Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Concentration-dependent HAT/ET mechanism of the reaction of phenols with 2,2-diphenyl-1-picrylhydrazyl (dpph˙) in methanol†
Paweł Przybylski a,
Adrian Konopko
a,
Adrian Konopko ab,
Piotr Łętowski
ab,
Piotr Łętowski a,
Katarzyna Jodko-Piórecka
a,
Katarzyna Jodko-Piórecka a and
Grzegorz Litwinienko
a and
Grzegorz Litwinienko *a
*a
aUniversity of Warsaw, Faculty of Chemistry, Pasteura 1, 02-093 Warsaw, Poland. E-mail: litwin@chem.uw.edu.pl
bPolish Academy of Science, Institute of Experimental Biology, Pasteura 3, 02-093 Warsaw, Poland
First published on 15th March 2022
Abstract
The reaction of a 2,2-diphenyl-1-picrylhydrazyl radical (dpph˙) with phenols carried out in alcohols is a frequently used assay for estimation of the antiradical activity of phenolic compounds. The rates of reactions of dpph˙ with five phenols (ArOH: unsubstituted phenol, 4-hydroxyacetophenone, two calix[4]resorcinarenes and baicalein) measured in methanol indicate the different kinetics of the process for very diluted phenols compared to their non-diluted solutions. This effect was explained as dependent on the ratio [ArO−]/[ArOH] and for diluted ArOH corresponds to an increased contribution of much faster electron transfer (ET, ArO−/dpph˙) over the Hydrogen Atom Transfer (HAT, ArOH/dpph˙). Simplified analysis of the reaction kinetics resulted in estimation of kET/kHAT ratios for each studied ArOH, and in calculation of the rate constants kET. Described results are cautionary examples of how the concentration of a phenol might change the reaction mechanism and the overall kinetics of the observed process.
Introduction
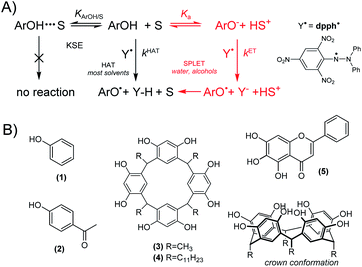
The stable 2,2-diphenyl-1-picrylhydrazyl radical, abbreviated as DPPH or dpph˙, is broadly employed for quick assessment of the radical scavenging abilities of natural and synthetic compounds. The methods are based on the monitoring of dpph˙ decoloration:| ArOH + dpph˙ → ArO˙ + dpph-H; overall kS | (1) |
In hydrocarbons and other non-polar solvents, reaction 1 proceeds as Hydrogen Atom Transfer (HAT). Since dpph˙ radicals are about 3 orders of magnitude less reactive than peroxyl radicals,1 reaction 1 can be easily employed for studies on reactivity of phenols and the kinetic solvent effect (KSE)1,2 (see Chart 1A), and even for estimation of O–H bond strengths. However, in ionization supporting solvents (water and alcohols) the HAT mechanism is “contaminated” by electron transfer (ET) from the ionized fraction of ArOH. This two-step mechanism is described as Sequential Proton-Loss Electron Transfer (SPLET),3,4 with kET >> kHAT, see Chart 1A, and even traces of ArO− causing an enormous increase of overall rate of reaction 1.2 Mixed mechanism in alcohols excludes dpph˙ as mimetic to peroxyl radicals but, regardless of this controversy,5 reaction 1 is one of the most frequently used colorimetric assays employed for quick assessment of the antiradical abilities of natural and synthetic compounds, with more than 40 thousand papers published during the last decade (SCOPUS, keywords “dpph” and “antioxidant”).
In contrast to simple kinetic measurements, typical non-kinetic assays are based on a titration of dpph˙ solution with phenol (or mixture of phenol extracts) in order to determine IC50 parameter, i.e., the concentration of phenol sufficient to scavenge 50% of the radicals present in the sample. However, reaction 1 can be (and usually is) reversible,6 because the bond dissociation enthalpy, BDE, in dpph-H (78.9 kcal mol−1)6 is lower than BDEO–H for majority of phenols, and IC50 measurements can be misleading. Another problem was noticed by Foti et al. who reported that for some phenols reaction 1 exhibits non-integer order in [ArOH].4–7 Such confusing phenomena needs to be further explored, and we selected series of ArOH accommodating two opposing features: enhanced acidity and a measurable reactivity (within stopped-flow time scale) toward dpph˙. The proper selection of compounds was not trivial because the introduction of an electron-withdrawing group into ArOH enhances its acidity but also causes an increase in the strength of the O–H bond. Here we present the results obtained for: phenol (1), 4-hydroxyacetophenone (2), C-methylcalix[4]-resorcinarene (3), C-undecylcalix[4] resorcinarene (4), and baicalein (5, the only natural compound within this series, being also an interesting example of ArOH with a strongly acidic catechol moiety), see Chart 1B.
