Open Access Article
Open Access Article2,5-Diisopropenylthiophene by Suzuki–Miyaura cross-coupling reaction and its exploitation in inverse vulcanization: a case study †
Christian Tavella a,
Giorgio Luciano
a,
Giorgio Luciano a,
Paola Lova
a,
Paola Lova b,
Maddalena Patrini
b,
Maddalena Patrini c,
Cristina D'Arrigo
c,
Cristina D'Arrigo a,
Davide Comoretto
a,
Davide Comoretto b and
Paola Stagnaro
b and
Paola Stagnaro *a
*a
aIstituto di Scienze e Tecnologie Chimiche “Giulio Natta”, Consiglio Nazionale delle Ricerche, SCITEC-CNR, Via De Marini 6, 16149 Genova, Italy. E-mail: paola.stagnaro@scitec.cnr.it
bDipartimento di Chimica e Chimica Industriale, Università degli Studi di Genova, DCCI-UNIGE, Via Dodecaneso 31, 16132 Genova, Italy
cDipartimento di Fisica, Università di Pavia, Via A. Bassi 6, 27100 Pavia, Italy
First published on 22nd March 2022
Abstract
A novel thiophene derivative, namely 2,5-diisopropenylthiophene (DIT) was synthetized by Suzuki–Miyaura cross-coupling reaction (SMCCR). The influence of reaction parameters, such as temperature, solvent, stoichiometry of reagents, role of the base and reaction medium were thoroughly discussed in view of yield optimization and environmental impact minimization. Basic design of experiment (DoE) and multiple linear regression (MLR) modeling methods were used to interpret the obtained results. DIT was then employed as a comonomer in the copolymerization with waste elemental sulfur through a green process, inverse vulcanization (IV), to obtain sulfur-rich polymers named inverse vulcanized polymers (IVPs) possessing high refractive index (n ≈ 1.8). The DIT comonomer was purposely designed to (i) favor the IV process owing to the high reactivity of the isopropenyl functionalities and (ii) enhance the refractive index of the ensuing IVPs owing to the presence of the sulfur atom itself and to the high electronic polarizability of the π-conjugated thiophene ring. A series of random sulfur-r-diisopropenylthiophene (S-r-DIT) copolymers with sulfur content from 50 up to 90 wt% were synthesized by varying the S/DIT feed ratio. Spectroscopic, thermal and optical characterizations of the new IVPs were carried out to assess their main chemical–physical features.
Introduction
In organic synthesis the formation of new carbon–carbon bonds allows to obtain products of higher molecular complexity starting from simple substrates. Classically, a new C–C bond is obtained by reacting an electrophilic substrate with a nucleophile counterpart whose reactive center is normally stabilized by a proper functional group. The major drawback of this methodology is the maintenance of the functional group(s), as such or modified, in the reaction product being formed, with consequent need for further synthetic steps.Modern cross-coupling reactions (CCRs) between the species R1–X (electrophilic partner) and R2–M (nucleophilic partner) allowed the formation of a new C–C bond in the absence of orienting functional groups, thus noticeably simplifying the synthetic protocol. The main advantages1 are: (i) high selectivity in the formation of new C–C bonds, with particular reference to regio- and diastereo-selectivity; (ii) excellent compatibility with many functional groups pre-existing on the starting substrates (that is, reduced need to use protective groups); (iii) variety of applications for the synthesis of multiple classes of compounds with non-specific reaction mechanism, the main stages being common to many different reactions.
Generally, CCRs involve the use of an organometallic reagent as the nucleophile in the presence of a catalytic system based on a properly complexed transition metal (often noble).2 The use of the latter in sub-stoichiometric quantities (typically 1–5 mol%) reduces the cost of the whole process allowing also for large-scale production. Advances in organometallic chemistry have led to the preparation of a variety of coordination compounds able to act as CCRs catalysts, among which the complexes of palladium stand out for versatility and efficiency.3
Amongst the numerous CCRs based on palladium catalysis, the condensation of Suzuki–Miyaura (SMCCR), who was awarded with the Nobel prize in 2010 arose particular interest in the academic field for its unique characteristics, and at industrial scale for its important applications.4 Often referred to more simply as Suzuki's coupling, this reaction uses as the carbon nucleophilic partner a boron derivative R–BZ2 (Z = R, OR) warranting the controlled formation of a new C–C single bond.5 Versatility and important applications of this SMCCR are ensured by its characterizing features such as, mild reaction conditions, availability of a variety of precursors, functional-group compatibility, stability towards air and moisture and, last but not least, use of non-toxic and easy-to-produce boron derivatives.5–8
Adapting a Suzuki's coupling protocol from literature,9 2,5-diisopropenylthiophene (DIT) molecule was synthesized for the first time by the authors,10,11 then, also for the first time, it was tested as the comonomer in the inverse vulcanization (IV) with elemental sulfur.
