean ± SD (n = 8). Groups with similar letters are not significantly different, while those with different letters are significantly different at p ≤ 0.05. GSH: glutathione; MDA: malondialdehyde; SOD: superoxide dismutase; CAT: catalase; TAC: total antioxidant capacity. | ||||||
| GSH (μg per mg protein) | 3100.00 ± 100.0a | 1200.60 ± 29.2b | 2650.60 ± 72.00d | 2210.00 ± 30.90c | 2720.0 ± 34.99d | 2490.00 ± 23.70d |
| % Change | −61.27 | −14.51 | −28.70 | −12.25 | −19.67 | |
| % Improvement | 46.70 | 32.50 | 49.09 | 41.60 | ||
| MDA (μg per mg protein) | 31.60 ± 2.10e | 80.00 ± 3.90f | 45.44 ± 2.90g | 50.00 ± 3.76g | 44.00 ± 2.70g | 50.76 ± 3.00g |
| % Change | 153.00 | 43.60 | 58.20 | 39.20 | 60.60 | |
| % Improvement | 109.30 | 94.90 | 113.90 | 92.60 | ||
| SOD (μmol per mg protein) | 1800.00 ± 50.77h | 800 ± 23.12i | 1750.33 ± 50.00h | 1480.67 ± 60.66j | 1690.33 ± 43.90h | 1550.30 ± 40.00j |
| % Change | −55.55 | −2.80 | −17.77 | −6.09 | −13.80 | |
| % Improvement | 52.77 | 37.70 | 49.44 | 41.60 | ||
| CAT (U per g tissue) | 200.00 ± 90.00k | 55.10 ± 4.80l | 180.90 ± 10.00n | 145.00 ± 8.00m | 167.61 ± 8.90n | 157.00 ± 9.00n |
| % Change | −72.45 | −9.55 | −27.5 | −16.20 | −21.50 | |
| % Improvement | 62.90 | 44.95 | 56.25 | 50.95 | ||
| TAC (U per g tissue) | 2.00 ± 0.023o | 1.00 ± 0.20p | 1.96 ± 0.42o | 1.80 ± 0.25o | 1.91 ± 0.20o | 1.91 ± 0.66o |
| % Change | −50.00 | −2.00 | −10.00 | −4.50 | −4.50 | |
| % Improvement | 48.00 | 40.00 | 45.50 | 45.50 | ||
3.8 Potential effects of Tamarindus indica extracts on acetylcholine and acetylcholine esterase levels in the brain
In AD-induced rats, the brain level of ACh was significantly lowered by 66.10%, compared to a normal control. A marked significant increase in ACh was shown in brain tissue after treatment with either donepezil or different extracts of T. indica (35.13, 41.98, 28.05, and 36.54%, for donepezil, n-hexane, dichloromethane, and ethyl acetate, respectively). On the other hand, by comparison to the control group, AChE activity in AlCl3-treated rats was found to be twice as high as that of the control group (182.20%). However, treatment of AD-induced rats with either donepezil or n-hexane, dichloromethane, or ethyl acetate fractions of T. indica resulted in a significant inhibition in the brain AChE activity by 58.4, 93.09, 56.15, and 83.7%, respectively, compared to AD-rats (Table 7) with the highest percentage improvement for donepezil followed by ethyl acetate extract (123.70 and 98.50%, respectively).| Parameter | Control | AlCl3-AD | n-Hexane | Dichloromethane | Ethyl acetate | Donepezil drug |
|---|---|---|---|---|---|---|
| a Data are expressed as means ± SD (n = 8). Groups with similar letters are not significantly different, while those with different letters are significantly different at p ≤ 0.05. Ach: acetylcholine; AchE: acetylcholine esterase. | ||||||
| Ach (nmol per g tissue) | 56.50 ± 3.22a | 19.15 ± 0.56b | 42.87 ± 3.30d | 35.00 ± 3.30c | 39.80 ± 3.00d | 39.00 ± 2.21d |
| % Change | −66.10 | −24.10 | −38.05 | −29.55 | −30.97 | |
| % Improvement | 41.98 | 28.05 | 36.54 | 35.13 | ||
| AchE (μmol per g tissue) | 3330.00 ± 300.00d | 9400.00 ± 300.90e | 6430 ± 455.00g | 5200.02 ± 400.02f | 6118.03 ± 360.00g | 5278.03 ± 265.70f |
| % Change | 182.2 | 93.09 | 56.15 | 83.70 | 58.4 | |
| % Improvement | 89.18 | 126.1 | 98.5 | 123.7 | ||
3.9 Potential effects of Tamarindus indica extracts on adrenaline, noradrenaline, dopamine, and serotonin levels in the brain
In AD-induced rats, there were significant reductions in A, NA, DA, and 5-HT neurotransmitter levels with percentage decreases of 54.2, 51.1, 62.3, and 57.7%, respectively, compared to normal control rats (Table 8). Treatment of AD-induced rats with either donepezil or different fractions of T. indica (n-hexane, dichloromethane, and ethyl acetate) showed a noticeable elevation in all levels of neurotransmitters (Table 8). The highest percentage improvements were recorded for A, DA, and 5-HT after treatment of AD rats with ethyl acetate followed by n-hexane and finally with donepezil. While NA showed the highest percentage improvement with n-hexane followed by ethyl acetate then donepezil.| Parameter | Control | AlCl3-AD | n-Hexane | Dichloromethane | Ethyl acetate | Donepezil drug |
|---|---|---|---|---|---|---|
| a Data are expressed as mean ± SD (n = 8). Groups with similar letters are not significantly different, while those with different letters are significantly different at p ≤ 0.05. A: adrenaline, NA: noradrenaline, DA: dopamine, 5-HT: serotonin. | ||||||