Results and discussion
The reaction was monitored in neat methanol or, in order to suppress phenol ionization, in methanol acidified with acetic acid (AcOH). Experimental, pseudo-first-order rate constants, kexp, were determined for series of increasing [ArOH] being always in stoichiometric excess over dpph˙, and bimolecular rate constant (kS) was obtained from the straight-line equation:| kexp = kS [ArOH] + const | (2) |
Fig. 1 indicates that 1 is the only phenol for which a straight-line dependence of kexp on [ArOH] was obtained within the whole [ArOH] range. For 2–5, after the initial linear increase in kexp against increasing [ArOH] there is a break in the trend, and the overall plot is not linear. This means that the rate law is more complex and the species other than ArOH are involved in the rate determining step. Foti et al.4b studied the reaction of quercetin (QH2) with dpph˙ in methanol/water, and interpreted non-integer order (kexp ∼ [QH2]0.4) as resulting from the reversible formation of π-stacked pre-reaction complex of quercetin anion with dpph˙, followed by fast ET:4b
| QH−+ dpph˙ ⇆ [QH−/dpph˙] → QH˙ + dpph− | (3) |
 | ||
| Fig. 1 Plots of kex versus [ArOH] for dpph˙ + phenols in neat methanol. Left panel: less reactive phenols 1 and 2. Right panel: much faster reacting phenols 3–5. | ||
A similar mechanism was also described for curcumin/dpph˙ pair in ethanol4d whereas for phenols with OH group internally H bonded to the N-base a formation of the contact ion pair (ET to dpph˙) was proposed:4c
| ArOH + dpph˙ ⇆ [ArOH˙+/dpph−] → ArO˙ + dpph-H | (4) |
Non-linear fitting of our data to a function kexp = a[ArOH]b gave reaction orders 0.38–0.60 with respect to concentration of 2–5, however, the goodness of fitting for 3 is rather moderate, with residuals as high as up to ±10%, see Fig. 2, S13, S25 and S30.† After addition of AcOH, the linear relationships (eqn (2)) were obtained‡ for the whole concentration range, see Fig. 2 and ESI,† proving the mixed HAT/SPLET mechanisms. Therefore, we limited our calculations of kS for apparently straight line sectors of kexp vs. [ArOH] plots, below the inflection point, as presented in Fig. 1, and the results are listed in Table 1.
| phenol | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
a Max. errors ± 20%, see ESI for statistical parameters calculated for eqn (2) for each analyzed system.b In mM.c Parameters for methanol: HB accepting ability βH2 = 0.41,8 relative permittivity εr = 32.7, and autoprotolysis constant pKSH = 17.2.9 For AcOH βH2 is assumed as 0.42 (the same value as for propionic acid).d Values pKa for 1, 210 and 511 in water, pKa1–3 for 3 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water/methanol.12e pKa1 in MeOH were calculated from the correlation: pKa (MeOH) = 1.08 pKa (H2O)+ 3.66 (R = 0.991).13 AcOH, with pKa = 9.63 in methanol,13 is still stronger acid than phenols 1–4 but has almost the same acidity as 5. The pH window for water is 0–14, and for methanol 1.8–17.2.9 1 water/methanol.12e pKa1 in MeOH were calculated from the correlation: pKa (MeOH) = 1.08 pKa (H2O)+ 3.66 (R = 0.991).13 AcOH, with pKa = 9.63 in methanol,13 is still stronger acid than phenols 1–4 but has almost the same acidity as 5. The pH window for water is 0–14, and for methanol 1.8–17.2.9 |
|||||
 d d |
9.99 | 7.87 | 8.82/10.8/11.7 | — | 5.4/9.8/11.3 |
| pKaMeOHe | 14.4 | 12.2 | 13.2 | — | 9.5 |