As known, the irreversible rubber vulcanization discovered by Goodyear,12 consists in cross-linking the elastomeric chains with minor amounts of sulfur. On the opposite, the recently introduced IV9,13–23 consists in a process where massive amount of sulfur are crosslinked with minor amounts of comonomer(s). In detail, sulfur is heated at 160–190 °C (that is well above its melting temperature, ca. 115 °C). At these temperatures the S8 rings open to form linear polysulfide chains made of S–S bonds that can react in bulk with the added comonomer(s). These comonomers are typically hydrocarbons bearing divinyl (or analogous) functionalities able to form bridges between the polysulfide chain via a radical reaction.
IV has risen great academic and industrial interest owing to a series of noticeable advantages it brings about. First, it is a solvent-free process where sulfur acts as both comonomer and solvent, thus preventing the use of low eco-friendly and costly organic solvents. This characteristic makes the IV process also easy to scale at the industrial level.18 Besides, employing sulfur in high proportions, IV contributes to reduce the ubiquitous wastes of this element, obtained in huge amounts as by-product from natural gas extraction and oil refinery.13,15,24,25 IV process is also intriguing for its synthetic versatility: small molecules, such as 1,3-diisopropenylbenzene,13,15,18 1,3,5-triisopropenylbenzene,9 divinylbenzene,19,20 1,3-diethinylbenzene,14 1,4-diphenylbutadiyne,26 limonene,21 dicyclopentadiene,27 and ethylidene norbornene,28 or more complex ones, as squalene29 and triglycerides,30 were in fact demonstrated to copolymerize with sulfur to give a variety of novel macromolecular architectures.
Preliminary results suggest 2,5-diisopropenylthiophene (DIT) molecule is promising to obtain a high refractive index IV polymer.10 The choice to synthesize DIT molecule as suitable comonomer for the IV process (Scheme 1), originates from various considerations: (i) the thiophene ring for its aromaticity and the presence of the sulfur atom itself (and therefore for the high electronic polarizability) should contribute to further increase the refractive index of the resulting polymeric material, which is interesting for photonic and more in general optical applications;31 (ii) as compared to the commonly used vinyl functionalities, the methyl groups borne by the isopropenyl substituents should ensure a higher reactivity in the IV radical process and, last but not least, (iii) DIT molecule, could be directly obtained through a double Suzuki–Miyaura cross-coupling reaction from the commercially available 2,5-dibromothiophene electrophile substrate and a non-toxic isopropenyl-substituted boron derivative, as suitable nucleophile.
 | ||
| Scheme 1 Double condensation of Suzuki–Miyaura on 2,5-dibromothiophene substrate to give DIT then used as a comonomer in the IV process with sulfur to give S-r-DIT copolymers. | ||
In this work, we present the DIT synthesis through Suzuki–Miyaura cross-coupling reaction as a case study with a twofold purpose: (i) to study the influence of reaction parameters including temperature, solvent, role of the base, and of the reaction medium on the reaction yield and (ii) to achieve novel polymers obtained via inverse vulcanization of sulfur exploiting the thiophene-based comonomer purposely designed to have very high refractive index. A deeper insight of the SMCCR leading to DIT molecule was undertaken using basic methods of design of experiment (DoE) to optimize the reaction parameters. Multiple linear regression (MLR) was useful to assess the importance of the factors under investigation and to interpret the results. The feasibility of DIT synthesis in SMCCR conditions as much as possible compliant with green chemistry principles was investigated as well. DIT molecule was then proven as suitable comonomer in the IV process. A series of random sulfur-r-diisopropenylthiophene (S-r-DIT) copolymers with increasing sulfur content from 60 up to 90 wt% was prepared. The synthesized IVPs were characterized by infrared spectroscopy, thermogravimetric analysis and differential scanning calorimetry. Finally, optical absorption and fluorescence of S-r-DIT copolymers have been recorded. Solutions were also used for preparing thin films by spin-coating and then to determine their refractive index and spectral dispersion.
Experimental
Materials
Manipulations of air and/or moisture sensitive materials were carried out under inert atmosphere using dual vacuum/argon lines. Elemental sulfur in powder (S, Ph. Eur., BP), 2,5-dibromothiophene (95%), 4,4,5,5-tetramethyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolane (isopropenylboronic acid pinacol ester, 95%), Cs2CO3 (99%), NaHCO3 (≥99.7%), KHCO3 (≥99.7%), NaOH (≥98%), KOH (85–100.5%), Pd(PPh3)4 (99%), 1,4-dioxane (≥99.5%), tetra-n-butylammonium iodide (≥99%), n-nonylphenol (technical grade, mixture of regioisomers), deuterated chloroform (CDCl3, 99.8% deut.) as well as other chemicals and solvents, were purchased from Merck-Sigma-Aldrich (Italy) and used as received.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to isolate 2,5-diisopropenylthiophene (DIT) as a bright yellow, low-melting crystalline solid in variable yields (see Table 3 in the Results and discussion).