| A (ng per gm tissue) | 350.00 ± 11.70a | 160.00 ± 10.10b | 285.50 ± 24.75d | 190.76 ± 15.60c | 290.78 ± 14.98d | 269.40 ± 210.21d |
| % Change | −54.20 | −18.40 | −45.40 | −16.92 | −23.02 | |
| % Improvement | 35.85 | 8.78 | 37.36 | 31.25 | ||
| NA (ng per g tissue) | 225.00 ± 11.30d | 110.00 ± 8.00e | 172.66 ± 6.60f | 160.00 ± 7.00f | 169.55 ± 7.61f | 167.00 ± 10.80f |
| % Change | −51.11 | −23.28 | −28.80 | −24.64 | −25.77 | |
| % Improvement | 27.82 | 22.22 | 26.46 | 25.33 | ||
| DA (ng per g tissue) | 75.55 ± 6.80g | 28.00 ± 1.90h | 55.23 ± 2.22i | 45.23 ± 2.22i | 58.10 ± 3.12i | 48.50 ± 2.10i |
| % Change | −62.39 | −26.89 | −40.13 | −23.09 | −35.80 | |
| % Improvement | 36.04 | 22.80 | 39.84 | 27.13 | ||
| 5-HT (ng per g tissue) | 95.20 ± 5.10j | 40.20 ± 3.02k | 69.40 ± 4.80l | 62.00 ± 3.20l | 72.30 ± 5.00l | 66.00 ± 3.00l |
| % Change | −57.77 | −27.10 | −34.87 | −24.05 | −30.67 | |
| % Improvement | 30.67 | 22.89 | 33.71 | 27.10 | ||
3.10 Potential effects of Tamarindus indica extracts on tau protein and amyloid-β protein levels
Significant increases in serum tau protein and amyloid-β protein were estimated in AD-induced rats, reaching 254, and 117%, respectively, compared to control rats (Table 9). Interestingly, treatment of AD-induced rats with either donepezil or different fractions of T. indica (n-hexane, dichloromethane, and ethyl acetate) showed significant decreases in serum tau protein and amyloid-β protein compared to AD rats, with the highest improvement percentages for tau protein treated with ethyl acetate (218.70%) and amyloid β treated with both n-hexane and ethyl acetate (103.60 and 103.30%, respectively) followed by donepezil and finally dichloromethane (Table 9).| Parameter | Control | AlCl3-AD | n-Hexane | Dichloromethane | Ethyl acetate | Donepezil drug |
|---|---|---|---|---|---|---|
| a Data are expressed as mean ± SD (n = 8). Groups with similar letters are not significantly different, while those with different letters are significantly different at p ≤ 0.05. | ||||||
| Tau protein (ng L−1) | 310.00 ± 12.00a | 1100.00 ± 29.45b | 512.00 ± 23.55d | 621.00 ± 30.18c | 422.50 ± 21.22e | 478.01 ± 34.90e |
| % Change | 254.83 | 65.16 | 100.32 | 36.12 | 54.19 | |
| % Improvement | 189.67 | 154.52 | 218.70 | 200.64 | ||
| Amyloid β (g L−1) | 665.00 ± 39.10h | 1445.50 ± 32.80i | 756.00 ± 22.23j | 870.00 ± 30.10j | 758.10 ± 3.12j | 834.19 ± 17.02j |
| % Change | 117.29 | 13.68 | 30.82 | 13.98 | 25.44 | |
| % Improvement | 103.60 | 86.46 | 103.30 | 91.87 | ||
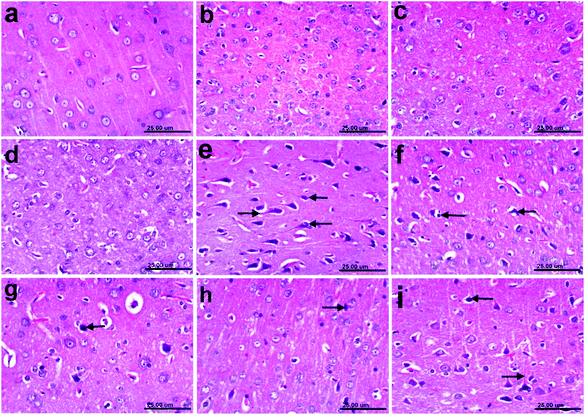
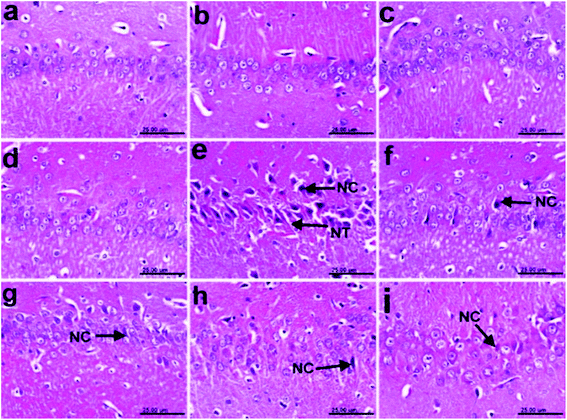
3.11 Histopathology results
| Histopathological lesion | Normal | Control n-hex. | Control DCM | Control EtOAc | +ve control | Treatment with n-hex. | Treatment with DCM | Treatment with EtOAc | Donepezil |
|---|---|---|---|---|---|---|---|---|---|
| a n-Hex.: n-hexane; DCM: dichloromethane; EtOAc: ethyl acetate. | |||||||||
| Cerebral cortex: necrosis of neurons | 0 | 0 | 0 | 0 | 3.6 ± 0.01a | 1.6 ± 0.02c | 1.6 ± 0.01c | 0.6 ± 0.01e | 0.6 ± 0.02e |
| Neuronophagia | 0 | 0 | 0 | 0 | 3.4 ± 0.02a | 1.8 ± 0.01c | 0.6 ± 0.02e | 0.6 ± 0.01e | 0.40 ± 0.01e |
| Neurofibrillary tangles | 0 | 0 | 0 | 0 | 3.4 ± 0.01a | 1.00 ± 0.01d | 0.4 ± 0.01e | 0.2 ± 0.01f | 0.2 ± 0.02f |
| Gliosis | 0 | 0 | 0 | 0 | 2.4 ± 0.02b | 0.6 ± 0.01e | 0.4 ± 0.01e | 0 | 0 |
| Hippocampus: necrosis of pyramidal neurons | 0 | 0 | 0 | 0 | 3.6 ± 0.04a | 1.6 ± 0.01c | 1.00 ± 0.02d | 0.4 ± 0.01e | 0.2 ± 0.01f |
| Neurofibrillary tangles | 0 | 0 | 0 | 0 | 2.6 ± 0.02b | 1.00 ± 0.04d | 0.4 ± 0.01e | 0 | 0 |
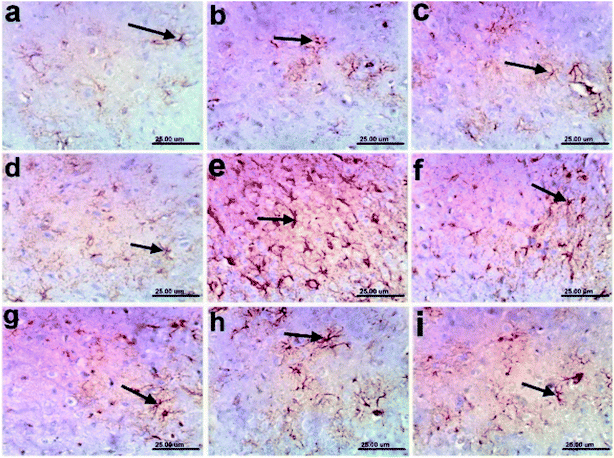
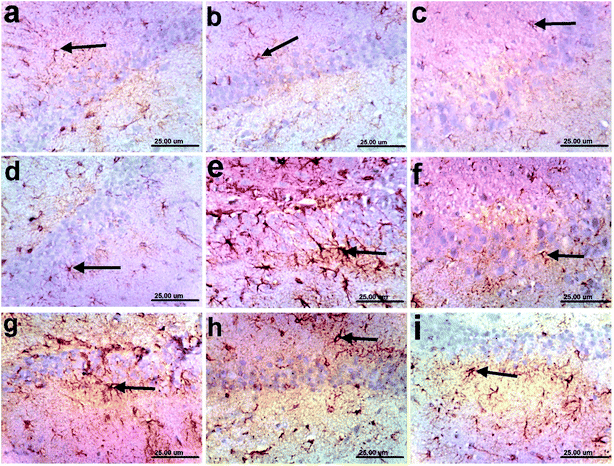
3.12 Immunohistochemistry
| Group | % area of positive GFAP staining in the cerebral cortex | % area of positive GFAP staining in the hippocampus |
|---|---|---|
| Normal control | 3.44 ± 0.12a | 3.14 ± 0.10a |
| Control (n-hexane) | 4.22 ± 0.23a | 4.14 ± 0.22a |
| Control (dichloromethane) | 4.11 ± 0.31a | 3.70 ± 0.19a |
| Control (ethyl acetate) | 3.96 ± 0.11a | 3.67 ± 0.33a |
| AD | 20.35 ± 0.21b | 23.10 ± 1.80b |
| Treat (n-hexane) | 12.46 ± 1.20c | 9.14 ± 0.92c |
| Treated (dichloromethane) | 7.85 ± 0.43d | 7.35 ± 0.62d |
| Treated (ethyl acetate) | 7.04 ± 0.52d | 5.03 ± 0.30e |
| Donepezil | 8.52 ± 0.61d | 6.29 ± 0.34e |