The value of kS for reaction of 1 with dpph˙ is in a reasonable agreement with our previously published 0.04 M−1 s−1,3a with some deviations that can be ascribed to a great sensitivity of the kinetics of the processes carried out in neat, non-buffered systems.§ BDEO–H for 1 is 87.2 kcal mol−1 (ref. 6) or 88.2 kcal mol−1,14 and for 2 is 90.3 kcal mol−1,14 and ΔBDE = BDEArO–H − BDEPhOH suggests that kHAT for 2 should be 10–25 times smaller than for 1, as it can be predicted from eqn (5).¶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kArOH/dpph˙ = −0.33 + 0.35(−ΔBDE) kArOH/dpph˙ = −0.33 + 0.35(−ΔBDE)
| (5) |
However, only 5-fold (instead of 25-fold) difference in kS indicates that kHAT is partially compensated with a greater participation of the SPLET mechanism for 2 than for 1. Interestingly, addition of AcOH causes a decrease in kS for 1, but not for 2 (even in methanol acidified with 1 M AcOH the parameter kS was the same as in neat MeOH). This observation does not exclude SPLET because 2 is a relatively strong acid and its concentration is moderately high. Using Ka for 2 in methanol (footnote e in Table 1), we obtained [2−] = 0.56 × 10−7 M and 1.8 × 10−7 M for 5 mM and 50 mM solution of 2, respectively (this concentration range was used for calculation of kS). Furthermore, in this particular case acetic acid can slightly accelerate the completion of SPLET by fast protonation of dpph− formed after ET from 2− to dpph˙ (pKa of dpph-H is 8.54 (ref. 15) or 8.59 (ref. 16) in methanol/water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Therefore, the equilibrium 2− + dpph˙ ⇆ 2˙ + dpph− will be shifted to the right. For reactive phenols this effect is kinetically not significant, but for a slowly and reversibly reacting electron deficient 2, the transfer of H+ from AcOH to dpph− drives the reaction to the products (2˙/dpph-H). We also measured kS for 1 and 2 reacting with dpph˙ in buffered methanol/water (1
1). Therefore, the equilibrium 2− + dpph˙ ⇆ 2˙ + dpph− will be shifted to the right. For reactive phenols this effect is kinetically not significant, but for a slowly and reversibly reacting electron deficient 2, the transfer of H+ from AcOH to dpph− drives the reaction to the products (2˙/dpph-H). We also measured kS for 1 and 2 reacting with dpph˙ in buffered methanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at pH 5.4 and 7.4. Both compounds react faster than in neat methanol, confirming the role of deprotonation in the reaction mechanism. Surprisingly, at pH 7.4 both phenols, 1 and 2, react with almost the same rate, k7.4 is 0.74 ± 0.07 M−1 s−1 for 1 and 0.66 ± 0.12 M−1 s−1 for 2, whereas at pH 5.4 phenol 2 is a bit more reactive (k5.4 = 0.25 ± 0.01 M−1 s−1) than 1 (k5.4 = 0.19 ± 0.03 M−1 s−1). At pH 5.4 the plots of kexp vs. [2] are linear, but some deviations from linearity occur at pH 7.4 (see ESI†).
1) at pH 5.4 and 7.4. Both compounds react faster than in neat methanol, confirming the role of deprotonation in the reaction mechanism. Surprisingly, at pH 7.4 both phenols, 1 and 2, react with almost the same rate, k7.4 is 0.74 ± 0.07 M−1 s−1 for 1 and 0.66 ± 0.12 M−1 s−1 for 2, whereas at pH 5.4 phenol 2 is a bit more reactive (k5.4 = 0.25 ± 0.01 M−1 s−1) than 1 (k5.4 = 0.19 ± 0.03 M−1 s−1). At pH 5.4 the plots of kexp vs. [2] are linear, but some deviations from linearity occur at pH 7.4 (see ESI†).
Phenols 3–5 are 105 times more reactive than 1 and 2, therefore, much smaller concentrations (<1 mM) were used for measurements, and the presence of 10 mM AcOH (large excess) causes a 100-fold suppression of ks for 5, and ca. 20-fold decrease for cyclic tetramers 3 and 4. A 10% better reactivity of 4 over 3 can be explained as an effect of a crown conformation of 4,12 with internal hydrogen bonds within the upper rim, see Chart 1B, facilitating the stabilization of a radical (although we cannot exclude other effects related to different conformations and causing small differences in acidity of both compounds).