1) to isolate 2,5-diisopropenylthiophene (DIT) as a bright yellow, low-melting crystalline solid in variable yields (see Table 3 in the Results and discussion).
| Factor | Coefficient value | Standard error | t-Value | (Pr>|t|) | p-Valuea |
|---|---|---|---|---|---|
| a Significance codes: 0 ‘***’, 0.001 ‘**’, 0.01 ‘*’, 0.05 ‘.’, 0.1 ‘·’.b Intercept which represents the average yield of the reaction (η% = 85.56, see below eqn (1.a) and (1.b)). | |||||
| Interceptb | 85.56250 | 0.37373 | 228.9421 | <2.2 × 10−16 | *** |
| A | −0.31250 | 0.37373 | −0.8362 | 0.405301 | · |
| S | 6.47917 | 0.37373 | 17.3365 | <2.2 × 10−16 | *** |
| T | 8.85417 | 0.37373 | 23.6914 | <2.2 × 10−16 | *** |
| A·S | 1.22917 | 0.37373 | 3.2889 | 0.001442 | ** |
| A·T | −1.81250 | 0.37373 | −4.8498 | 5.218 × 10−6 | *** |
| S·T | −3.56250 | 0.37373 | −9.5323 | <2.2 × 10−16 | *** |
ATR-FTIR: ν (cm−1) 3084 (vinyl and aromatic = C–H stretch), 2975–2922 (aliphatic –C–H stretch), 1618 (vinyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch), 1453 (breathing vibration of 2,5-disubstituted thiophene ring), 874 (out-of-plane angular deformation of thiophene ring), 799 (out-of-plane = C–H wag in 2,5-disubstituted thiophene ring).
C stretch), 1453 (breathing vibration of 2,5-disubstituted thiophene ring), 874 (out-of-plane angular deformation of thiophene ring), 799 (out-of-plane = C–H wag in 2,5-disubstituted thiophene ring).
1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm) 2.04 (dd, 6H, J = −1.5 Hz and J = −1.0 Hz, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 4.86–4.87 (app quint, 2H, J = −1.5 Hz, vinyl C
3), 4.86–4.87 (app quint, 2H, J = −1.5 Hz, vinyl C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) cis to CH3), 5.27–5.28 (app quint, 2H, J = −1.0 Hz, vinyl C
cis to CH3), 5.27–5.28 (app quint, 2H, J = −1.0 Hz, vinyl C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) trans to CH3), 6.81 (s, 2H, thiophene ring C
trans to CH3), 6.81 (s, 2H, thiophene ring C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) (Fig. S1 of ESI†).
) (Fig. S1 of ESI†).
2.80 mL of n-nonylphenol (NP, mixture of regioisomers, 12.0 mmol, 1 eq.) were introduced into a 50 mL flask where the prepared Bu4N+ OH− was previously transferred. Then 20 mL of a MeOH/toluene mixture in azeotropic proportion were added, the reaction mixture was kept under magnetic stirring for 1 h at room temperature, and then concentrated by rotoevaporation to recover a solid product which was filtered on a Büchner funnel, washed with MeOH, and dried in a ventilated oven at 100 °C for 12 h. TBANP was obtained as a white crystalline solid in 74% yield. 1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm) 0.5–1.8 (complex m, 45H, aliphatic C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3 and C
3 and C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 6.74–6.78 (m, 2H, aromatic C
2), 6.74–6.78 (m, 2H, aromatic C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) and 7.09–7.22 (m, 2H, aromatic C
) and 7.09–7.22 (m, 2H, aromatic C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) (Fig. S2 of ESI†).
) (Fig. S2 of ESI†).
Characterization methods
Fourier transform infrared spectroscopy in attenuated total reflectance mode (ATR-FTIR) was performed on DIT comonomer and S-r-DIT copolymers operating with a PerkinElmer Spectrum TwoTM spectrometer and recording spectra in the wavenumber range 4000–400 cm−1 (16 scans, resolution 0.5 cm−1).1H NMR spectrum of DIT comonomer was acquired at room temperature in CDCl3 (internal chemical shift reference 0.03% TMS) with a Varian Gemini 300 spectrometer on a 10 mg sample using a 5 mm probe.
Dynamic thermogravimetric analysis (TGA) measurements on S-r-DIT copolymers were carried out with a PerkinElmer TGA8000 instrument. Specimens of about 15 mg were heated at 10 °C min−1 in the temperature range 25–700 °C under N2 and under O2 from 700 to 850 °C (gas flow 40 mL min−1). Two or three replicas were performed for each sample. Effective S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DIT ratios were derived by the carbonaceous residue (wt%) measured at 700 °C.
DIT ratios were derived by the carbonaceous residue (wt%) measured at 700 °C.
Differential scanning calorimetry (DSC) measurements were performed with a Mettler DSC 821e calorimeter operating under N2 atmosphere (flow 40 mL min−1). S-r-DIT samples of ca. 10–15 mg were heated at 20 °C min−1 from 25 up to 150 °C, cooled at the same scan rate to −50 °C and subjected to a second heating from −50 up to 200 °C The reported values of glass transition temperature (Tg) were averaged from two or three replicas for each sample.
Thin films have been prepared by dissolving 10 mg mL−1 of S-r-DIT copolymer in toluene solution and spin-casting 100 μL on substrates rotating at speeds ranging between 60 and 120 rps (rounds per second).