According to the study results in Table 4, the behavioral test data agree with previously obtained results which demonstrated that AlCl3-neurointoxicated rats took more time to catch food in a T-maze, compared to control rats, denoting deteriorated neurocognitive function.63 Whereas various T. indica fractions (n-hexane, and dichloromethane) showed a significant decrease in time taken by rats to reach food in the T-maze, in comparison to the AD-induced group, indicating improved cognitive abilities, with percentage improvements reaching 116.14 and 101.10% (6 weeks of treatment), respectively. Additionally, the group treated with the T. indica ethyl acetate fraction showed the most significant decrease in time to achieve the task, compared to the AD-induced group, with percentage improvement reaching 155.30% (6 weeks of treatment) (Table 4).
On the other hand, a beam balance test is used to analyze rodent gait in a testing environment that challenges their ability to maintain balance given that the animals must cross an elevated beam with a narrow diameter. Beam balance test results showed that AlCl3 caused significant deterioration in brain cognitive functions for AlCl3-neurotoxicant rats (AD-group) (Table 5) with a percentage decrease of 80%. However, treatment of rats with donepezil or different fractions of Tamarindus indica (n-hexane, dichloromethane, and ethyl acetate) resulted in an improvement in behavioral status represented by improved motor coordination and improved cognition, with percentage improvements reaching 68.18, 63.64, 70.36%, respectively, compared to the standard drug donepezil which recorded 72.73% (6 weeks of treatment).
According to the literature, disruption in the antioxidant defense mechanism and excessive generation of reactive oxygen species (ROS) are considered the main causes of mitochondrial dysfunction induced intracellular damage.64 The current results (Table 6) revealed significant elevation in oxidative damage biomarkers in brain tissue following AlCl3 induction. This result is in agreement with Aly et al. 2015,64 who declared that AlCl3-linked neurotoxicity may lead to a rise in lipid peroxidation. Further reports added a marked elevation in thiobarbituric acid reactive substances in rat brains after AlCl3 induction which was related to Fe3+-carrying protein transferring binding, hence lowering Fe2+ binding and raising free intracellular Fe2+ that produces membrane lipids, protein peroxidation and later membrane destruction, though loss of membrane fluidity, alteration in the potential of the membrane, elevation in the permeability of the membrane and disturbances in the function of receptors.65 In addition, the present study showed that the rise in MDA in AD-induced rats was associated with the inhibition of antioxidant enzymes that is implicated in the removal of ROS, such as SOD, CAT and GSH in brain tissue, suggesting the pro-oxidant action of AlCl3. Instead, Sumathi et al. 2013 (ref. 65) declared that AlCl3 exposition promotes destruction in neuronal lipids associated with modifications in the enzymatic antioxidant defense system. Moreover, the presented results showed a significant decrease in GSH level in the brain tissues of rats induced with AlCl3, which may be attributed to a high level of H2O2-induced cytotoxicity in brain endothelial cells because of inhibition of glutathione reductase.64 The significant reduction in brain TAC in AlCl3-induced AD rats may be attributed to long-term exposure to AlCl3 leading to an increase in lipid peroxidation with depletion and exhaustion of several antioxidant enzymes.64 In addition, Aly et al. 2018 (ref. 64) explained the reduction in TAC in AD-induced rats by the decrease in axonal mitochondria transformation, impairment of Golgi and reduction of synaptic vesicles, which results in the release of oxidative products like hydroperoxide and carbonyls as well peroxyl nitrites, while there is a decrease in antioxidant enzymes and glutathione within the neurons. In addition to the high level of Fe promoting ROS due to the high brain content of polyunsaturated fatty acids which can easily interact with elaborated radicals and afford oxidative destruction in AD-induced rats.64 Treatment of rats with different fractions of T. indica (n-hexane, dichloromethane, and ethyl acetate) showed their potent antioxidant activities through increasing the levels of the antioxidant defense system GSH, CAT SOD, TAC as well as reducing MDA in brain tissues (Table 6).