Foti et al. used a model of π-stacking pre-reaction complex (eqn (3)) to explain a non-linear, concentration-dependent kinetic behavior of quercetin but in the same work a similar peculiarity was observed also for catechin, which does not form such complex.4b Our results confirm that the mixed order of reaction is a more general phenomenon caused by HAT/ET competition and supported by ArOH/ArO− ratio. The rate law is:
| −d[dpph˙]/dt = n (kHAT[ArOH] + kET[ArO−])[dpph˙] | (6) |
For diluted solutions, the reaction order is very close to 1.0 (a straight line in Fig. 1). From the comparison of the reactions rates carried out for two different concentrations [ArOH]1 and [ArOH]2, the proportion kexp1/kexp2 (pseudo-first order conditions) is obtained:
 | (7) |
Eqn (7) can be solved after introducing 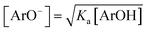 , with Ka values in methanol taken from Table 1, and separation of the variables, see ESI.† By introducing kexp and [ArOH] pairs from straight-line sections of the plots of eqn (2), we obtained kET/kHAT ratio listed in Table 2. Such estimation gives the opportunity to compare the overall ET/HAT rates, without any additional knowledge about a formation of dpph˙/ArOH complexes.
, with Ka values in methanol taken from Table 1, and separation of the variables, see ESI.† By introducing kexp and [ArOH] pairs from straight-line sections of the plots of eqn (2), we obtained kET/kHAT ratio listed in Table 2. Such estimation gives the opportunity to compare the overall ET/HAT rates, without any additional knowledge about a formation of dpph˙/ArOH complexes.
| phenol | kET/kHAT | kET | E0 |
|---|---|---|---|
| a From ref. 14 In the same work redox potentials (at pH > 12) for 1˙/1− and 2˙/2− are 790 mV, and 1000 mV, respectively.14b This is the upper value, the lower kET can be 35 M−1 s−1, see explanation in the text.c Measured in water with Britten–Robinson buffer vs. Hg|Hg2SO4|K2SO4 electrode and recalculated into NHE.17d From ref. 18. This value is very close to E0 for quercetin at pH 7.19 | |||
| 1 | 1.5 × 106 | 8.8 × 103 | 970a |
| 2 | 1.4 × 105 | 8.9 × 102 b | 1060a |
| 3 | 5.4 × 104 | 3.1 × 106 | 617c |
| 5 | 1.6 × 104 | 3.7 × 105 | 290d |
Values of kET listed in second column of Table 2 were calculated assuming that ET is eliminated (kS ≈ kHAT) for reactions carried out in methanol containing 1 M AcOH. Obtained values kET for 1−, 3−, and 5− (anions) are in reasonable agreement with 1 × 104 M−1 s−1 calculated by Foti et al. (for QH−/dpph˙ in methanol)4b and with 3.5 × 106 M−1 s−1 for ET from electron rich anion of 4-methoxyphenol derivative to dpph˙ in acetonitrile.4c Table 2 presents also the reduction potentials for studied phenols taken from literature (E0 for dpph˙/dpph−,H+ in water at pH 7 is 545 mV).20 E0 for 5 is 290 mV18 making this flavonoid stronger reducing agent than quercetin (E0(pH 7) = 330 mV),19b that could be reasoned also as an effect of greater participation of 5− than QH− in ET at pH 7 (5 is stronger acid than quercetin). The presented data do not allow to obtain a nice linear correlation of kET versus E0, perhaps, because of large error of estimation of kET values. For electron deficient 2, the upper value of kET was estimated assuming that kHAT for 2 and for 1 are the same (that could be reasoned also due to similar values of E0, see Table 2), however, basing on eqn (5),¶ kHAT for 2 should be ca. 25 times smaller than for 1, thus, kET for 2− might be as low as 35 M−1 s−1. Such a small kET is not surprising because for 2− this process is the most endergonic among all five ArO−/dpph˙ pairs.
The presented kET values are not strictly quantitative and include propagation of experimental errors (especially for 2) but they allow to estimate the concentration-dependent contribution of HAT and ET mechanisms to the overall rate of reaction, see Fig. S41–S43† and graphical abstract. Reaction of dpph˙ with the most acidic and most reactive 5 is dominated by ET within the whole concentration range (Fig. 2 and S43†) with the reaction order close to 0.5 in [5] as a consequence of the Ostwald law of dilution. For 1 (the weakest acid) HAT dominates over ET, whereas for 2 there is an inversion of dominating mechanism at [2] ≈ 50 mM.