Spectroscopic ellipsometry was performed such films to retrieve the material refractive index on thin films cast on fused silica substrates using a VASE instrument (J. A. Woollam Co, Lincoln, NE, USA) in the range 250–2500 nm at different incidence angles from 55 to 75°. Reflectance and transmittance at normal incidence were also measured with a Varian Cary 6000i spectrometer in the spectral range of 200–1800 nm for fitting purposes. As a result, the complex refractive index for all materials was evaluated by the WVASE32® software (J. A. Woollam Co, Version 3.774, Lincoln, NE, USA) while adopting oscillator models to guarantee the Kramers–Kronig consistency.
Absorbance and photoluminescence spectra for IVP solutions were measured employing a home-made optical set-up. For absorbance, a Deuterium–Halogen light source (DH 2000 BAL) and an Avantes Avaspec 2048-EVO spectrometer (250–1050 nm with resolution 1.4 nm) connected through optical fibers were used. Photoluminescence was instead collected with the same detector exciting the solution with a 405 nm Oxxius continuous wave laser source with power up to 50 mW. The fluorescence signal was filtered with a 450 nm long-pass filter to eliminate the excitation source from the measured spectra.
Results and discussion
Optimization of the SMCCR protocol to prepare DIT comonomer
The design of organic syntheses is a particularly complex task that begins well before carrying out the laboratory tests. Indeed, to give the best possible results, any chemical reaction needs the definition of several factors including temperature, time, pressure, type of suitable substrates, quantities of reagents, rate of addition, catalyst, solvent medium, concentration and pH conditions. All these parameters, singularly or combined can have a relevant influence on the reaction result in terms of yield, purity and selectivity. Due to the large number of variables affecting the SMCRR synthesis, a scouting of the most useful conditions to be adopted has been performed by DoE, which supports the rational choice of experimental conditions. DoE allows to plan the tests in the experimental domain (defined as the variability domain of the considered factors, that is the input variables) to obtain the maximum of information regarding the response (dependent or output variable) using the minimum number of experimental tests.32–35The simplest DoE procedure for the analysis of a property (for instance the reaction yield) is the two-level factorial design, in which the single variables assume two values: the maximum and the minimum one with respect to an average value (which represents the expected average value for that factor). Typically, the input variables are normalized in the interval (−1, +1). The number of tests needed to “cover” the full range of variability is equal to 2n, where n is the number of variables considered. From the comparative examination of the values of the model coefficients, it is possible to identify the relative importance of the various variables under investigation. In fact, the construction of a model curve allows to highlight anomalies or reverse trends otherwise not appreciable.
Here, to individuate the best SMCCR conditions for the optimization of the yield in the desired DIT molecule, a DoE based on a multivariate approach was applied by considering the substrate typology (A), solvent (S) and reaction temperature (T) as the input variables (x), and the percentage reaction yield (η%) as the response (y).
As initial experimental conditions for the synthesis of DIT, we used a procedure reported for the preparation of 1,3,5-triisopropenylbenzene from 1,3,5-tribromobenzene,9 here adapted on smaller quantities (1.0–1.5 g, 4.5–6.5 mmol) of 2,5-dibromothiophene substrate. However, the reaction conditions reported in the literature (8 eq. of Cs2CO3, solvent system made of a mixture dimethoxyethane/EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2), concentration 0.2 M of the brominated substrate, temperature of 80 °C, reaction time of 24 h in argon atmosphere) were found not suitable for a reagent system scaled down by about an order of magnitude. In fact, various critical issues aroused: (i) demixing of the constituents of the solvent system with pauperization of the organic fraction; (ii) inefficient stirring caused by the increase in viscosity due in particular to the large excess of cesium carbonate base; (iii) poor dispersion of the brominated substrate in the reaction medium and consequent reduction of the contact surface between reactants and catalyst; (iv) tendency of the Pd(PPh3)4 catalyst to segregate, further worsening the effectiveness of the reactant interaction.
1.2), concentration 0.2 M of the brominated substrate, temperature of 80 °C, reaction time of 24 h in argon atmosphere) were found not suitable for a reagent system scaled down by about an order of magnitude. In fact, various critical issues aroused: (i) demixing of the constituents of the solvent system with pauperization of the organic fraction; (ii) inefficient stirring caused by the increase in viscosity due in particular to the large excess of cesium carbonate base; (iii) poor dispersion of the brominated substrate in the reaction medium and consequent reduction of the contact surface between reactants and catalyst; (iv) tendency of the Pd(PPh3)4 catalyst to segregate, further worsening the effectiveness of the reactant interaction.
Considering the several difficulties encountered, the empirical approach has been replaced with a more systematic investigation aimed at optimizing the preparation of DIT through the SMCCR and better understanding the influence of the experimental parameters. To this end, the investigation was focused on reactions involving as the nucleophile species a boronic ester (as mentioned boronic esters are typically used in SMCCR)5–8 bearing one isopropenyl moiety, namely the pinacolic ester of the isopropenylboronic acid.
Specifically, by using the Reaxys database36 available online for searching compounds, properties and chemical reactions, amongst the numerous SMCCRs described in the literature 704 articles involving the pinacolic ester of isopropenylboronic acid were found. Within this large group, about a hundred were selected that showed greater similarity with the electrophilic substrate under study: that is, non-condensed aromatic rings, zero or unitary number of ring heteroatoms, substitutions with halogens (essentially bromine and iodine) and absence of other groups orienting and/or with important electronic effects.