Besides that, AlCl3 is reported to be a cholinotoxin that provokes functional alterations in cholinergic, dopaminergic, and noradrenergic neurotransmission. Therefore, it has the propensity to cause impaired cholinergic transmission by affecting the synthesis and release of neurotransmitters.66 Impaired cholinergic transmission occurs in two ways: first, it occurs either due to a decline in ACh release or due to decreased choline acetyltransferase activity, which results in scarcity of ACh. Second, elevated AChE activity further adds to the scarcity of ACh at the synapse by accelerating the decomposition of available ACh; this degradation of ACh is abolished by effective RIVA (AChE-inhibitor).67 Moreover, acetyl Co-A synthesis relies on pyruvate formation through energy-dependent glycolysis, which was also found to be altered and justified the deterioration in ACh levels and AChE activity.68 Furthermore, AlCl3-induced oxidative disruption in membrane fluidity/composition can also affect the membrane-bound AChE activity; thus, it also corroborated the decreased AChE activity.67 Our data showed that administration of AlCl3-induced cholinergic impairment in AD-induced rats was represented by a marked decrease in brain and serum ACh levels and a significant increase in brain serum AChE activities, compared to a control group. These results run in accordance with those of Mohamd et al. 2011 (ref. 69) who stated that AlCl3 administration produced a significant elevation in AChE activity in the brain, compared to neurologically normal control rats. A markedly significant increase in ACh was shown in brain tissue after treatment with different extracts of T. indica (41.98, 28.05, and 36.54%, for n-hexane, dichloromethane, and ethyl acetate, respectively). On the other hand, treatment of AD-induced rats with n-hexane, dichloromethane, and ethyl acetate fractions of T. indica resulted in a significant inhibition in brain AChE activity by 93.09, 56.15, and 83.7%, respectively, compared to AD-rats (Table 7) with the highest percentage improvement for ethyl acetate extract (98.50%).
In addition, AlCl3 exposure to rats induced a significant decrease in dopamine (DA) level, and this runs in parallel with Singla and Dhawan,70 who indicated the negative impact of AlCl3 on memory function. Adverse effects of AlCl3 on cognitive behavior in rats and mice were observed.71 This may be due to the intervention of AlCl3 in the dopaminergic system,70 and also to its ability to induce oxidative stress, where oxidative stress and inflammation cause deficiency in several major neurotransmitters, including ACh and DA.72 Furthermore, the significant alterations in brain neurotransmitters in AlCl3-exposed rats may be related to increased formation of O2 and H2O2, and aggregation of Lewy bodies in the brain; thus increasing the risk of neurodegenerative diseases.73 Further, DA oxidation is enhanced by the increased concentration of iron in Al-exposed rats, leading to the production of DA quinones, that covalently interact with cysteine residues of glutathione (GSH) enzymes inhibiting its antioxidative activity.74 Moreover, AlCl3 promotes the aggregation of a-synuclein and decreases DA-binding receptors (D1 and D2) in the brain cortex and striatum of AlCl3-exposed subjects, as well as exerting an inhibiting activity on different levels, including inhibition of DA b-hydroxylase (responsible for the conversion of DA into norepinephrine) and inhibition of tryptophan decarboxylase activity (responsible for DA formation).75 The decreased level of DA and altered cholinergic function might also be attributed to increased monoamine oxidase (MAO) activity, that led to increased degradation of dopamine.70 Collectively, neurotransmission is negatively modified by AlCl3; either by directly inhibiting the enzymes involved in neurotransmitter synthesis and/or utilization or by affecting the structural properties of synaptic membranes that could affect the release and/or uptake of these molecules.71
Supplementations of different fractions of T. indica pulp (n-hexane, dichloromethane, and ethyl acetate), in AD-induced rats showed a marked elevation in all levels of neurotransmitters (adrenaline, noradrenaline, dopamine, and serotonin) (Table 8). The recovery levels of neurotransmitters may be attributed to the role of polyphenols in restoring the activity of enzymes involved in the synthesis of neurotransmitters.
The significant increase in serum amyloid-β protein was able to differentiate between AD-induced rats and neurologically normal controls. This runs in agreement with previous studies by Nayak and Yokel et al. 1999, 2002,76,77 which demonstrated that AlCl3 promotes the accumulation of insoluble Aβ (1-42) protein and Aβ plaque formation. Moreover, a study performed by Pesini et al. 2019 (ref. 78) supported the concept that the vascular system is a major player in controlling Aβ levels in the brain; Aβ-plaques appear to be formed if their levels in brain extracellular space surpass the transport capacity of the clearance mechanism across the blood–brain barrier (BBB), or if the vascular transport of the peptide had deteriorated and proved that increased blood Aβ levels is an early event that precedes the onset of cognitive decline and increases the risk of developing AD. The current significant increase in serum Aβ peptide levels in untreated AD-induced rats indicated neuronal cytoskeleton disruption induced by AlCl3 intoxication led to abnormal accumulation of Aβ peptide in the brain and was reflected in its high serum level. Consequently, its clearance is considered a primary therapeutic target for managing AD. The neuroprotective potential of polyphenol through reducing neuronal damage and loss induced by neurotoxins or neuroinflammation, altering ROS production, as well as attenuating the accumulation of neuropathological hallmarks, such as Aβ, might take place through the capacity of polyphenols to interact with molecular signaling pathways and related cellular mechanisms such as inflammation,79,80 or to interact with neuronal and glial signalling.81 However, it is not clear whether this anti-amyloidogenic activity of polyphenols is attributable to the antioxidant activity and/or to its direct interaction with Aβ.82 Interestingly, T. indica pulp extracts showed significant decrease in Aβ levels compared to AD rats, reflecting the possible role of polyphenols in serum Aβ peptide decrease and clearance.
In addition, the pronounced improvement in Alzheimer rate exhibited by different fractions of T. indica (n-hexane, dichloromethane, and ethyl acetate) was confirmed by histological examination of the cerebral cortexes and hippocampi of the brains of AD-induced rats, indicating higher amelioration in different lesion scores of the cerebral cortexes and hippocampi than the standard drug (Tables 10, 11 and Fig. 2–4).
