Open Access Article
Open Access ArticleIn vivo characterization of electroactive biofilms inside porous electrodes with MR Imaging†
Luca Häuser a,
Johannes Erben
a,
Johannes Erben b,
Guillaume Pillot
b,
Guillaume Pillot a,
Sven Kerzenmacher
a,
Sven Kerzenmacher *a,
Wolfgang Dreher
*a,
Wolfgang Dreher c and
Ekkehard Küstermann
c and
Ekkehard Küstermann c
c
aCenter for Environmental Research and Sustainable Technology (UFT), University of Bremen, 28359 Bremen, Germany. E-mail: kerzenmacher@uni-bremen.de
bElectrochaea GmbH, 82152 Planegg, Germany
cIn-vivo-MR Group, Faculty 02 (Biology/Chemistry), University of Bremen, 28359 Bremen, Germany
First published on 15th June 2022
Abstract
Identifying the limiting processes of electroactive biofilms is key to improve the performance of bioelectrochemical systems (BES). For modelling and developing BES, spatial information of transport phenomena and biofilm distribution are required and can be determined by Magnetic Resonance Imaging (MRI) in vivo, in situ and in operando even inside opaque porous electrodes. A custom bioelectrochemical cell was designed that allows MRI measurements with a spatial resolution of 50 μm inside a 500 μm thick porous carbon electrode. The MRI data showed that only a fraction of the electrode pore space is colonized by the Shewanella oneidensis MR-1 biofilm. The maximum biofilm density was observed inside the porous electrode close to the electrode-medium interface. Inside the biofilm, mass transport by diffusion is lowered down to 45% compared to the bulk growth medium. The presented data and the methods can be used for detailed models of bioelectrochemical systems and for the design of improved electrode structures.
Introduction
In bioelectrochemical systems (BES), oxidation and/or reduction reactions are catalysed by enzymes or microbes using an electrode as an electron sink or source, respectively.1–4 Applications for BES comprise the synthesis of chemicals, bioremediation, and energy harvesting.5–11 Most applications rely on electroactive microbes with the ability of extracellular electron transfer (EET) forming electroactive biofilms (EAB) on electrodes.12,13 High current densities are generally required for a high production rate and thus for future commercialization of BES.14–16 While a high amount of biocatalyst i.e. a high biofilm density X is a prerequisite for high current densities, the transport of nutrients and reaction products is limited by the biofilm itself.17–19The transport of substrate and reaction products i.e. fluxes into and out of the biofilm are linked to the current density according to Faraday's law.14,20 The fluxes are mainly governed by diffusion which is driven by gradients and coupled with the diffusion coefficient D according to Fick's laws. The gradients result in local chemical microenvironments e.g. substrate concentration and pH that effect metabolic activity and therefore current production.21–23 However, the chemical microenvironment in biofilms has only been measured directly in biofilms grown on flat electrodes mostly using microelectrodes.24–26 The inside of most porous electrodes cannot be characterized because the material is too fragile (for the use of microelectrodes) or opaque (for characterization with optical tomographic methods, see below). Compared to porous electrodes8,10 which enable more space for the biofilm and enhanced mass transport, flat electrodes support relatively low current densities and low productivity, but are accessible for such measurements and simple to model.
Such computational biofilm models that include mass transport allow a deeper understanding of the processes occurring in biofilms.27–29 Existing models of biofilms grown on flat electrodes either rely on parametrization of the biofilm structure, diffusion coefficients and substrate conversion kinetics10,20,30–34 or make use of spatial information (abstracted profiles of diffusion coefficients) acquired by tomographic imaging techniques.13,35
Modelling of complex biofilm structures and its validation requires in-depth information that can be acquired using various tomographic measuring techniques such as Confocal Laser Scanning Microscopy (CLSM),36–41 Optical Coherence Tomography (OCT)42–47 and Magnetic Resonance Imaging (MRI).48 While optical tomographic techniques allow fast and high resolution imaging, only MRI enables the characterization of optically opaque porous electrode/biofilm structures.45,49–51
1H-MRI visualizes predominantly water as it is the most abundant molecule with 1H-nuclei. The contrast in MR images depends on structure specific properties of water molecules such as relaxation (e.g. T1, T2,  ), concentration and diffusion. While water signals are detected from the intra- or extracellular space, the electrode itself does not contribute to the 1H-signal measured and appears in the data as a region with reduced signal intensity. Besides imaging of biofilm structures, MRI enables the monitoring of chemical species, metabolic processes and transport phenomena non-destructively, in situ and in vivo.52–59 Thus, it allows the measurement of a variety of quantities relevant to characterization and modelling of electroactive biofilms on porous electrodes. For instance, the MRI measurands – such as relaxation times T1, T2 and apparent diffusion coefficients D* – are indicators for the biofilm density as they differ significantly from the bulk water due to the different physical environments.53,60 Because the biofilm density X cannot be quantified directly by MRI, empirical correlations of MRI measurands and X are required.
), concentration and diffusion. While water signals are detected from the intra- or extracellular space, the electrode itself does not contribute to the 1H-signal measured and appears in the data as a region with reduced signal intensity. Besides imaging of biofilm structures, MRI enables the monitoring of chemical species, metabolic processes and transport phenomena non-destructively, in situ and in vivo.52–59 Thus, it allows the measurement of a variety of quantities relevant to characterization and modelling of electroactive biofilms on porous electrodes. For instance, the MRI measurands – such as relaxation times T1, T2 and apparent diffusion coefficients D* – are indicators for the biofilm density as they differ significantly from the bulk water due to the different physical environments.53,60 Because the biofilm density X cannot be quantified directly by MRI, empirical correlations of MRI measurands and X are required.
Recently, an ex vivo study showed that the biofilm volume (extracted from colonized carbon beads) specified by T1 correlates with total nitrogen content of the biofilm and the total produced electrical charge.61 Previously Renslow et al. used apparent diffusion coefficients D* and an empiric correlation17 to quantify the local biofilm density X.35 However, the biofilm quantification was not validated. The depth profiles of D* and X derived from these MRI data were subsequently used to simulate substrate flux, current production and substrate concentration profiles within a G. sulfurreducens biofilm grown on a flat electrode.
So far, no study has been published that exploits the benefits of MRI – the in vivo imaging of complex EABs inside porous electrodes that are relevant for applications. The present study demonstrates the feasibility of MRI as an in vivo, in situ, in operando and non-invasive method to characterize electroactive biofilms inside porous electrodes. High performance carbon nanofiber electrodes are used which enable high biofilm and current density with S. oneidensis.62–64 The biofilm density and its distribution are analyzed with qualitative indicators, the transversal relaxation time T2 and the (effective) apparent diffusion coefficient D* of water. The acquired data, especially the D* as transport coefficient may, support the 3D modelling of complex biofilms in porous structures.