Besides the typology of substrate, attention was focused on two other important variables: the solvent medium (S) and the reaction temperature (T). From the analyzed data, it was possible to classify the reactions as follows: those where 1,4-dioxane is used as reaction medium or those where another solvent, single or mixed, is selected. Furthermore, the temperatures at which these SMCCRs are carried out can be grouped in a relatively low range (T ≈ 70 °C) or in a higher one (T ≈ 100 °C).
In such a way, a set of variables that can be defined as “categorical” or “Boolean”, as they do assume discrete values in a given interval, was constructed. By defining two alternatives of “maximum” (+) and “minimum” (−) for each of the variables studied (A = aromaticity of the substrate; S = solvent; T = temperature), the reactions selected from the literature were classified considering all possible combinations of the three above mentioned variables, thus creating a factorial design of type 23 for a total of 8 categories of reaction according to the following criteria:
A (+) = substrate of typology A
A (−) = substrate of typology B
S (+) = solvent 1,4-dioxane
S (−) = other solvent, single or mixed
T (+) = high temperature (≈100 °C)
T (−) = low temperature (≈70 °C)
For each series of reactions, an average of the percentage yields (η%) was calculated to have final numerical data as little as possible affected by experimental errors and as much independent as possible from the single specific case, in other words, as much representative as possible of the whole system.
A MLR model was then adopted to assess the importance of the factors under investigation. The evaluation of this model required analysing the coefficients assumed by each term both in absolute value, to quantify the impact of the corresponding variable, and for their sign, that is for their positive or negative effect with respect to the response variable (reaction yield, η%). The resulting output is summarized in Table 2, which also evidences if the effect of a factor is attributable to random experimental errors, in other words if it is with no or little significance for the reaction yield.
From the data reported in Table 2 and considering the following results obtained for the model: Multiple R-squared: 0.9173, Adjusted R-squared: 0.9118, F-statistic: 164.6 on 6 coefficients and 89 DF, p-value: <2.2 × 10−16, we inferred four considerations, which have been of great practical utility for this experimental work and are listed in the following.
(i) The condensation of Suzuki–Miyaura is substantially independent of the type of aromatic substrate (very low effect of the A factor), indeed it finds wide applicability without significantly changing the reaction performance.
(ii) The temperature is certainly the predominant factor on the reaction yield: its lowering, even relatively slight, heavily affects the result which sometimes may not be appreciable (in the sense that the reaction does not take place at all).
(iii) The solvent also plays a decisive role, mostly because the reagent system is often not homogeneous with consequent problems of interaction between reactants and catalyst.
(iv) The terms of double interaction A·S and A·T are of little importance, this being in line with the conclusions of point (i). In addition, it is worth noting that the sign of the S·T double factor suggests a non-negligible correlation between the two variables in a decreasing sense. This means it is not sufficient to optimize the solvent or the temperature separately, but it is required to evaluate whether a variation made to the first does not negatively affect the second and vice versa.
Considering the observations made above, for η% the following response function (eqn (1.a) and (1.b)) in the two relevant variables (S, T) was obtained (depending on the coded value of the solvent used):
| η%(S, T) = 85.56 + 6.48S + 8.85T − 3.56ST | (1.a) |
| η%(S, T) = 85.56 − 6.48S + 8.85T + 3.56ST | (1.b) |
By analysing the response function, interesting information can be obtained because of the boundary hypotheses for the constructed model. First, the choice of 1,4-dioxane (S = +1) as the solvent leads to an improvement in terms of reaction yield. Secondly, it can be shown that to obtain a theoretical yield of 100% it would be necessary to introduce, using the normalized variables, T ≈ 1.45, that is a value well outside the discrete interval [−1:1], and therefore not experimentally accessible. Instead, by imposing a yield η% ≈ 95, a temperature T ≈ 90 °C is obtained, slightly lower than the extreme (100 °C) for the investigated range.
Using reaction conditions as much as possible similar like suggested by the multivariate analysis, that are using 1,4-dioxane as the relevant solvent and 90 °C as the reaction temperature, while doing a proper merge with conditions reported to prepare 1,3,5-triisopropenylbenzene,9 where water was employed in the solvent mixture to help dissolution of the inorganic base, we obtained DIT monomer, focus of this work, in 89% yield (see Scheme 2 and Table 3, entry 1 in the following).
Table 3 shows the results obtained by varying the employed inorganic base in terms of reaction yield and presence or absence of the mono-substituted intermediate.
| Entry | Base | Mono-substituted intermediate | DIT yield (η%) |
|---|---|---|---|
a Reaction conditions: 2,5-dibromothiophene 0.2 M; solvent mixture 1,4-dioxane/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); boronic ester 1.5 eq.; Pd(PPh3)4, catalyst 1.5 mol%; base 4 eq.; temperature 90 °C; time 24 h; argon atmosphere. 1); boronic ester 1.5 eq.; Pd(PPh3)4, catalyst 1.5 mol%; base 4 eq.; temperature 90 °C; time 24 h; argon atmosphere. |
|||
| 1 | Cs2CO3 | Present | 89 |
| 2 | NaHCO3 | Present | 78 |
| 3 | KHCO3 | Present | 82 |
| 4 | NaOH | Absent | 88 |
| 5 | KOH | Absent | 91 |
From the collected data one can first observe that a strong alkaline base (entry 4 and 5 in Table 3) (higher pH of the reaction system) allows to obtain better reaction yields if compared with those obtained using basic salts (entry 2 and 3). At equal cation, the yield is 10% higher for the stronger bases (entry 4 with respect to 2 and entry 5 with respect to 3).