3.13 Phytochemical investigation of Tamarindus indica pulp
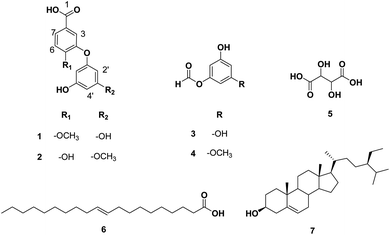
Based on the physicochemical and chromatographic properties, the spectral analyses from UV, 1H, and DEPT-Q NMR, as well as comparisons with the literature and some authentic samples, the crude ethanolic extract of T. indica pulp offered two new diphenyl ether derivatives: 4-phloroglucinol, 5-methoxybenzoic acid 1, 4-(3′-methoxyphloroglucinol), 5-hydroxybenzoic acid 2, along with the known 3,5-dihydroxyphenyl formate 3,83 5-methoxy, 3-hydroxyphenyl formate 4,83 tartaric acid 5,84 gondoic acid 6,85 and β-sitosterol 7 (ref. 35) (Fig. 5). Compounds 3 and 4 were isolated herein for the first time from the genus Tamarindus (Fig. S1–S14† and 5).The obtained HRESIMS data, along with NMR data for compounds 1 and 2, suggest the characteristic structure of the diphenyl ether unit.35 Compound 1 (Table 1, Fig. 5 and see ESI Fig. S1–S3†) was obtained as a yellow amorphous solid. The HRESIMS data for compound 1 showed an adduct pseudo-molecular ion peak at m/z 277.0712 [M + H]+, consistent with the molecular formula C14H13O6 and suggesting 9 degrees of unsaturation. The 1H, DEPT-Q, HSQC, and HMBC NMR data (Table 1, ESI Fig. S1–S3,† 5 and 6), showed 7 characteristic resonances: three methine groups at δH 7.33, dd (1H, 2.4, 8.0) δC 119.0, 6.88, d (1H, 2.4) δC 115.3, and δH 7.13, d (1H, 8.0) δC 113.6, with four quaternary carbons at δC 172.8, 122.6, 146.2, and 163.1, suggesting the characteristic structure of the 3,4-dihydroxybenzoic acid unit.79 Moreover, the acquired NMR data (Table 1 and Fig. 6) showed 5 characteristic resonances: three methine groups at δH 6.61, dd (2H, 2.4, 3.1) δC 104.9, δH 6.93, dd (1H, 2.4, 3.1) δC 102.8, with three quaternary carbons at δC 149.7, 157.8, 157.8, suggesting the characteristic structure of a phloroglucinol unit.86 1H, DEPT-Q, and HSQC showed an additional characteristic resonance methoxy group at δH 3.81, s (3H) δC 52.1, where HMBC showed the characteristic 3J HMBC correlation of proton δH 3.81, s (3H) with C-5 δC 163.1. Accordingly, compound 1 was identified as 4-phloroglucinol, 5-methoxybenzoic acid.
The molecular formula of compound 2 was identical to that of 1 based on HRESIMS (C14H13O6).
But the 1H and 13C NMR data differed in the resonating chemical shifts of the methine groups of the core diphenyl ether moiety besides the appearance of six methine groups instead of five, compared with 1. This suggested a positional difference in the location of the methoxy group in the diphenyl ether moiety versus 1 (Table 1, Fig. 2, and S4–S6†). The assignment of the location of the methoxy group in 2 was aided by the HMBC experiment. HMBC showed the characteristic 3J HMBC correlation of proton δH 3.81, s (3H) with C-3′ δC 163.1. Accordingly, compound 2 was identified as 4-(3′-methoxyphloroglucinol), 5-hydroxybenzoic acid.
3.14 Molecular modeling
Subsequently, the retrieved docking results were arranged as a list of affinity scores from the highest negative values to the lowest one. To identify the best targets for each input structure (i.e. isolated compounds from T. indica crude extract), we set a score of −7 kcal mol−1 as a cut-off value. Being a key protein in Alzheimer's disease, acetyl choline esterase (AchE) was found to be a potential target protein for compounds 1 and 2 with docking scores of −11.3 and −10.8 kcal mol−1, respectively. It will be established that inhibiting the hydrolytic activity of this enzyme leads to maintaining a higher level of acetyl choline in the brain's synapses, which in turn led to reduced CNS-related symptoms.87
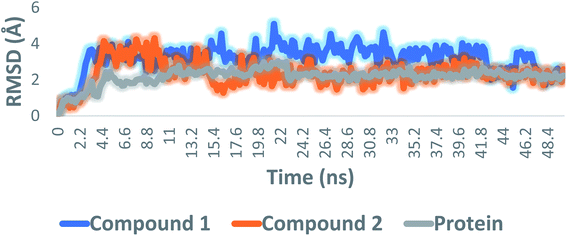
All compounds were then re-docked against the active site of AchE (PDB: 4EY6)88 using AutoDock Vina. The Vina docking results were comparable with those of idTarget, where compounds 1 and 2 showed similar binding modes and affinities (Vina scores = −10.4 and −9.9 kcal mol−1, respectively). To further validate the previous docking-based screening, the resulting docking poses of both compounds 1 and 2 were subjected to molecular dynamic simulations (MDS) and binding free energy (ΔG) estimation.
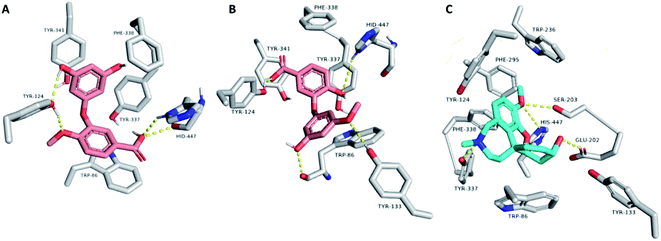
Consequently, the last snapshot of MDS of each compound was extracted and visualized to investigate the binding mode of each compound inside the AchE binding site, which was highly comparable to that of the co-crystallized inhibitor (Fig. 8). TRP-86, TYR-124, TYR-133, and HID-447 were found to be the key amino acid residues involved in H-bonding with both compounds 1 and 2, while TRP-86, TYR-337, PHE-338, and TYR-341 were the key residues involved in the hydrophobic interactions. Binding interactions of compounds 1 and 2 were comparable to that of the co-crystallized inhibitor galantamine, in which they were H-bonded to GLU-202, SER-203, TYR-337, and HID-447. It also established hydrophobic interactions with TRP-86, TYR-337, PHE-338, and TYR-341 (Fig. 8). All the previous in silico investigations putatively indicated that both compounds 1 and 2 were the most probable active anti-Alzheimer agents in T. indica crude extract.