Conclusions
There are many well-documented shortcomings of the dpph˙ assay, including poor or no correlation with the antioxidant activity of phenols measured under physiologically relevant conditions (reactivity toward peroxyl radicals).5 Another problem with the dpph˙ assay is that IC50 parameter reflects the position of redox equilibrium between dpph˙ and the tested compound, established after the incubation time, and gives no information about the kinetics and stoichiometry of the reaction.5b,dKinetic measurements (stopped flow technique, initial rates of reaction) can provide information on the structure-reactivity relationship of putative antioxidants reacting via HAT in non-polar solvents,6 However, in polar solvents much faster electron transfer is competitive or dominating mechanism and we demonstrated that contribution of HAT/ET to the overall rate is strongly dependent on the concentration of a tested phenol. A careful interpretation of both, kinetic and quasi-kinetic (IC50) results, has to be always performed, and mixed HAT/ET mechanism of reaction 1 might produce false results not only in the kinetic experiments but also in IC50 assay, as IC50 is a parameter strongly related to the concentration of tested phenols (and even expressed in phenol concentration units !). IC50 parameter is frequently used for comparison of “antioxidant properties” of phenols and other phytochemicals but our findings indicate a serious limitation of such methodology.
General experimental procedures
Commercially available phenols 1, 2, and 5 were of the highest purity and were used as received. Macrocyclic polyphenols: C-methylcalix[4]resorcinarene (3) and C-undecylcalix[4]resorcinarene (4) were prepared following the method proposed by Weinelt and Schneider21 by condensation of resorcinol and appropriate aldehyde in ethanol containing aqueous HCl as in our previous work.12 1H NMR of C-alkyl[4]resorcinarenes were recorded on a Varian spectrometer at 300 MHz and 298 K and were compared with literature.22 3 was obtained by condensation of resorcinol with ethanal. 0.5 mol of resorcinol dissolved in 500 mL of ethanol/water (1/1, v/v) was immersed in an ice bath, then 125 mL of concentrated hydrochloric acid was added. Subsequently, 0.5 mol of ethanal was added dropwise. Then, the reaction mixture was kept at rt and the reaction was carried out for 96 hours with continuous stirring under nitrogen. The mixture was cooled and concentrated under reduced pressure. The obtained precipitate was washed several times with cold ethanol, crystallized form ethanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and dried (yield 40–50%) 1H NMR (300 MHz, acetone-d6) δ 8.48 (s, 8H), 7.64 (s, 4H), 6.21 (s, 4H), 4.52 (q, J = 7.3 Hz, 4H), 1.76 (d, J = 7.4 Hz, 12H), see Fig. S1.† 4 was obtained by condensation of resorcinol (0.69 mol) with dodecanal (0.69 mol). The compounds were dissolved in 690 mL of ethanol and cooled in the ice bath to temperature close to 0 °C. Subsequently, 111 mL of concentrated hydrochloric acid was added dropwise to the mixture. Then, the temperature was increased to 70 °C and kept for 12 hours with continuous stirring and nitrogen flow. The mixture was cooled and concentrated under reduced pressure. The obtained precipitate was washed several times with cold ethanol, crystallized from methanol, and dried (yield 20–30%). 1H NMR (300 MHz, CDCl3) δ 9.56 (t, J = 20.0 Hz, 4H), 9.28 (t, J = 25.0 Hz, 4H), 7.20 (s, 4H), 6.11 (s, 4H), 4.29 (t, J = 7.7 Hz, 4H), 2.21 (s, 8H), 1.27 (s, 72H), 0.88 (t, 12H), see Fig. S2.†
1), and dried (yield 40–50%) 1H NMR (300 MHz, acetone-d6) δ 8.48 (s, 8H), 7.64 (s, 4H), 6.21 (s, 4H), 4.52 (q, J = 7.3 Hz, 4H), 1.76 (d, J = 7.4 Hz, 12H), see Fig. S1.† 4 was obtained by condensation of resorcinol (0.69 mol) with dodecanal (0.69 mol). The compounds were dissolved in 690 mL of ethanol and cooled in the ice bath to temperature close to 0 °C. Subsequently, 111 mL of concentrated hydrochloric acid was added dropwise to the mixture. Then, the temperature was increased to 70 °C and kept for 12 hours with continuous stirring and nitrogen flow. The mixture was cooled and concentrated under reduced pressure. The obtained precipitate was washed several times with cold ethanol, crystallized from methanol, and dried (yield 20–30%). 1H NMR (300 MHz, CDCl3) δ 9.56 (t, J = 20.0 Hz, 4H), 9.28 (t, J = 25.0 Hz, 4H), 7.20 (s, 4H), 6.11 (s, 4H), 4.29 (t, J = 7.7 Hz, 4H), 2.21 (s, 8H), 1.27 (s, 72H), 0.88 (t, 12H), see Fig. S2.†