Materials and methods
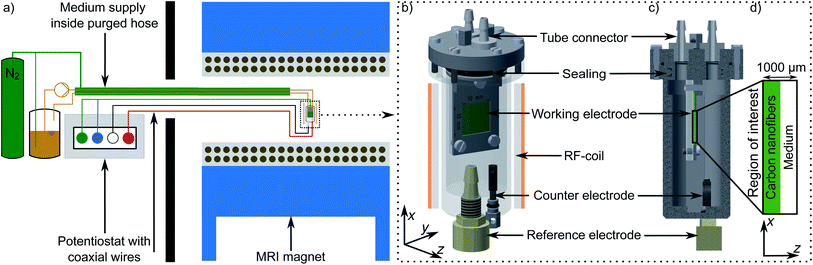
We developed a bioelectrochemical reactor suitable for MRI as shown in Fig. 1a and b. The reactor was placed inside the MRI magnet while the nitrogen-purged medium reservoir including medium pump (Ismatec REGLO Digital MS-4/6, Cole-Parmer GmbH, Wertheim, Germany) as well as the potentiostat (Gamry Interface 1010E, C3 Prozess- und Analysentechnik GmbH, Haar, Germany) were located at 4 m distance from the magnet in an adjacent room. The medium feed tube was placed inside a nitrogen gas purged jacket to minimize oxygen intrusion by diffusion. The potentiostat was connected to the electrodes by grounded coaxial wires with non-magnetizing terminal resistors (SRT Resistor Technology GmbH, Cadolzburg, Germany) and tailor-made titanium connectors. The working electrode was mounted in the electrode holder and exposed to the medium at one side. The working electrode (10 × 10 × 0.5 mm3) was a high-performance electrode made of electrospun and carbonized nanofibers with a mean diameter of 108 nm and a porosity of 94% (mesopore volume Vmeso = 0.24 × 103 cm3, mean macropore diameter dmacro = 0.4 μm, for more details see at Erben et al.63). A platinum mesh served as counter electrode. The Ag/AgCl reference electrode was made from a silver wire coated with AgCl-suspension inside a LuerLock connector filled with saturated KCl-solution and capped with a glass frit (Gamry, C3 Prozess- und Analysentechnik GmbH, Haar, Germany). The reactor was designed to minimize magnetic field distortions in the region of interest (ROI) by reducing the conductive materials and optimize the alignment of working electrode to the RF coil.The reactor, the reservoir and lines were filled with 300 ml medium and sterilized by autoclaving at 121 °C for 20 min. Two variants of the medium were used: a standard medium M and an improved medium M* with high phosphate-buffered saline (PBS) content and additional riboflavin (see Table 1 for details). All chemicals were purchased from commercial suppliers, in particular from Sigma-Aldrich (Taufkirchen, Germany), Carl Roth (Karlsruhe, Germany) and Merck (Darmstadt, Germany). After autoclaving the reference electrode was sterilised with 10% hydrogen peroxide solution and then mounted in the reactor under sterile conditions. Before inoculation with Shewanella oneidensis MR-1, the contrast agent Gd-DTPA (Magnevist, Bayer AG, Leverkusen, Germany) was added via a sterile filter and septum. Magnevist, which is assumed not to enter the bacteria, reduces the T1 of the medium and total measurement time considerably. By using a TR of 0.5 s the signal originating from the bacterial cytoplasmic compartment, which is expected to have a longer T1 relaxation time, will be attenuated. This attenuation favours the characterization of the bacterial microenvironment inside the porous electrode. The working electrode was polarized at a potential of 0 V vs. Ag/AgCl for at least 12 h prior to inoculation to minimize non-coulombic contributions to the measured current.
| Component | Concentration c in mmol L−1 | |
|---|---|---|
| Medium | Standard M | Improved M* |
| NaCl | 137 | 77 |
| KCl | 2.7 | 2.7 |
| NaHPO | 10 | 40 |
| KHPO4 | 1.76 | 7.04 |
| (NH4)2SO4 | 9 | 9 |
| MgSO4·7H2O | 1 | 1 |
| CaCl2 | 0.1 | 0.1 |
| Trace elements | ||
| Casein hydrolysate | 0.1 | 0.1 |
| Na-D/L lactate (50% in H2O) | 50 | 50 |
| Additional components | ||
| Riboflavin | 0 | 0.001 |
| Contrast agent Magnevist | 2 | 2 |
S. oneidensis MR-1 cells were precultivated aerobically in Lysogeny Broth (LB medium) and prepared for the use in the bioelectrochemical reactor according to Kipf et al.65 The sterilized reactor was finally inoculated through a sterile septum to get an initial optical density (OD600) of approximately 0.05.
The entire bioreactor was imaged prior to the inoculation for control purposes (abiotic status) and after the bioproduced current density reached its maximum (biotic status) using the same MR sequences and parameters. MR imaging of water was done at 300 MHz (7 T) on a horizontal small-bore MRI system (Bruker Biospec 70/20 USR, Bruker BioSpin MRI, Ettlingen, Germany) with a gradient system of 12 cm inner diameter (B-GA 12S2). For signal transmission and reception, a custom-made, inductively coupled RF-coil (linearly polarized slotted tube resonator) was used which encloses the entire reactor. After adjusting the field homogeneity and RF-power levels the reactor was imaged with two 3D spin-echo (SE) sequences, one with T2- and a second with diffusion weighting comprising the same field-of-view (FOV) of 19.20 mm × 19.20 mm × 20.0 mm (z y x direction). The FOV is mapped with a resolution of at least 384 × 32 × 33 voxels. This results in a resolution of 50 μm in the z-direction. Local T2-values were calculated from SE images obtained with a TR of 0.5 s at 8 echo times TE of 20 ms, 40 ms, 80 ms, 100 ms, 120 ms, 140 ms, 160 ms by fitting the observed signal decay to the equation S(TE) = S(0)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−TE/T2}. The corresponding 3D-maps of the local self-diffusion constant D* of water were calculated from 4 images obtained with the so called b-values of 50, 350, 650, and 950 s mm−2 fitted to the equation S(b) = S(0)
exp{−TE/T2}. The corresponding 3D-maps of the local self-diffusion constant D* of water were calculated from 4 images obtained with the so called b-values of 50, 350, 650, and 950 s mm−2 fitted to the equation S(b) = S(0)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−bD*}, where the b-value describes the diffusion weighting, which can be tuned by strength and duration of the two diffusion sensitizing magnetic field gradients. Prior to data fitting the recorded time domain data was Fourier transformed, masked, filtered, analyzed and graphically represented using custom ImageJ, Matlab and LaTeX scripts. Detailed information on the processing steps is available in the supplementary information (Fig. S1† in ESI).
exp{−bD*}, where the b-value describes the diffusion weighting, which can be tuned by strength and duration of the two diffusion sensitizing magnetic field gradients. Prior to data fitting the recorded time domain data was Fourier transformed, masked, filtered, analyzed and graphically represented using custom ImageJ, Matlab and LaTeX scripts. Detailed information on the processing steps is available in the supplementary information (Fig. S1† in ESI).
Relative slice diffusion coefficients 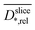 are (in z-slices/parallel to electrode) averaged slice diffusion coefficients
are (in z-slices/parallel to electrode) averaged slice diffusion coefficients  normalized to their respective value in the medium
normalized to their respective value in the medium 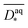 .
.  and relative slice relaxation times
and relative slice relaxation times 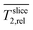 calculated analogously are unitless and range from 0 to 1.
calculated analogously are unitless and range from 0 to 1.