4. Conclusions
In this study, different fractions of T. indica pulp (n-hexane, dichloromethane, and ethyl acetate) displayed remarkable neuroprotective, antiapoptotic, and anti-amnesic effects against AlCl3-induced cerebral damage and cognitive decline, an action that could be related to their antioxidant and anti-AchE properties. Seven compounds were isolated and identified from different fractions of T. indica, including two new ones. Molecular docking analysis of the AchE molecular target predicted the possible mode of action relating to the anti-Alzheimer activities of the isolated compounds. This study recommends the application of T. indica pulp extract as a promising therapy for AD treatment. However, future detailed mechanistic studies and quantification of secondary metabolites in the extract are still required to confirm these results.Author contributions
Conceptualization: AHE, URA, HFA, DEA, methodology: AHE, HFA, SGS, EAY, KAA, AMG, AMS; software: AHE, HFA, SGS, EAY, KAA, FA, AMG, AMS; formal analysis: AHE, HFA, FA, SGS, EAY, KAA, AMG, AMS; investigation: AHE, HFA, SGS, EAY, KAA, AMG, AMS, DEA; resources: AHE, HFA, SGS, EAY, KAA, AMG, AMS; data curation: AHE, HFA, SGS, EAY, KAA, AMG, AMS; writing – original draft: AHE, HFA, SGS, KAA, AMG, AMS; writing – review and editing: AHE, URA, HFA, SGS, EAY, KAA, AMG, AIO, NI, FA, DEA, AMS; supervision: AHE, URA, HFA, DEA. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors are grateful to Umm Al-Qura University for their support.References
- S. Babri, G. Mohaddes, I. Feizi, A. Mohammadnia, A. Niapour, A. Alihemmati and M. Amani, Eur. J. Pharmacol., 2014, 732, 19–25 CrossRef CAS PubMed.
- A. Dement, Alzheimer's Dementia, 2016, 12, 459–509 CrossRef PubMed.
- J. Zhang, Y.-f. Zhen, L.-g. Song, W.-n. Kong, T.-m. Shao, X. Li and X.-q. Chai, Behav. Brain Res., 2013, 244, 70–81 CrossRef CAS PubMed.
- A. C. Pereira, J. D. Gray, J. F. Kogan, R. L. Davidson, T. G. Rubin, M. Okamoto, J. H. Morrison and B. S. McEwen, Mol. Psychiatry, 2017, 22, 296–305 CrossRef CAS PubMed.
- R. Raschetti, E. Albanese, N. Vanacore and M. Maggini, PLoS Med., 2007, 4, e338 CrossRef PubMed.
- K. G. Yiannopoulou and S. G. Papageorgiou, J. Cent. Nerv. Syst. Dis., 2020, 12, 1179573520907397 Search PubMed.
- N. Kimura, Int. J. Mol. Sci., 2016, 17, 503 CrossRef PubMed.
- S. Beheshti and R. Aghaie, Avicenna J. Phytomed., 2016, 6, 468 CAS.
- G. M. Cole, G. P. Lim, F. Yang, B. Teter, A. Begum, Q. Ma, M. E. Harris-White and S. A. Frautschy, Neurobiol. Aging, 2005, 26, 133–136 CrossRef PubMed.
- A. A. Boligon, R. P. Pereira, A. C. Feltrin, M. M. Machado, V. Janovik, J. B. T. Rocha and M. L. Athayde, Bioresour. Technol., 2009, 100, 6592–6598 CrossRef CAS PubMed.
- T. T. Bui and T. H. Nguyen, J. Basic Clin. Physiol. Pharmacol., 2017, 28, 413–423 CAS.
- X. Chen, J. Drew, W. Berney and W. Lei, Cells, 2021, 10, 1309 CrossRef CAS PubMed.
- G. H. Abdelrahman and A. A. Mariod, Wild Fruits: Composition, Nutritional Value and Products, Springer, 2019, pp. 229–238 Search PubMed.
- P. Kuru, Asian Pac. J. Trop. Biomed., 2014, 4, 676–681 CrossRef.
- S. S. Parvez, M. M. Parvez, Y. Fujii and H. Gemma, Jpn. J. Trop. Agric., 2003, 47, 243–249 CAS.
- R. Maiti, D. Jana, U. Das and D. Ghosh, J. Ethnopharmacol., 2004, 92, 85–91 CrossRef CAS PubMed.
- F. Martinello, S. Soares, J. Franco, A. d. Santos, A. Sugohara, S. Garcia, C. Curti and S. Uyemura, Food Chem. Toxicol., 2006, 44, 810–818 CrossRef CAS PubMed.
- M. Abukakar, A. Ukwuani and R. Shehu, Asian J. Biochem., 2008, 3, 134–138 CrossRef CAS.
- M. M. T. do Rosário, M. M. Kangussu-Marcolino, A. E. do Amaral, G. R. Noleto and C. L. de Oliveira Petkowicz, Chem.-Biol. Interact., 2011, 189, 127–133 CrossRef PubMed.
- S. Ushanandini, S. Nagaraju, K. Harish Kumar, M. Vedavathi, D. Machiah, K. Kemparaju, B. Vishwanath, T. Gowda and K. Girish, Phytother. Res., 2006, 20, 851–858 CrossRef CAS PubMed.
- J. Fook, L. Macedo, G. Moura, F. Teixeira, A. Oliveira, A. Queiroz and M. Sales, Life Sci., 2005, 76, 2881–2891 CrossRef CAS PubMed.
- A. P. L. Librandi, T. N. Chrysostomo, A. E. Azzolini, C. Recchia, S. A. Uyemura and A. I. de Assis-Pandochi, Food Chem. Toxicol., 2007, 45, 1487–1495 CrossRef PubMed.
- R. Khan, S. Siddiqui, I. Azhar and S. Ahmed, J. Basic Appl. Sci., 2005, 1, 3–11 Search PubMed.