Since rate of ArOH/dpph˙ reaction in neat methanol is highly sensitive to traces of acids and bases, prior to the use, methanol was fractionally distilled over a small amount of dpph˙ and a few beads of an acidic ion-exchange resin. Measurements were made following the procedure described previously.3c,23 Decays of dpph˙ (ε ∼ 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) were monitored 517 nm on an Applied Photophysics SX 20 stopped flow spectrometer, equipped with a xenon arc lamp source and photodiode array detector. The mixing cell (10 mm optical path length, dead-time of mixing 1.1 ms) and the tubes delivering the reactants were thermostated at temperature 25 °C. Initial concentrations of dpph˙ were 6–12 × 10−5 M for reactions with 1 and 2 and 1–15 × 10−5 M for reactions with much more reactive 3–5, i.e., always in the presence of a stoichiometric excess of ArOH. Measurements were made in neat and acidified methanol (with 10, 100 and 1000 mM AcOH) and in mixed 1
000 M−1 cm−1) were monitored 517 nm on an Applied Photophysics SX 20 stopped flow spectrometer, equipped with a xenon arc lamp source and photodiode array detector. The mixing cell (10 mm optical path length, dead-time of mixing 1.1 ms) and the tubes delivering the reactants were thermostated at temperature 25 °C. Initial concentrations of dpph˙ were 6–12 × 10−5 M for reactions with 1 and 2 and 1–15 × 10−5 M for reactions with much more reactive 3–5, i.e., always in the presence of a stoichiometric excess of ArOH. Measurements were made in neat and acidified methanol (with 10, 100 and 1000 mM AcOH) and in mixed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) methanol–water with pH adjusted to 5.4 (acetate buffer) and pH 7.4 (phosphate buffer). In all experiments, the initial rates (usually determined for 5–10% of dpph˙ conversion) were taken for calculations of kexp; for example, the conversion of dpph˙ 0.5 s after mixing was 1%, 0.38%, 17%, 29% and 31% for 1–5, respectively, and after 2 seconds the conversion was 3.2%, 0.8%, 50% and 71% for compounds 1–4. The pseudo-first-order rate constants, kexp, were calculated as average values from at least two independent sets of measurements. Values of bimolecular rate constants, kS, were calculated as a slope of the straight-line eqn (2). EPR measurements by Staško et al.24 for mixed ethanol/water systems indicated that dpph˙ behaves as typical solute for a lower water ratio of 0–60% (v/v) but at a higher water content (above 60%) dpph˙ forms microaggregates (still without precipitation). We assume, therefore, that dpph˙ forms a homogeneous system with water/methanol (1
1 (v/v) methanol–water with pH adjusted to 5.4 (acetate buffer) and pH 7.4 (phosphate buffer). In all experiments, the initial rates (usually determined for 5–10% of dpph˙ conversion) were taken for calculations of kexp; for example, the conversion of dpph˙ 0.5 s after mixing was 1%, 0.38%, 17%, 29% and 31% for 1–5, respectively, and after 2 seconds the conversion was 3.2%, 0.8%, 50% and 71% for compounds 1–4. The pseudo-first-order rate constants, kexp, were calculated as average values from at least two independent sets of measurements. Values of bimolecular rate constants, kS, were calculated as a slope of the straight-line eqn (2). EPR measurements by Staško et al.24 for mixed ethanol/water systems indicated that dpph˙ behaves as typical solute for a lower water ratio of 0–60% (v/v) but at a higher water content (above 60%) dpph˙ forms microaggregates (still without precipitation). We assume, therefore, that dpph˙ forms a homogeneous system with water/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) during our experiments at pH 5.4 and 7.4.
1, v/v) during our experiments at pH 5.4 and 7.4.