The depth dependent biofilm density  was calculated assuming the relative slice diffusion coefficients
was calculated assuming the relative slice diffusion coefficients  as diffusion coefficient using the empirical correlation from Fan et al.:17
as diffusion coefficient using the empirical correlation from Fan et al.:17
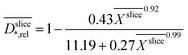 | (1) |
The biofilm density 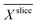 inside the electrode was normalized using the biofilm density
inside the electrode was normalized using the biofilm density  of the abiotic uncolonized electrode.
of the abiotic uncolonized electrode.
After the MRI experiments the electrode was removed from the reactor, the biofilm was fixated and characterized with fluorescence microscopy and qPCR as control for the biofilm density and its distribution (details in ESI†).66
Results and discussion
Two S. oneidensis biofilms respiring on and inside porous electrodes in two different media were characterized using T2-weighted and diffusion-weighted MR imaging to obtain spatial information on biofilm distribution and diffusion coefficients. To demonstrate the MRI capabilities and to visualize biofilm differences, two biofilms were grown using a standard medium M64,65,67 and an improved medium M* with increased buffer concentration (40 mM PBS) and supplementary riboflavin (1 μM) that stimulates biofilm growth.67The biofilms grown on the electrodes were characterized once the current production had become stable (t > 120 h, solid line in Fig. 2a and b). The dotted line represents the current density of the biofilm outside the MRI scanner. Optical density OD600 of the media was always below 0.1 (Fig. S8† in ESI) indicating that oxygen input through the reactor and peripherals and the resulting planktonic growth of S. oneidensis was negligible.64
 | ||
| Fig. 2 Chronoamperometry at a potential of 0 mV vs. Ag/Cl reveals the rising current as result of S. oneidensis use of the electrode as electron acceptor (a) and the feasibility of MRI measurements while operating the bioelectrochemical reactor (b). In the standard medium M an electric defect of the reference electrode (marked with + in (b)) caused the breakdown of the current density, but a control measurement revealed that biofilm was still attached (in ESI†). | ||
Unfortunately, the reference electrode in the experiment with standard medium M was defective, leading to breakdown of the current production (marked with + in Fig. 2b). However, the subsequent control MR imaging (Fig. S7† in ESI) did reveal that the biofilm was still attached to the anode. Therefore, we assume that these experimental results are valid. Our focus was on the biofilm in the improved medium M* while the weak biofilm in the standard medium M should only demonstrate the higher resolution.
Current production during MR imaging
The maximum observed current density imax was 68 μA cm−2 in the standard medium M and 280 μA cm−2 in the improved medium M*. These current densities are slightly lower than values reported for electron spun material in the literature,62,64 but higher than Renslows S. oneidensis biofilm grown on a flat gold electrode (1.83 μA mm−2).35 Higher current densities (in both media M and M*) reported by Erben et al. are most likely caused by the higher temperature of 30 °C compared to our study (∼21 °C). The current increase by a factor of 2 can be well explained by lower metabolic rates due to the temperature difference of about 10 °C to the optimal temperature of 30 °C.68 The drop in current production after the reactor was positioned from the adjacent room into the MRI magnet (black arrows in Fig. 2b) was probably caused by the temperature difference between the two rooms. The observations described above demonstrate that in operando MR image acquisition of live electroactive biofilms within porous electrodes is possible.T2-weighted imaging of the bioanode
Fig. 3a shows four representative slices of the region of interest (ROI) covering the porous electrode (slice A) to the growth medium (slices C and D). Slice B represent the electrode with biofilm (visualized for the improved medium M*, standard medium M not shown). The transversal relaxation T2 is presented as heat maps. The slices are parallel to the electrode/biofilm starting at the back of the electrode, reaching the center and the interface of electrode/medium (z ≤ 500 μm) and the medium (z ≥ 600 μm). As the electrode is highly porous and filled with medium the boundary between them is unsharp and illustrated in shades of grey. By averaging all T2 values in each slice and dividing by Taq2 of water/medium, relative relaxation times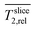 of each slice were calculated. Fig. 3b shows a comparison of depth profiles of these
of each slice were calculated. Fig. 3b shows a comparison of depth profiles of these  for the electrode without biofilm (abiotic control) and with the biofilm in both media M and M*.
for the electrode without biofilm (abiotic control) and with the biofilm in both media M and M*.
In the following the regions of the electrode with biofilm in the improved medium M* are discussed:
• Electrode without biofilm (slice A, z = 200 μm):
Slice A is placed at the center of the electrode and shows a rather homogenous T2 distribution.  in this region (slice A) was lower than in the medium (slice C and D,
in this region (slice A) was lower than in the medium (slice C and D,  ∼ 1).
∼ 1).
• Electrode with (maximum) biofilm (slice B, z = 400 μm):
The averaged 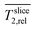 with biofilm (in both media M* and M) show a minimum close to the electrode growth medium interface. The minima are not present in the abiotic control (slight decrease is not significant) revealing that the biofilm reduces T2 relaxation time in accordance with other MRI studies of biofilms.48 The T2 heat maps in this region (slice B and Fig. S2† in ESI) show an inhomogeneous distribution indicating an uneven biofilm distribution inside the electrode.
with biofilm (in both media M* and M) show a minimum close to the electrode growth medium interface. The minima are not present in the abiotic control (slight decrease is not significant) revealing that the biofilm reduces T2 relaxation time in accordance with other MRI studies of biofilms.48 The T2 heat maps in this region (slice B and Fig. S2† in ESI) show an inhomogeneous distribution indicating an uneven biofilm distribution inside the electrode.
• Growth medium (slice C and D, z ≤ 600 μm):
 is defined as 1 in this region (normalization).
is defined as 1 in this region (normalization).  in this region is not reduced compared to the abiotic control neither by biofilm nor planktonic cells, indicating low cell densities.
in this region is not reduced compared to the abiotic control neither by biofilm nor planktonic cells, indicating low cell densities.
Fig. 3b reveals differences in the biofilm formation for biofilms grown in both media. The biofilm in the improved medium M* is denser and thicker than in the standard medium M according to its lower  minimum (0.490 ± 0.018 vs. 0.687 ± 0.035) and its broader peak (300 μm vs. 250 μm). Please note: all given deviations (value after ±) were calculated based on the standard deviation of their corresponding values in the medium and do not reflect inhomogeneities of the biofilm. The width of the Tslice2,rel peak in the improved medium M* is mainly caused by the inhomogeneity of the biofilm and in particular by its curved shape (affecting the value Tslice2,rel minima, see ESI†). The lower
minimum (0.490 ± 0.018 vs. 0.687 ± 0.035) and its broader peak (300 μm vs. 250 μm). Please note: all given deviations (value after ±) were calculated based on the standard deviation of their corresponding values in the medium and do not reflect inhomogeneities of the biofilm. The width of the Tslice2,rel peak in the improved medium M* is mainly caused by the inhomogeneity of the biofilm and in particular by its curved shape (affecting the value Tslice2,rel minima, see ESI†). The lower  minimum in the improved medium M* might be related to the higher concentration of buffer and riboflavin and thus more biofilm mass.67 In both media, cells were found in the entire electrode (see fluorescence microscopy images Fig. S3† in ESI), but most of the biomass is almost exclusively present close to the interface of electrode to medium.
minimum in the improved medium M* might be related to the higher concentration of buffer and riboflavin and thus more biofilm mass.67 In both media, cells were found in the entire electrode (see fluorescence microscopy images Fig. S3† in ESI), but most of the biomass is almost exclusively present close to the interface of electrode to medium.