- N. Dighe, S. Pattan, S. Nirmal, R. Kalkotwar, V. Gaware and M. Hole, Res. J. Pharmacogn. Phytochem., 2009, 1, 69–71 Search PubMed.
- A. Souza and K. Aka, Afr. J. Tradit., Complementary Altern. Med., 2007, 4, 261–266 CrossRef CAS.
- N. Povichit, A. Phrutivorapongkul, M. Suttajit, C. Chaiyasut and P. Leelapornpisid, Pak. J. Pharm. Sci., 2010, 23, 403–408 CAS.
- L. Raimondi, M. Lodovici, F. Guglielmi, G. Banchelli, M. Ciuffi, E. Boldrini and R. Pirisino, J. Pharm. Pharmacol., 2003, 55, 333–338 CrossRef CAS PubMed.
- S. Aravind, M. M. Joseph, S. Varghese, P. Balaram and T. Sreelekha, Sci. World J., 2012, 2012, 5–13 Search PubMed.
- D. H. Rao and L. R. Gowda, J. Agric. Food Chem., 2008, 56, 2175–2182 CrossRef CAS PubMed.
- A. Romero, E. Ramos, C. de Los Ríos, J. Egea, J. Del Pino and R. J. Reiter, J. Pineal Res., 2014, 56, 343–370 CrossRef CAS PubMed.
- A. Kumar, A. Prakash and S. Dogra, Pharmacol. Rep., 2011, 63, 915–923 CrossRef CAS PubMed.
- C.-Y. Yuan, Y.-J. Lee and G.-S. W. Hsu, J. Biomed. Sci., 2012, 19, 1–9 CrossRef PubMed.
- M. R. F. Ashworth and E. Stahl, Thin-layer chromatography: a laboratory handbook, Springer Science & Business Media, 2013 Search PubMed.
- A. H. Elmaidomy, M. M. Mohyeldin, M. M. Ibrahim, H. M. Hassan, E. Amin, M. E. Rateb, M. H. Hetta and K. A. El Sayed, Phytother. Res., 2017, 31, 1546–1556 CrossRef CAS PubMed.
- A. H. Elmaidomy, R. Mohammed, H. M. Hassan, A. I. Owis, M. E. Rateb, M. A. Khanfar, M. Krischke, M. J. Mueller and U. Ramadan Abdelmohsen, Metabolites, 2019, 9, 223 CrossRef CAS PubMed.
- A. H. Elmaidomy, R. Mohammed, A. I. Owis, M. H. Hetta, A. M. AboulMagd, A. B. Siddique, U. R. Abdelmohsen, M. E. Rateb, K. A. El Sayed and H. M. Hassan, RSC Adv., 2020, 10, 10584–10598 RSC.
- K. Biswas, A. Azad, T. Sultana, F. Khan, S. Hossain, S. Alam, R. Chowdhary and Y. Khatun, J. Intercult. Ethnopharmacol., 2017, 6, 115 CrossRef CAS PubMed.
- H. Singh, M. Hebert and M. Gault, Clin. Chem., 1972, 18, 137–144 CrossRef CAS.
- M. Kawahara, M. Kato-Negishi and K. Tanaka, Metallomics, 2017, 9, 619–633 CrossRef CAS PubMed.
- E. Y. Pioli, B. N. Gaskill, G. Gilmour, M. D. Tricklebank, S. L. Dix, D. Bannerman and J. P. Garner, Behav. Brain Res., 2014, 261, 249–257 CrossRef PubMed.
- M. Altun, E. Bergman, E. Edström, H. Johnson and B. Ulfhake, Physiol. Behav., 2007, 92, 911–923 CrossRef CAS PubMed.
- M. Yerima, J. Anuka, O. Salawu and I. Abdu-Aguye, Pak. J. Biol. Sci., 2014, 17, 414–418 CrossRef CAS PubMed.
- M. Abubakar, Res. J. Pharm., Biol. Chem. Sci., 2010, 1, 104–111 Search PubMed.
- R. Komakech, Y.-g. Kim, G. M. Matsabisa and Y. Kang, Integr. Med. Res., 2019, 8, 181–186 CrossRef PubMed.
- K.-M. Baek, O.-D. Kwon, H. S. Kim, S.-J. Park, C.-H. Song and S.-K. Ku, Int. J. Pharmacol., 2015, 11, 343–350 Search PubMed.
- W. Underwood and R. Anthony, AVMA Guidelines for the Euthanasia of Animals: 2020 Edition, Retrieved on March, 2020, 2013, pp. 2020–2021 Search PubMed.
- M. B. Close, K. Banister, V. Baumans, E.-M. Bernoth, N. Bromage, J. Bunyan, W. Erhardt, P. Flecknell, N. Gregory and H. Hackbarth, Lab. Anim., 1996, 30, 293–316 CrossRef PubMed.
- H. Aune, P.-A. Hals, B. Hansen and J. Aarbakke, Pharmacology, 1984, 28, 67–73 CrossRef CAS PubMed.
- H. M. Awad, H. I. Abd-Alla, M. A. Ibrahim, E. R. El-Sawy and M. M. Abdalla, Med. Chem. Res., 2018, 27, 768–776 CrossRef CAS.
- L. Zhu, L. Xu, X. Wu, F. Deng, R. Ma, Y. Liu, F. Huang and L. Shi, ACS Appl. Mater. Interfaces, 2021, 13, 23328–23338 CrossRef CAS PubMed.
- E. Engvall and P. Perlman, Immunochemistry, 1971, 8, 871–874 CrossRef CAS PubMed.
- M. Giday, Z. Asfaw and Z. Woldu, J. Ethnopharmacol., 2009, 124, 513–521 CrossRef PubMed.
- D. Koracevic, G. Koracevic, V. Djordjevic, S. Andrejevic and V. Cosic, J. Clin. Pathol., 2001, 54, 356–361 CrossRef CAS PubMed.
- E. Beutler, J. Lab. Clin. Med., 1963, 61, 882–888 CAS.
- H. Ohkawa, N. Ohishi and K. Yagi, Anal. Biochem., 1979, 95, 351–358 CrossRef CAS PubMed.
- M. Nishikimi, N. A. Rao and K. Yagi, Biochem. Biophys. Res. Commun., 1972, 46, 849–854 CrossRef CAS PubMed.