In our system, this result can be explained assuming that the concentration of the hydroxyl ions is relevant in the acid–base interaction with the boronic ester (Scheme 3, Path A) or in the formation of the oxo–palladium complex (Scheme 3, Path B). In Path A (via boronate) a boron tetra-coordinate anionic species is formed which associates by means of a dative bond with the complex resulting from the oxidative addition. In this way, the reactive species entering the catalytic cycle is the boronate, which is formed by acid–base reaction with OH−. In Path B (via oxo–palladium) the oxo-anionic species acts as a nucleophile by replacing the ligand X (a good leaving group) leading to the formation of an oxo–palladium complex. Then, the ligand (OH− as in the present case or analogue) acts as a Lewis base by means of a non-bonding electron pair interacting with the boron derivative.
Initially Suzuki and Miyaura6,37 proposed the catalytic cycle proceeded through Path A, but more detailed studies subsequently conducted by the same authors38 also suggested the possibility of Path B. Despite the ambivalence of the mechanism, the base certainly plays a decisive role as a real stoichiometric species whose total consumption, regardless of the reaction path, is at least equal to two equivalents with respect to the boron derivative. Indeed, in both cases, the reactive intermediate that leads to transmetallation reaction is a zwitterionic complex containing the sequence of Pd–O–B bonds.
Another observation concerns the presence of small quantities of the mono-substituted intermediate, that the use of strong bases overcomes, according to the output reported in Table 3 (entry 4 and 5). Further, in entry 1, although a carbonate base, whose basic strength is intermediate with respect to the pairs 2/3 and 4/5, is used, the resulting reaction yield is comparable to those of entry 4 and 5 carried out with the stronger OH− base. To understand this finding, the role of the cation (namely Cs+) must also be considered. Independently of the reaction path, to have oxidative addition it is essential the cleavage of the carbon–halogen bond of the substrate occurring in the transmetallation step, when the halogen is removed from the palladium complex to bind the oxygen deriving from the base. To speed up this process, an appropriate cation, that can electrostatically associate with the leaving anion, can be introduced into the reaction medium. If this takes place with a high stability constant, as in the case of Cs+ (entry 1), the heterolytic cleavage of the bond will occur faster thus favoring the reaction and, in turn, increasing the yield despite the basic strength of the carbonate is lower than that of the OH−. This can be inferred also for the runs with the same anion (entry pairs 2/3 and 4/5), where a slight increase in the yield is observed when the cation is K+ compared to Na+.
As described before, the choice of the base represents a crucial aspect in Suzuki's condensation. The possibility of using non-toxic salts or inorganic bases, which are easy to find and provide excellent results, has shifted the study on the optimization of synthetic protocols that make extensive use of them. An important issue accompanying the choice of these oxygen nucleophiles is the need for an aqueous medium in which they can be easily dissociated. On the other hand, most organic substrates have good solubility only in non-polar solvents. Therefore, heterogeneous systems are often used by combining the two opposite needs. Under these conditions, contact problems between the reactants and difficulties in finely suspending the palladium catalyst are observed; as a consequence, condensation only occurs at the phase separation surface with consequent increasing of the reaction times.
In this respect, Sanzone et al.40 demonstrated that the addition of surfactant substances, acting as phase transfer catalysts (PTCs) able of forming micellar structures within which contact efficiency among reactants is increased, can be used as a method to favor and accelerate Suzuki–Miyaura coupling in heterogeneous reaction systems. In the case of anionic reactants such as OH−, a quaternary ammonium salt can generally simplify the transfer of the reactants from one phase to the other where the reaction occurs more easily.
Such a strategy was here employed to further improve the SMCCR conditions for DIT synthesis. As regards the solvent medium, although the preliminary investigation based on DoE from literature data suggested 1,4-dioxane as the solvent of choice for the cross-coupling under study, we opted for the use of a mixed 1,4-dioxane/water solvent system to favor the dissolution of the inorganic base. Thus, with a view to a more sustainable and less impacting chemistry, an attempt was made to optimize new conditions that possibly reduce the use of organic solvents in favor of water, without affecting the reaction performance.