Accordingly, a large portion of the electrode is not or only marginally colonized by the biofilm. Hence, the total biofilm density and thereby the amount of biocatalyst in the electrode could be higher. The location of the biofilm at the electrode surface might be related to limited diffusive transport of nutrients into the depth of the fiber-biofilm structure and products, especially protons, out of it. To investigate this issue in the next section spatially resolved relative diffusion coefficients  were determined and calculated analogously to the determination of relative relaxation times
were determined and calculated analogously to the determination of relative relaxation times 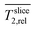 .
.
Diffusion inside the porous electrode without and with biofilm
Fig. 4a shows depth profile of the relative diffusion coefficient (see Materials and methods section) without and with biofilms in the standard medium M and in the improved medium M*. The profiles of the relative diffusion coefficients are similar to the relaxation times (Fig. 3b): For the uncolonized and the colonized electrode,
(see Materials and methods section) without and with biofilms in the standard medium M and in the improved medium M*. The profiles of the relative diffusion coefficients are similar to the relaxation times (Fig. 3b): For the uncolonized and the colonized electrode,  inside the center of the electrode (z ≤ 300 μm) is similar to
inside the center of the electrode (z ≤ 300 μm) is similar to  in the growth medium (z ≤ 600 μm) as the pores of the electrode are filled with medium (porosity >90%). A relative diffusion coefficient of 1 corresponds to a water diffusion coefficient
in the growth medium (z ≤ 600 μm) as the pores of the electrode are filled with medium (porosity >90%). A relative diffusion coefficient of 1 corresponds to a water diffusion coefficient  of 2.00 10−9 ± 0.17 10−9 m2 s−1 in the standard medium M and 1.94 10−9 ± 0.09 10−9 m2 s−1 in the improved medium M* related to temperatures of approximately 22 °C and 21 °C.69
of 2.00 10−9 ± 0.17 10−9 m2 s−1 in the standard medium M and 1.94 10−9 ± 0.09 10−9 m2 s−1 in the improved medium M* related to temperatures of approximately 22 °C and 21 °C.69
The diffusion near the boundary between electrode and medium is reduced as compared to the electrode center and the growth medium. The minimum of the relative diffusion coefficient 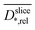 in the improved medium M* is lower as compared to the standard medium M (0.448 ± 0.056 < 0.713 ± 0.087). The minima of the diffusion coefficients are in the same distance from the electrode surface as the minima of the relaxation times
in the improved medium M* is lower as compared to the standard medium M (0.448 ± 0.056 < 0.713 ± 0.087). The minima of the diffusion coefficients are in the same distance from the electrode surface as the minima of the relaxation times 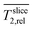 . T2 and D* heat maps as well as the
. T2 and D* heat maps as well as the  and
and  profiles show a very high similarity. This similarity is in part caused by the diffusion weighting bias brought by the strong magnetic field gradients required for high resolution imaging.70 This effect attenuates T2-values of areas with unhindered diffusion more than those of diffusion restricted areas. Nevertheless, the well-known link of biofilm density and restricted diffusion17,19 is confirmed by the correlation of
profiles show a very high similarity. This similarity is in part caused by the diffusion weighting bias brought by the strong magnetic field gradients required for high resolution imaging.70 This effect attenuates T2-values of areas with unhindered diffusion more than those of diffusion restricted areas. Nevertheless, the well-known link of biofilm density and restricted diffusion17,19 is confirmed by the correlation of  and
and 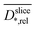 (Fig. S6† in ESI).
(Fig. S6† in ESI).
The morphology and diffusion coefficients of the EABs grown inside porous electrodes in our study differ from the S. oneidensis biofilm grown on a flat surface analyzed with MRI by Renslow et al.35 Since biofilm formation from S. oneidensis on gold electrodes is often difficult, Renslow et al. pre-cultured a biofilm with a constant depth film fermenter, harvested it and transplanted it into the MRI reactor. For this reason, their biofilm exhibited a heterogenous morphology with individual biofilm clusters that settled on the flat gold electrode. In our case it has to be considered, that the colonization of individual S. oneidensis cells is different to the attachment of cell clusters, and that the colonization of cells inside electrospun carbon fibers is enhanced compared to a flat electrode. Therefore, the structure and the diffusion coefficients of our biofilms are more comparable to the uniform shape of Renslow's Geobacter sulfurreducens biofilm: it has a dense core, but in contrast to the G. sulfurreducens biofilm, the diffusion coefficients inside the S. oneidensis biofilm decrease on both, the fluid facing and the opposite sides. This underlines: biofilm properties such as morphology and diffusion coefficients vary substantially depending on the electrode and the cultivation conditions. While on a flat surface the EAB only has a limited surface for extracellular electron transfer (EET), an EAB inside a porous electrode can use more electrode volume for EET and that could support uniform biofilm growth within the electrode and more current production.
In porous materials with a high biofilm density and thus a high current production, proton transport from the individual cells to the bulk can become rate-limiting. Under such conditions, not only the electrode has an influence on the biofilm formation, but also the medium:67 assuming the same biofilm density, higher buffer capacity in the improved medium M* enhances diffusive transport of protons or the corresponding buffer molecules and thus provides lower local acidification of the anodic biofilm in the improved medium M* as compared to the standard medium M (lower buffer concentration). This allows the biofilm in the improved medium M* to continue to grow and to increase its density until its local acidification reaches the level of the biofilm in the standard medium M. Thus, high puffer capacities allow high diffusive proton transport rates and thus more biofilm mass.67
The higher biofilm mass could lead to a homogeneous distribution in the electrode (thicker biofilm) or to an increase in biofilm density in the front region (denser biofilm). The local increase of the biofilm density in the front region is equivalent to an increase in the biocatalyst in the region. Thus, the volumetric production of electrons and protons also increases. In this case, the protons are more concentrated in the front region. According to Fick's 1st law of diffusion, a steeper gradient in denser biofilms results in higher transport fluxes and thus higher currents.
We observed exactly that: the biofilm is mainly located around the interface of electrode and growth medium (see also fluorescence microscopy images in Fig. S3† in ESI) and higher buffer capacity result in a denser, but not thicker biofilms. This is well in line with Erben et al.64 who investigated the biofilm formation on the same material: under anaerobic conditions (OD < 0.1, similar to our experiments) more biofilm was found at the front (medium facing) side than on the backside as observed by scanning electron microscopy (SEM).64 However, the imaging techniques like SEM or fluorescence microscopy used in this context only provide information about the first few micrometers in depth. In contrast to these methods, MR imaging covers the entire volume and has previously been used to quantify the biofilm density.35,58 Unlike other bulk-based methods using dry weight, total nitrogen content, qPCR or protein analysis,13,46,50,61 the determination of biofilm density based on correlation of MRI parameters is in situ and non-destructive.