- G. Ehrenbrink, F. S. Hakenhaar, T. B. Salomon, A. P. Petrucci, M. R. Sandri and M. S. Benfato, Exp. Gerontol., 2006, 41, 368–371 CrossRef CAS PubMed.
- E. Kidd, A. Banerjee, S. Ferrier, C. Longbottom and Z. Nugent, Caries Res., 2003, 37, 125–129 CrossRef CAS PubMed.
- O. M. Farag, R. M. Abd-Elsalam, H. A. Ogaly, S. E. Ali, S. A. El Badawy, M. A. Alsherbiny, C. G. Li and K. A. Ahmed, Neurochem. Res., 2021, 46, 819–842 CrossRef CAS PubMed.
- S. Youssef, O. A. Abd-El-Aty, H. H. Mossalam and A. M. Tolba, J. Am. Sci., 2011, 7, 139–152 Search PubMed.
- F. Alsenani, A. M. Ashour, M. A. Alzubaidi, A. F. Azmy, M. H. Hetta, D. H. Abu-Baih, M. A. Elrehany, A. Zayed, A. M. Sayed and U. R. Abdelmohsen, Mar. Drugs, 2021, 19, 605 CrossRef CAS PubMed.
- A. M. Sayed, H. A. Alhadrami, S. S. El-Hawary, R. Mohammed, H. M. Hassan, M. E. Rateb, U. R. Abdelmohsen and W. Bakeer, Microorganisms, 2020, 8, 293 CrossRef CAS PubMed.
- I. H. Borai, M. K. Ezz, M. Z. Rizk, H. F. Aly, M. El-Sherbiny, A. A. Matloub and G. I. Fouad, Biomed. Pharmacother., 2017, 93, 837–851 CrossRef CAS PubMed.
- H. Aly, N. Elrigal, S. Ali, M. Rizk and N. Ebrahim, J. Mater. Environ. Sci., 2018, 9, 1931–1941 CAS.
- T. Sumathi, C. Shobana, V. Mahalakshmi, R. Sureka, M. Subathra, A. Vishali and K. Rekha, Asian J. Pharm. Clin. Res., 2013, 7, 80–90 Search PubMed.
- J. John, M. Nampoothiri, N. Kumar, J. Mudgal, G. K. Nampurath and M. R. Chamallamudi, Pharmacogn. Mag., 2015, 11, 327 CrossRef CAS PubMed.
- M. Singh, M. Kaur, H. Kukreja, R. Chugh, O. Silakari and D. Singh, Eur. J. Med. Chem., 2013, 70, 165–188 CrossRef CAS PubMed.
- N. Singla and D. Dhawan, J. Neurosci. Res., 2012, 90, 698–705 CrossRef CAS PubMed.
- E. Mohamd, H. Ahmed, S. Estefan, A. Farrag and R. Salah, Eur. Rev. Med. Pharmacol. Sci., 2011, 15, 1131–1140 CAS.
- N. Singla and D. Dhawan, Mol. Neurobiol., 2017, 54, 406–422 CrossRef CAS PubMed.
- G. M. Abu-Taweel, J. S. Ajarem and M. Ahmad, Pharmacol., Biochem. Behav., 2012, 101, 49–56 CrossRef CAS PubMed.
- M. J. Crockett, L. Clark, G. Tabibnia, M. D. Lieberman and T. W. Robbins, Science, 2008, 320, 1739 CrossRef CAS PubMed.
- W. J. Burke, S. W. Li, H. D. Chung, D. A. Ruggiero, B. S. Kristal, E. M. Johnson, P. Lampe, V. B. Kumar, M. Franko and E. A. Williams, Neurotoxicology, 2004, 25, 101–115 CrossRef CAS PubMed.
- Y. Xu, A. H. Stokes, R. Roskoski Jr and K. E. Vrana, J. Neurosci. Res., 1998, 54, 691–697 CrossRef CAS PubMed.
- S. Kim, J. Nam and K. Kim, Hum. Exp. Toxicol., 2007, 26, 741–746 CrossRef CAS PubMed.
- R. A. Yokel, D. D. Allen and D. C. Ackley, J. Inorg. Biochem., 1999, 76, 127–132 CrossRef CAS PubMed.
- P. Nayak, Environ. Res., 2002, 89, 101–115 CrossRef CAS PubMed.
- V. Pérez-Grijalba, J. Arbizu, J. Romero, E. Prieto, P. Pesini, L. Sarasa, F. Guillen, I. Monleón, I. San-José and P. Martínez-Lage, Alzheimer's Res. Ther., 2019, 11, 1–9 CrossRef PubMed.
- R. González, I. Ballester, R. López-Posadas, M. Suárez, A. Zarzuelo, O. Martinez-Augustin and F. S. D. Medina, Crit. Rev. Food Sci. Nutr., 2011, 51, 331–362 CrossRef PubMed.
- V. Habauzit and C. Morand, Appl. Environ. Microbiol., 2012, 3, 11–16 Search PubMed.
- J. Bensalem, A. Dal-Pan, E. Gillard, F. Calon and V. Pallet, Nutr. Aging, 2015, 3, 89–106 CAS.
- V. Chauhan and A. Chauhan, Pathophysiology, 2006, 13, 195–208 CrossRef CAS PubMed.
- A. Takagaki, Y. Kato and F. Nanjo, Arch. Microbiol., 2014, 196, 681–695 CrossRef CAS PubMed.
- G. Wagner and F. A. Loewus, Plant Physiol., 1974, 54, 784–787 CrossRef CAS PubMed.
- M. Fromm, S. Bayha, R. Carle and D. R. Kammerer, Eur. Food Res. Technol., 2012, 234, 1033–1041 CrossRef CAS.
- I. P. Singh, J. Sidana, P. Bansal and W. J. Foley, Expert Opin. Ther. Pat., 2009, 19, 847–866 CrossRef CAS PubMed.
- M. M. Koola, Psychiatry Res., 2020, 113409 CrossRef CAS PubMed.
- J. Cheung, M. J. Rudolph, F. Burshteyn, M. S. Cassidy, E. N. Gary, J. Love, M. C. Franklin and J. J. Height, J. Med. Chem., 2012, 55, 10282–10286 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1–S16: 1D and 2D NMR spectra of compounds 1–7. See https://doi.org/10.1039/d2ra01340a |
| ‡ Those authors are equally contributed. |
| This journal is © The Royal Society of Chemistry 2022 |