To this purpose a phase transfer catalyst, namely tetra-n-butylammonium n-nonylphenoxide (TBANP) was specifically prepared in two simple synthetic steps (see the Experimental section). Indeed, the n-tetrabutylammonium cation effectively performs the function of catalyst for phase transfer, favoring the contact between the hydroxyl ion (in the aqueous medium) and the boron reactive or the palladium catalyst, depending on the reaction mechanism undertaken (Path A or B). To favor the interaction between the substrate (2,5-dibromothiophene) and the palladium phosphine complex (Pd(PPh3)4), both non-polar compounds poorly miscible in the reaction medium, the conjugate base of a long-chain alkyl phenol, namely n-nonylphenoxide, was here chosen as the counter anion. The contact between the two mentioned reactants is indeed important since it involves oxidative addition, the first step of the catalytic cycle. TBANP is a synthetic organic compound used industrially in surfactant chemistry. Its amphiphilic properties allow to lower the surface tension of a liquid, facilitating its miscibility with others of different polarity. The PTC percentages here adopted should allow to reach, at least locally, the critical micellar concentration, as suggested in the literature.40,41
The series of complicated equilibria of ionic association and diffusion occurring in the studied reagent system is summarized in Scheme 4. As exemplified, the hydrophobic tails of the n-nonylphenoxide create an apolar region in the internal core of the micelle in which 2,5-dibromothiophene substrate, Pd(PPh3)4 catalyst and the acid form of the boronic ester are better solvated. Whereas the Bu4N+ cation favors the migration of anionic species (OH− and boronate). It is worth noting that the proposed scheme takes into account both possibilities of condensation mechanism (Path A or B) previously discussed. Indeed, the described supramolecular aggregates (micelle) can be viewed as nano-reactors where the rate of cross-coupling reaction is enhanced by the increase of local concentration of the reactant species.
 | ||
| Scheme 4 Possible associative and diffusive phenomena of the ions and reacting species involved in the SMCCR in the presence of TBANP as PTC. | ||
To evaluate the effect of the presence in different percentage of PTC and water in the reaction medium, some targeted tests were then carried out following a very basic DoE of the type called central composite design (CCD).42 Through the CCD approach the effect of continuous variables, the percentage of water in the solvent medium and that of the PTC (TBANP), ranging between a minimum and a maximum value can be investigated, also highlighting how these parameters interact, by means a reduced number of costly and time-consuming experiments. It must be pointed out that operating in such way, the second order model for the response variable (reaction yield in our case) is constructed without a complete factorial design, thus greatly simplifying the laboratory work but, at the same time, reducing its predictive capacity.
The tests, listed in Table 4, were carried out using as solvent medium 1,4-dioxane/water mixtures at various ratios and different percentages (mol% with respect to the 2,5-dibromothiophene substrate) of the PTC ad hoc synthesized. On the base of the results previously discussed, the concentration of the 2,5-dibromothiophene substrate was kept constant (0.2 M), as did the temperature (T = 90 °C), the catalyst (Pd(PPh3)4, 1.5 mol%), the boronic ester (1.5 eq.) and the duration (24 h). As for the inorganic base, 4 eq. KOH, which was proven to provide the best results in terms of reaction yield (see Table 3) were used.
Data elaboration by MLR allowed the construction of a quadratic model (eqn (2)), where α indicates the percentage of water (vol/vol) in the reaction medium and β the percentage (mol%) of the TBANP phase transfer catalyst.
| η%(α,β) = 87.00 − 1.10α + 3.78β + 0.52αβ + 0.12α2 + 0.89β2 | (2) |
By questioning the simple model elaborated, some interesting observations can be made:
(i) the known term of eqn (2), equal to 87.00, confirms the efficiency of the Suzuki condensation once the experimental conditions have been optimized. A good agreement of this value with that found in eqn (1.a) and (1.b) (equal to 85.5) employed in the preliminary phase for the reaction optimization is observable;
(ii) the increase in the percentage of water, in the absence of PTC, leads to a decrease in the yield (Table 4, entry 3 with respect to 1) due to the increasing segregation of the reactants and the palladium catalyst. This aspect is quantitatively evidenced by the negative coefficient of the variable α. This is assigned also to the tendency, in an aqueous environment, of Pd(0) to undergo oxidative processes leading to non-reactive Pd(II) species increase;
(iii) the increase in the amount of the phase transfer catalyst, as expected, in any case (entry 4 with respect to 1 and entry 5 with respect to 3) improves the reaction yield. For high PTC values, the yield remains substantially unchanged passing from 30 to 100% water in the solvent medium (entry 5 with respect to 4).
(iv) from eqn (2) one can infer that it is possible to obtain very good yields (η% ≈ 90–92%) by keeping the amount of the PCT in the range 18–22 mol% for practically the entire variability of the water percentage. This result is justifiable considering the discrete value of the positive coefficient of the double interaction term α·β.
The importance of the use of surfactants in Suzuki–Miyaura cross coupling reactions was emphasized in a recent work of Sanzone et al.,40 where PTCs were used for Suzuki polymerization. The implementation of these systems also makes possible to adopt substantially aqueous solvents with reducing environmental impact. Indeed, in a more and more diffuse green chemistry framework, this environmental aspect has assumed strategic importance following the introduction of the E-factor.43,44 This is a quantifier of the sustainability of chemical methods defined as the ratio between the quantity of organic waste produced (in kg) and that of the product obtained, expressed with the same unit of measurement. The evaluation of this descriptor requires however production on a semi-industrial scale, here not investigated because out of the scope of the present work.