Biofilm density distribution inside the porous electrode
Assuming the validity of the frequently used empirical correlation of Fan et al. (see eqn (1), Materials and methods section) the biofilm density was calculated with
was calculated with 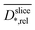 . In Fig. 4b for both media, the profile of the biofilm density
. In Fig. 4b for both media, the profile of the biofilm density 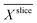 is plotted against the z-axis. The empirical relation translates low
is plotted against the z-axis. The empirical relation translates low  to high biofilm densities. Thus, the maximum biofilm density coincides with
to high biofilm densities. Thus, the maximum biofilm density coincides with  minima inside the electrode. Due to the lower
minima inside the electrode. Due to the lower 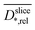 values, in the improved medium M* the maximum biofilm density
values, in the improved medium M* the maximum biofilm density 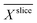 = 32.8 (28.8, 40.7) kg m−3 is higher than 9.6 (7.1, 17.1) kg m−3 determined in the standard medium M with lower buffer concentration. The values in brackets reflect the deviation of the biofilm density calculations and are based on the standard deviations of the relative diffusion coefficients in the medium. The upper limit is higher because the empirical Fan correlation is not linear.
= 32.8 (28.8, 40.7) kg m−3 is higher than 9.6 (7.1, 17.1) kg m−3 determined in the standard medium M with lower buffer concentration. The values in brackets reflect the deviation of the biofilm density calculations and are based on the standard deviations of the relative diffusion coefficients in the medium. The upper limit is higher because the empirical Fan correlation is not linear.
The results of the Fan's empirical relationship and in particular the quantitative results should be treated with caution for at least three reasons:
• First, this relationship was derived from the characterization of aerobic biofilms on smooth surfaces, rotating cylinders, and bioflocs.17 The conditions in our experiments differ significantly: the use of porous 3D electrodes and a facultative anaerobic organism with a metabolism based on external electron transfer might result in different cell densities, Extracellular Polymeric Substance (EPS) and the production of conductive appendages, affecting the density of the biomass.
• Second, the diffusion coefficients for the entire biofilm were specified mostly using diffusion reaction models and diffusion cells, which both measure the transport of a specific species trough a compartment.17 This might differ significantly from the MRI approach determining the self-movement of water molecules in a compartment/sub volume/voxel.
• Third, compared to bulk diffusion coefficients the spatially resolved diffusion coefficients might be biased due to the non-linearity of the empiric correlation (Fig. S4f and S5† in ESI). Thus, variances introduced by higher noise levels at high resolution, subvolumes and the biofilm inhomogeneity can lead to different biofilm densities (discussed in ESI†).
• Fourth, the model from Fan et al. has been derived from data measuring the diffusion in the extracellular space of the biofilm. Similarly, in the present study the signal from the bacterial cytoplasm is attenuated due to the addition of the contrast agent Gd-DTPA, which reduces only the T1-relaxation time of the medium. Thus, the estimated diffusion coefficients are biased to the extracellular space which justifies to use the Fan model in order to calculate the biofilm density.
Therefore, it is even more important to validate the biofilm densities and their distribution determined by diffusion weighted MRI and the Fan correlation. For example, the cell count (not including EPS-matrix) or the total biomass in the electrode can be determined using qPCR assays or determination of the total nitrogen content.61,71,72 The validation we applied by qPCR and correlation of current per biomass (see ESI† for details) cannot confirm quantitatively the biofilm density but show a similar trend of a higher biofilm density in the improved medium M*.
Limitations of an electroactive biofilm in a porous fiber electrode
The amount of current produced depends on the amount of biocatalyst or biofilm mass.64 Certain materials with a high attractiveness of the anodic habitat, such as the porous carbon nanofiber electrode used here, stimulate biofilm formation and current production, especially for S. oneidensis.62,64,73,74In this study, MRI demonstrated that the ability of the porous electrospun electrode to host biofilm is not fully exploited. The determined biofilm density distribution within electrodes is inhomogeneous, as mostly the region around the interface of electrode to medium is colonized. The porous electrode could thus host higher biofilm densities in the deeper region, but also in the front region the biofilm density could thus be higher as a comparison to other biofilms shows.35 The question remains why only a part of the electrode is colonized and what can be deduced from the shape of the biofilm.
The transport through the biofilm is restricted by the biofilm itself. The consequence of the restricted diffusive transport in the biofilm and metabolism of the cells is the formation of a concentration gradient. Gradients in the electrode create microenvironments which influence the metabolism, the current production and the biofilm formation.2,13,23,24,75
For instance, if fresh medium with dissolved oxygen is present at the liquid facing side, in the anaerobic depth of the biofilm the terminal electron acceptor (TEA) is the electrode where the aerobic toplayer uses dissolved oxygen as TEA.76 Moreover, the directional motility of cells may prevent penetrating deeper due to the higher redox potential of oxygen compared to the potential of the anode.77 However, the observed optical density OD600 < 0.1 in the growth medium does not indicate a high amount of dissolved oxygen (Fig. S7† in ESI).
Biofilm formation in deep layers could also be prevented by the arising pH gradients/local acidification in deep layers of the biofilm/electrode.67 However, increasing the buffer concentration (from standard medium M to improved medium M*) does not, or only slightly, cause the biofilm to penetrate deeper into the electrode – instead, the biofilm density increases. But even in the improved medium M*, the biofilm density is not high enough to restrict transport of protons in the electrode completely. This can be deduced from the fact that the relative relaxation times  and diffusion coefficients
and diffusion coefficients  are reduced by a maximum of 55% inside the biofilm. Denser and not thicker biofilms enhance diffusive transport fluxes because the driving forces i.e., concentration gradients are higher. The higher transport flux of protons and their corresponding buffer molecules lead to higher current densities.
are reduced by a maximum of 55% inside the biofilm. Denser and not thicker biofilms enhance diffusive transport fluxes because the driving forces i.e., concentration gradients are higher. The higher transport flux of protons and their corresponding buffer molecules lead to higher current densities.
Thus, further research is needed to understand the specific connection between concentration gradients and biofilm formation in porous electrodes. Advanced NMR methods such as spectroscopic imaging, chemical exchange saturation transfer (CEST) and electrophoretic NMR in combination with electrochemical methods can further elucidate the dynamics of and inside the biofilm and its performance in bioelectrochemical systems.2,78–81
Conclusions
In this study we present a biochemical reactor for the non-destructive, in vivo and in situ characterization of electroactive biofilms with Magnetic Resonance Imaging. For the first time, we show the feasibility of MRI inside porous electrodes. We demonstrate that the MR image acquisition is possible while the reactor serves as a bioelectrochemical cell. The biofilm reduced both transversal relaxation times T2 and apparent diffusion coefficients D*. The spatial biofilm density can be qualitatively estimated from the T2 or D* values. Furthermore, a quantitative determination via relative T2 and D* and empirical correlations is possible but requires further research. In an improved medium M* with a higher buffer concentration the biofilm produced more biomass (lower T2 and D*) and higher current density. The MRI method was able to reveal that only a fraction – the upper, fluid facing side – of the electrode was colonized by the biofilm leading to low current densities. In conclusion, this study showed that MRI is a versatile method for the characterization and development of electroactive biofilms inside porous, opaque electrodes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the German Research Association DFG through the priority programme SPP 2240: eBiotech (project: 445813859, grant numbers: KE 1576/5-1 and DR 298/15-1). Furthermore, the authors want to thank SRT Resistor Technology GmbH for providing non-magnetizing resistors and Hanseatische Waren Handelsgesellschaft mbH & Co. KG for providing titanium raw materials.Notes and references
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schröder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Microbial fuel cells: methodology and technology, Environ. Sci. Technol., 2006, 40, 5181–5192 CrossRef CAS PubMed.