Author contributions
Conceptualization (GL and PP), funding acquisition (GL), investigation (PP, AK, PŁ), methodology, supervision and project administration (GL), visualization (KJP and GL), writing (all authors).Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. K.: This work implemented as a part of Operational Project Knowledge Education Development 2014–2020 co-financed by European Social Fund, Project No POWR.03.02.00-00-I007/16-00 (POWER 2014–2020). G. L. gratefully acknowledges financial support from the National Science Center of Poland, grant OPUS No. 2018/31/B/ST4/02354.Notes and references
- M. C. Foti, E. R. Johnson, M. R. Vinqvist, J. S. Wright, L. R. C. Barclay and K. U. Ingold, J. Org. Chem., 2002, 67, 5190–5196 CrossRef CAS PubMed.
- (a) L. Valgimigli, J. T. Banks, K. U. Ingold and J. Lusztyk, J. Am. Chem. Soc., 1995, 117, 9966–9971 CrossRef CAS; (b) D. W. Snelgrove, J. Lusztyk, J. T. Banks, P. Mulder and K. U. Ingold, J. Am. Chem. Soc., 2001, 123, 469–477 CrossRef CAS; (c) G. Litwinienko and K. U. Ingold, Acc. Chem. Res., 2007, 40, 222–230 CrossRef CAS PubMed.
- (a) G. Litwinienko and K. U. Ingold, J. Org. Chem., 2003, 68, 3433–3438 CrossRef CAS PubMed; (b) G. Litwinienko and K. U. Ingold, J. Org. Chem., 2004, 69, 5888–5896 CrossRef CAS PubMed; (c) M. Musialik, R. Kuzmicz, T. S. Pawłowski and G. Litwinienko, J. Org. Chem., 2009, 74, 2699–2709 CrossRef CAS PubMed; (d) K. Jodko-Piórecka, B. Sikora, M. Kluzek, P. Przybylski and G. Litwinienko, J. Org. Chem., 2022, 87, 1791–1804 CrossRef PubMed.
- (a) M. C. Foti, C. Daquino and C. Geraci, J. Org. Chem., 2004, 69, 2309–2314 CrossRef CAS PubMed; (b) M. C. Foti, C. Daquino, G. A. DiLabio and K. U. Ingold, Org. Lett., 2011, 13, 4826–4829 CrossRef CAS PubMed; (c) R. Amorati, S. Menichetti, C. Viglianisi and M. C. Foti, Chem. Commun., 2012, 48, 11904–11906 RSC; (d) M. C. Foti, A. Slavova-Kazakova, C. Rocco and V. D. Kancheva, Org. Biomol. Chem., 2016, 14, 8331–8337 RSC.
- (a) R. Amorati and L. Valgimigli, Free Radical Res., 2015, 49, 633–649 CrossRef CAS PubMed; (b) M. C. Foti, J. Agric. Food Chem., 2015, 63, 8765–8776 CrossRef CAS PubMed; (c) R. Shah, L. A. Farmer, O. Zilka, A. T. M. Van Kessel and D. A. Pratt, Cell Chem. Biol., 2019, 26, 1594–1607 CrossRef CAS PubMed; (d) A. Baschieri and R. Amorati, Antioxidants, 2021, 10, 1551 CrossRef CAS PubMed.
- M. C. Foti and C. Daquino, Chem. Commun., 2006, 3252–3254 RSC.
- (a) I. Nakanishi, T. Kawashima, K. Ohkubo, T. Waki, Y. Uto, T. Kamada, T. Ozawa, K. I. Matsumoto and S. Fukuzumi, Chem. Commun., 2014, 50, 814–816 RSC; (b) I. Nakanishi, T. Kawashima, K. Ohkubo, H. Kanazawa, K. Inami, M. Mochizuki, K. Fukuhara, H. Okuda, T. Ozawa, S. Itoh, S. Fukuzumi and N. Ikota, Org. Biomol. Chem., 2005, 3, 626–629 RSC.
- M. H. Abraham, P. L. Grellier, D. V. Prior, J. J. Morris and P. J. Taylor, J. Chem. Soc., Perkin Trans. 2, 1990, 521–529 RSC.
- K. Izutsu, Electrochemistry in nonaqueous solutions, John Wiley & Sons, 2009 Search PubMed.
- E. P. Serjeant and B. Dempsey, Ionisation constants of organic acids in aqueous solution, Pergamon Press, Oxford, 1979 Search PubMed.