- Bioelectrochemical systems. From extracellular electron transfer to biotechnological application, K. Rabaey, L. T. Angenent, U. Schröder and J. Keller, ed. IWA Publishing, London, New York, 2010, ISBN: 9781843392330 Search PubMed.
- F. Harnisch and U. Schröder, From MFC to MXC: chemical and biological cathodes and their potential for microbial bioelectrochemical systems, Chem. Soc. Rev., 2010, 39, 4433–4448 RSC.
- H. V. M. Hamelers, A. ter Heijne, T. H. J. A. Sleutels, A. W. Jeremiasse, D. P. B. T. B. Strik and C. J. N. Buisman, New applications and performance of bioelectrochemical systems, Appl. Microbiol. Biotechnol., 2010, 85, 1673–1685 CrossRef CAS PubMed.
- V. Shah, Emerging environmental technologies, Springer, New York, 2008 Search PubMed.
- H. Wang and Z. J. Ren, A comprehensive review of microbial electrochemical systems as a platform technology, Biotechnol. Adv., 2013, 31, 1796–1807 CrossRef CAS PubMed.
- A. Schievano, T. Pepé Sciarria, K. Vanbroekhoven, H. de Wever, S. Puig, S. J. Andersen, K. Rabaey and D. Pant, Electro-Fermentation - Merging Electrochemistry with Fermentation in Industrial Applications, Trends Biotechnol., 2016, 34, 866–878 CrossRef CAS PubMed.
- C. Santoro, C. Arbizzani, B. Erable and I. Ieropoulos, Microbial fuel cells: From fundamentals to applications. A review, J. Power Sources, 2017, 356, 225–244 CrossRef CAS PubMed.
- G. Kumar, R. G. Saratale, A. Kadier, P. Sivagurunathan, G. Zhen, S.-H. Kim and G. D. Saratale, A review on bio-electrochemical systems (BESs) for the syngas and value added biochemicals production, Chemosphere, 2017, 177, 84–92 CrossRef CAS PubMed.
- F. Harnisch and D. Holtmann, Bioelectrosynthesis, Springer International Publishing, Cham, 2019 Search PubMed.
- T. J.-P. Ivase, B. B. Nyakuma, O. Oladokun, P. T. Abu and M. N. Hassan, Review of the principal mechanisms, prospects, and challenges of bioelectrochemical systems, Environ. Prog. Sustainable Energy, 2020, 39, 13298 CrossRef CAS.
- A. P. Borole, G. Reguera, B. Ringeisen, Z.-W. Wang, Y. Feng and B. H. Kim, Electroactive biofilms: Current status and future research needs, Energy Environ. Sci., 2011, 4, 4813 RSC.
- H. Beyenal and J. T. Babauta, Biofilms in bioelectrochemical systems. From laboratory practice to data interpretation, John Wiley & Sons Inc, Hoboken, 2015 Search PubMed.
- P. N. Bartlett, Bioelectrochemistry. Fundamentals, experimental techniques and applications, John Wiley & Sons, Chichester England, Hoboken NJ, 2008 Search PubMed.
- T. H. J. A. Sleutels, A. ter Heijne, C. J. N. Buisman and H. V. M. Hamelers, Bioelectrochemical systems: an outlook for practical applications, ChemSusChem, 2012, 5, 1012–1019 CrossRef CAS PubMed.
- M. M. Ghangrekar and P. Chatterjee, A Systematic Review on Bioelectrochemical Systems Research, Curr. Pollut. Rep., 2017, 3, 281–288 CrossRef CAS.
- L. S. Fan, R. Leyva-Ramos, K. D. Wisecarver and B. J. Zehner, Diffusion of phenol through a biofilm grown on activated carbon particles in a draft-tube three-phase fluidized-bed bioreactor, Biotechnol. Bioeng., 1990, 35, 279–286 CrossRef CAS PubMed.
- P. S. Stewart, A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms, Biotechnol. Bioeng., 1998, 59, 261–272 CrossRef CAS PubMed.
- H. Horn and E. Morgenroth, Transport of oxygen, sodium chloride, and sodium nitrate in biofilms, Chem. Eng. Sci., 2006, 61, 1347–1356 CrossRef CAS.
- C. I. Torres, A. K. Marcus, P. Parameswaran and B. E. Rittmann, Kinetic experiments for evaluating the Nernst-Monod model for anode-respiring bacteria (ARB) in a biofilm anode, Environ. Sci. Technol., 2008, 42, 6593–6597 CrossRef CAS PubMed.
- C. I. Torres, A. Kato Marcus and B. E. Rittmann, Proton transport inside the biofilm limits electrical current generation by anode-respiring bacteria, Biotechnol. Bioeng., 2008, 100, 872–881 CrossRef CAS PubMed.
- A. K. Marcus, C. I. Torres and B. E. Rittmann, Analysis of a microbial electrochemical cell using the proton condition in biofilm (PCBIOFILM) model, Bioresour. Technol., 2011, 102, 253–262 CrossRef CAS PubMed.
- S. C. Popat and C. I. Torres, Critical transport rates that limit the performance of microbial electrochemistry technologies, Bioresour. Technol., 2016, 215, 265–273 CrossRef CAS PubMed.
- J. T. Babauta, H. D. Nguyen, T. D. Harrington, R. Renslow and H. Beyenal, pH, redox potential and local biofilm potential microenvironments within Geobacter sulfurreducens biofilms and their roles in electron transfer, Biotechnol. Bioeng., 2012, 109, 2651–2662 CrossRef CAS PubMed.
- J. T. Babauta and H. Beyenal, Local Current Variation by Depth in Geobacter Sulfurreducens Biofilms, J. Electrochem. Soc., 2014, 161, H3070–H3075 CrossRef.
- J. Hou, Z. Liu, Y. Zhou, W. Chen, Y. Li and L. Sang, An experimental study of pH distributions within an electricity-producing biofilm by using pH microelectrode, Electrochim. Acta, 2017, 251, 187–194 CrossRef CAS.
- D. Deb, R. Patel and V. E. Balas, A Review of Control-Oriented Bioelectrochemical Mathematical Models of Microbial Fuel Cells, Processes, 2020, 8, 583 CrossRef CAS.
- E. M. Gaffney, M. Grattieri, Z. Rhodes and S. D. Minteer, Editors' Choice—Review—Exploration of Computational Approaches for Understanding Microbial Electrochemical Systems: Opportunities and Future Directions, J. Electrochem. Soc., 2020, 167, 65502 CrossRef CAS.