- R. Liang, C. H. Chen, R. M. Han, J. P. Zhang and L. H. Skibsted, J. Agric. Food Chem., 2010, 58, 9221–9227 CrossRef CAS PubMed.
- P. Ziaja, A. Krogul, T. S. Pawłowski and G. Litwinienko, Thermochim. Acta, 2016, 623, 112–119 CrossRef CAS.
- F. Rived, M. Rosés and E. Bosch, Anal. Chim. Acta, 1998, 374, 309–324 CrossRef CAS.
- J. Lind, X. Shen, T. E. Eriksen and G. Merenyi, J. Am. Chem. Soc., 1990, 112, 479–482 CrossRef CAS.
- C. Luca, P. Ionita and T. Constantinescu, Rev. Roum. Chim., 1994, 39, 1141–1149 CAS.
- O. Friaa and D. Brault, Org. Biomol. Chem., 2006, 4, 2417–2423 RSC.
- P. Ziaja, PhD thesis, University of Warsaw, 2013.
- M. Vestergaard, K. Kerman and E. Tamiya, Anal. Chim. Acta, 2005, 538, 273–281 CrossRef CAS.
- (a) R. Sokolová, Š. Ramešová, I. Degano, M. Hromadová, M. Gál and J. Žabka, Chem. Commun., 2012, 48, 3433–3435 RSC; (b) S. V. Jovanovic, S. Steenken, Y. Hara and M. G. Simic, J. Chem. Soc., Perkin Trans. 2, 1996, 2497–2504 RSC.
- Q.-k. Zhuang, F. Scholz and F. Pragst, Electrochem. Commun., 1999, 1, 406–410 CrossRef CAS.
- F. Weinelt and H. J. Schneider, J. Org. Chem., 1991, 56, 5527–5535 CrossRef CAS.
- (a) D. J. Cram, S. Karbach, H. E. Kim, C. B. Knobler, E. F. Maverick, J. L. Ericson and R. C. Helgeson, J. Am. Chem. Soc., 1988, 110, 2229–2237 CrossRef CAS; (b) Y. Aoyama, Y. Tanaka and S. Sugahara, J. Am. Chem. Soc., 1989, 111, 5397–5404 CrossRef CAS; (c) A. S. Högberg, J. Am. Chem. Soc., 1980, 102, 6046–6050 CrossRef.
- E. Van Wenum, R. Jurczakowski and G. Litwinienko, J. Org. Chem., 2013, 78, 9102–9112 CrossRef CAS PubMed.
- A. Staško, V. Brezová, S. Biskupič and V. Mišík, Free Radical Res., 2007, 41, 379–390 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: synthesis and 1H NMR for 3 and 4, details for kinetic measurements, kinetic traces for dpph˙ bleaching during reaction with 1–5, tables with series of kexp obtained for increasing [ArOH] in methanol and acidified methanol and in buffered methanol/water at pH 5.4 and 7.4, plots and kinetic parameters for linear and non linear fitting of eqn 2, dissociation diagrams, derivation of kET/kHAT ratio. See DOI: 10.1039/d2ra01033j |
| ‡ Kinetic data from the inset can be also fitted for non-integer order model kexp = a[ArOH]b, giving artificial (in our opinion) b = 0.76 ± 0.10. For reaction of 5 with dpph˙ in methanol containing 100 and 1000 mM acetic acid the calculated b are scattered from 0.63 to 0.36 because even very small deviation of single experimental point produce substantial decrease of b, than can be either an error or an effect of mixed HAT/ET mechanism. Therefore, for acidified systems we did not push the reaction order into nonintegral orders in [ArOH]. Moreover, data for non-acidifiers 1 (straight line in Fig. 1A) can also be fitted to non linear function, giving reaction order 0.83. |
| § To remove traces of phenols/stabilizers and other compounds that could contaminate the kinetics, we distilled methanol over a small amount of dpph˙ and a few beads of ion-exchange resin. |
| ¶ This empirical equation is valid for non-hindered phenols reacting with dpph˙ radical in hydrocarbons (pure HAT mechanism, with no KSE). In other solvents some small differences can be observed due to different ability of ArOH and PhOH to form H bond with solvent, see discussion in footnote 34 in ref. 1. Predicted differences in kS can be even bigger, because 2, with strong EW substituent, will be better HB donating agent than unsubstituted phenol 1. |
| This journal is © The Royal Society of Chemistry 2022 |