- S. Luo, H. Sun, Q. Ping, R. Jin and Z. He, A Review of Modeling Bioelectrochemical Systems: Engineering and Statistical Aspects, Energies, 2016, 9, 111 CrossRef.
- C. Picioreanu, I. M. Head, K. P. Katuri, M. C. M. van Loosdrecht and K. Scott, A computational model for biofilm-based microbial fuel cells, Water Res., 2007, 41, 2921–2940 CrossRef CAS PubMed.
- A. Kato Marcus, C. I. Torres and B. E. Rittmann, Conduction-based modeling of the biofilm anode of a microbial fuel cell, Biotechnol. Bioeng., 2007, 98, 1171–1182 CrossRef PubMed.
- A. K. Marcus, C. I. Torres and B. E. Rittmann, Evaluating the impacts of migration in the biofilm anode using the model PCBIOFILM, Electrochim. Acta, 2010, 55, 6964–6972 CrossRef CAS.
- B. Korth, L. F. M. Rosa, F. Harnisch and C. Picioreanu, A framework for modeling electroactive microbial biofilms performing direct electron transfer, Bioelectrochemistry, 2015, 106, 194–206 CrossRef CAS PubMed.
- W.-F. Cai, J.-F. Geng, K.-B. Pu, Q. Ma, D.-W. Jing, Y.-H. Wang, Q.-Y. Chen and H. Liu, Investigation of a two-dimensional model on microbial fuel cell with different biofilm porosities and external resistances, Chem. Eng. J., 2018, 333, 572–582 CrossRef CAS.
- R. Renslow, J. Babauta, P. Majors and H. Beyenal, Diffusion in biofilms respiring on electrodes, Energy Environ. Sci., 2013, 6, 595–607 RSC.
- D. de Beer, P. Stoodley, F. Roe and Z. Lewandowski, Effects of biofilm structures on oxygen distribution and mass transport, Biotechnol. Bioeng., 1994, 43, 1131–1138 CrossRef CAS PubMed.
- H. Beyenal, C. Donovan, Z. Lewandowski and G. Harkin, Three-dimensional biofilm structure quantification, J. Microbiol. Methods, 2004, 59, 395–413 CrossRef CAS PubMed.
- F. Waharte, K. Steenkeste, R. Briandet and M.-P. Fontaine-Aupart, Diffusion measurements inside biofilms by image-based fluorescence recovery after photobleaching (FRAP) analysis with a commercial confocal laser scanning microscope, Appl. Environ. Microbiol., 2010, 76, 5860–5869 CrossRef CAS PubMed.
- A. Jain, G. Gazzola, A. Panzera, M. Zanoni and E. Marsili, Visible spectroelectrochemical characterization of Geobacter sulfurreducens biofilms on optically transparent indium tin oxide electrode, Electrochim. Acta, 2011, 56, 10776–10785 CrossRef CAS.
- A. A. Carmona-Martínez, F. Harnisch, U. Kuhlicke, T. R. Neu and U. Schröder, Electron transfer and biofilm formation of Shewanella putrefaciens as function of anode potential, Bioelectrochemistry, 2013, 93, 23–29 CrossRef PubMed.
- J. Hauth, J. Chodorski, A. Wirsen and R. Ulber, Improved FRAP measurements on biofilms, Biophys. J., 2020, 118, 2354–2365 CrossRef CAS PubMed.
- M. Wagner, B. Manz, F. Volke, T. R. Neu and H. Horn, Online assessment of biofilm development, sloughing and forced detachment in tube reactor by means of magnetic resonance microscopy, Biotechnol. Bioeng., 2010, 107, 172–181 CrossRef CAS PubMed.
- M. Wagner, D. Taherzadeh, C. Haisch and H. Horn, Investigation of the mesoscale structure and volumetric features of biofilms using optical coherence tomography, Biotechnol. Bioeng., 2010, 107, 844–853 CrossRef CAS PubMed.
- F. Blauert, H. Horn and M. Wagner, Time-resolved biofilm deformation measurements using optical coherence tomography, Biotechnol. Bioeng., 2015, 112, 1893–1905 CrossRef CAS PubMed.
- M. Wagner and H. Horn, Optical coherence tomography in biofilm research: A comprehensive review, Biotechnol. Bioeng., 2017, 114, 1386–1402 CrossRef CAS PubMed.
- S. D. Molenaar, T. Sleutels, J. Pereira, M. Iorio, C. Borsje, J. A. Zamudio, F. Fabregat-Santiago, C. J. N. Buisman and A. ter Heijne, In situ Biofilm Quantification in Bioelectrochemical Systems by using Optical Coherence Tomography, ChemSusChem, 2018, 11, 2171–2178 CrossRef CAS PubMed.
- M. Hackbarth, T. Jung, J. E. Reiner, J. Gescher, H. Horn, A. Hille-Reichel and M. Wagner, Monitoring and quantification of bioelectrochemical Kyrpidia spormannii biofilm development in a novel flow cell setup, Chem. Eng. J., 2020, 390, 124604 CrossRef CAS.
- M. P. Herrling, S. Lackner, H. Nirschl, H. Horn and G. Guthausen, in Annual Reports on NMR Spectroscopy, ed. G. A. Webb, Academic Press [Imprint]; Elsevier Science & Technology, Oxford, 2019, pp. 163–213 Search PubMed.
- T. R. Neu, B. Manz, F. Volke, J. J. Dynes, A. P. Hitchcock and J. R. Lawrence, Advanced imaging techniques for assessment of structure, composition and function in biofilm systems, FEMS Microbiol. Ecol., 2010, 72, 1–21 CrossRef CAS PubMed.
- J. Azeredo, N. F. Azevedo, R. Briandet, N. Cerca, T. Coenye, A. R. Costa, M. Desvaux, G. Di Bonaventura, M. Hébraud, Z. Jaglic, M. Kačániová, S. Knøchel, A. Lourenço, F. Mergulhão, R. L. Meyer, G. Nychas, M. Simões, O. Tresse and C. Sternberg, Critical review on biofilm methods, Crit. Rev. Microbiol., 2017, 43, 313–351 CrossRef CAS PubMed.
- Y. Zhang, A. Xu, X. Lv, Q. Wang, C. Feng and J. Lin, Non-Invasive Measurement, Mathematical Simulation and In Situ Detection of Biofilm Evolution in Porous Media: A Review, Appl. Sci., 2021, 11, 1391 CrossRef CAS.
- E. E. Beuling, D. van Dusschoten, P. Lens, J. C. van den Heuvel, H. van As and S. P. P. Ottengraf, Characterization of the diffusive properties of biofilms using pulsed field gradient-nuclear magnetic resonance, Biotechnol. Bioeng., 1998, 60, 283–291 CrossRef CAS PubMed.
- B. C. Hoskins, L. Fevang, P. D. Majors, M. M. Sharma and G. Georgiou, Selective imaging of biofilms in porous media by NMR relaxation, J. Magn. Reson., 1999, 139, 67–73 CrossRef CAS PubMed.
- B. Manz, F. Volke, D. Goll and H. Horn, Measuring local flow velocities and biofilm structure in biofilm systems with magnetic resonance imaging (MRI), Biotechnol. Bioeng., 2003, 84, 424–432 CrossRef CAS PubMed.
- P. D. Majors, J. S. McLean, G. E. Pinchuk, J. K. Fredrickson, Y. A. Gorby, K. R. Minard and R. A. Wind, NMR methods for in situ biofilm metabolism studies, J. Microbiol. Methods, 2005, 62, 337–344 CrossRef CAS PubMed.
- J. S. McLean, O. N. Ona and P. D. Majors, Correlated biofilm imaging, transport and metabolism measurements via combined nuclear magnetic resonance and confocal microscopy, ISME J., 2008, 2, 121–131 CrossRef CAS PubMed.
- J. S. McLean, P. D. Majors, C. L. Reardon, C. L. Bilskis, S. B. Reed, M. F. Romine and J. K. Fredrickson, Investigations of structure and metabolism within Shewanella oneidensis MR-1 biofilms, J. Microbiol. Methods, 2008, 74, 47–56 CrossRef CAS PubMed.
- R. S. Renslow, P. D. Majors, J. S. McLean, J. K. Fredrickson, B. Ahmed and H. Beyenal, In situ effective diffusion coefficient profiles in live biofilms using pulsed-field gradient nuclear magnetic resonance, Biotechnol. Bioeng., 2010, 106, 928–937 CrossRef CAS PubMed.
- B. Zhang and R. Powers, Analysis of bacterial biofilms using NMR-based metabolomics, Future Med. Chem., 2012, 4, 1273–1306 CrossRef CAS PubMed.
- H. van As and P. Lens, Use of 1 H NMR to study transport processes in porous biosystems, J. Ind. Microbiol., 2001, 26, 43–52 CrossRef CAS PubMed.
- L. Caizán-Juanarena, J. R. Krug, F. J. Vergeldt, J. M. Kleijn, A. H. Velders, H. van As and A. ter Heijne, 3D biofilm visualization and quantification on granular bioanodes with magnetic resonance imaging, Water Res., 2019, 167, 115059 CrossRef PubMed.
- S. A. Patil, S. Chigome, C. Hägerhäll, N. Torto and L. Gorton, Electrospun carbon nanofibers from polyacrylonitrile blended with activated or graphitized carbonaceous materials for improving anodic bioelectrocatalysis, Bioresour. Technol., 2013, 132, 121–126 CrossRef CAS PubMed.
- J. Erben, A. Heußner, S. Thiele and S. Kerzenmacher, Activation of electrospun carbon fibers: the effect of fiber diameter on CO2 and steam reaction kinetics, J. Polym. Res., 2021, 28, 1–14 CrossRef.
- J. Erben, X. Wang and S. Kerzenmacher, High Current Production of Shewanella Oneidensis with Electrospun Carbon Nanofiber Anodes is Directly Linked to Biofilm Formation, ChemElectroChem, 2021, 8, 1836–1846 CrossRef CAS.
- E. Kipf, J. Koch, B. Geiger, J. Erben, K. Richter, J. Gescher, R. Zengerle and S. Kerzenmacher, Systematic screening of carbon-based anode materials for microbial fuel cells with Shewanella oneidensis MR-1, Bioresour. Technol., 2013, 146, 386–392 CrossRef CAS PubMed.
- M. Loferer-Krössbacher, J. Klima and R. Psenner, Determination of bacterial cell dry mass by transmission electron microscopy and densitometric image analysis, Appl. Environ. Microbiol., 1998, 64, 688–694 CrossRef PubMed.
- J. Erben, Z. A. Pinder, M. S. Lüdtke and S. Kerzenmacher, Local Acidification Limits the Current Production and Biofilm Formation of Shewanella oneidensis MR-1 With Electrospun Anodes, Front. Microbiol., 2021, 12, 660474 CrossRef PubMed.
- R. Abboud, R. Popa, V. Souza-Egipsy, C. S. Giometti, S. Tollaksen, J. J. Mosher, R. H. Findlay and K. H. Nealson, Low-temperature growth of Shewanella oneidensis MR-1, Appl. Environ. Microbiol., 2005, 71, 811–816 CrossRef CAS PubMed.
- J. H. Simpson and H. Y. Carr, Diffusion and Nuclear Spin Relaxation in Water, Phys. Rev., 1958, 111, 1201–1202 CrossRef CAS.
- H. Edzes, D. van Dusschoten and H. Van As, Quantitative T2 imaging of plant tissues by means of multi-echo MRI microscopy, Magn. Reson. Imag., 1998, 16, 185–196 CrossRef CAS.
- A. Gędas and M. A. Olszewska, in Recent trends in biofilm science and technology, ed. M. Simões, A. Borges and L. C. Simões, Academic Press, London, United Kingdom, San Diego, CA, 2020, pp. 1–21 Search PubMed.
- L. Gao, X. Lu, H. Liu, J. Li, W. Li, R. Song, R. Wang, D. Zhang and J. Zhu, Mediation of Extracellular Polymeric Substances in Microbial Reduction of Hematite by Shewanella oneidensis MR-1, Front. Microbiol., 2019, 10, 575 CrossRef PubMed.
- J. Wei, P. Liang and X. Huang, Recent progress in electrodes for microbial fuel cells, Bioresour. Technol., 2011, 102, 9335–9344 CrossRef CAS PubMed.
- K. Dolch, J. Danzer, T. Kabbeck, B. Bierer, J. Erben, A. H. Förster, J. Maisch, P. Nick, S. Kerzenmacher and J. Gescher, Characterization of microbial current production as a function of microbe-electrode-interaction, Bioresour. Technol., 2014, 157, 284–292 CrossRef CAS PubMed.
- K. Muffler and R. Ulber, Productive Biofilms, Springer International Publishing, Cham, 2014 Search PubMed.
- J. Yang, S. Cheng, P. Li, H. Huang and K. Cen, Sensitivity to Oxygen in Microbial Electrochemical Systems Biofilms, iScience, 2019, 13, 163–172 CrossRef CAS PubMed.
- M. Lu, S. Chan, S. Babanova and O. Bretschger, Effect of oxygen on the per-cell extracellular electron transfer rate of Shewanella oneidensis MR-1 explored in bioelectrochemical systems, Biotechnol. Bioeng., 2017, 114, 96–105 CrossRef CAS PubMed.
- W. Bogner, R. Otazo and A. Henning, Accelerated MR spectroscopic imaging—a review of current and emerging techniques, NMR Biomed., 2021, 34, 0952–3480 CrossRef PubMed.
- P. van Zijl and N. Yadav, Chemical exchange saturation transfer (CEST): what is in a name and what isn't?, Magn. Reson. Med., 2011, 65, 927–948 CrossRef CAS PubMed.
- M. Holz, Electrophoretic NMR, Chem. Soc. Rev., 1994, 23, 165–174 RSC.
- P. Stilbs and I. Furó, Electrophoretic NMR, Curr. Opin. Colloid Interface Sci., 2006, 11, 3–6 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01162j |
| This journal is © The Royal Society of Chemistry 2022 |