S-r-DIT copolymers by inverse vulcanization
The new DIT comonomer was then employed in the synthesis of sulfur-rich copolymers via inverse vulcanization. As described in the Introduction, the IV process is a synthetic method recently burst out in the literature.9,13–23,26–30 It allows to obtain polymers with a high sulfur content (from 50–90 wt%) with peculiar and intriguing properties, in particular very high refractive index and excellent transparency in the near infrared region, this making them appealing for photonic applications. We would like to further stress sulfur is a waste of oil and gas industry, thus making IV a strategy for recycling such elements to produce polymers for novel technological applications.13,24,25In general, the experimental procedures of IV foresee the introduction of the elemental sulfur in a reaction vessel and subsequent heating well above its melting temperature under stirring, usually in an inert environment. Once the complete fusion of sulfur is obtained, the organic comonomer, typical examples are divinylbenzene and diisopropenylbenzene, is added. Since the IV process is still in its early stage of development, various experimental set-up and conditions are described in the literature: different reaction times (strongly dependent on the substrate nature), variable order of reagents addition, use or not of controlled atmosphere, process temperatures variable in a certain range; post-reaction thermal treatments.
Here, a IV procedure preliminary suggested by us10 for preparing sulfur-rich copolymers containing 2,5-diisopropenylthiophene comonomer was further developed and optimized. Such a procedure (see the Experimental section) guarantees to control temperature and atmosphere in the reaction vessel to avoid DIT comonomer losses while efficiently stirring the reaction mixture. A series of random S-r-DIT copolymers at increasing sulfur contents from 60 up to 90 wt% with increments of 5 wt% was prepared (see Table 5).
S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DIT (wt DIT (wt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt) wt) |
TDa (°C) | TVmaxb (°C) | Residue@700 °Cc (wt%) | Tgd (°C) |
|---|---|---|---|---|
| a Corresponding to a mass loss of 1–2%.b Corresponding to the maximum rate of mass loss.c Before the introduction of oxygen into the instrument furnace.d Measured at heating rate of 20 °C min−1.e From ref. 51. | ||||
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
222 | 258 | 35 | 1 |
65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
225 | 262 | 34 | −3 |
70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
226 | 264 | 31 | −8 |
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
228 | 266 | 25 | −15 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
230 | 272 | 23 | −18 |
85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
234 | 275 | 16 | −23 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
244 | 288 | 7 | −27 |
| Sulfur | 244 | 287 | 0.1 | −30e |
The copolymers were first characterized by FTIR spectroscopy in the ATR mode. A selection of spectra collected for copolymers at various S/DIT increasing ratios from top down are shown in Fig. 1; spectra of elemental sulfur and DIT comonomer are reported as well for comparison purpose. The infrared spectrum of pure sulfur exhibits a main peak at 465 cm−1, related to the stretching of the S–S bond.45–47 As visible in the figure, a peak with similar feature is also present in all the spectra of the synthetized S-r-DIT copolymers, confirming the presence in their macromolecular architecture of sulfur in the form of polysulfide (S–S)n chains.47
 | ||
| Fig. 1 ATR-FTIR spectra of selected S-r-DIT copolymers. Spectra of DIT comonomer and elemental sulfur are reported as well for comparison purpose. | ||
Upon the IV reaction, the appearance in all spectra of the broad band centered at about 590 cm−1 ensues the inverse vulcanized copolymerization; this band can be reasonably assigned to the stretching of the new formed C–S bond.44,47 In this specific system, the C–S bond is already present in the thiophene ring of DIT; however, since such an absorption often appears as a weak (if not faint) band,46,48 it is difficult to identify it with certainty for DIT molecule. The infrared spectrum of DIT instead clearly shows the stretching of the unsaturated = C–H, borne by both the heteroaromatic ring and isopropenyl moieties, centered at 3084 cm−1, the aliphatic –C–H stretching bands (due to the methyl groups) in the range 3000–2800 cm−1, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the isopropenyl moiety at 1618 cm−1, and a strong absorption at 874 cm−1, reasonably ascribable to the out-of-plane = C–H bending of the isopropenyl groups.46 By comparing the spectra of the DIT comonomer with those of the copolymers, one can observe: (i) the decrease in intensity of the peak at 3084 cm−1 due to the vanishing of the isopropenyl = C–H stretching band, (ii) the disappearance of the peaks at 1618 cm−1, characteristic of isopropenyl C
C stretching of the isopropenyl moiety at 1618 cm−1, and a strong absorption at 874 cm−1, reasonably ascribable to the out-of-plane = C–H bending of the isopropenyl groups.46 By comparing the spectra of the DIT comonomer with those of the copolymers, one can observe: (i) the decrease in intensity of the peak at 3084 cm−1 due to the vanishing of the isopropenyl = C–H stretching band, (ii) the disappearance of the peaks at 1618 cm−1, characteristic of isopropenyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching, and (iii) of the strong band at 874 cm−1, upon their reaction with sulfur during the IV process. On the other hand, the S-r-DIT samples show spectra which retain the features of the thiophene ring and methyl groups of the starting DIT. Indeed, the vibration so-called of ring breathing and that due to the out-of-plane heteroaromatic = C–H wagging at, respectively, 1453 and 799 cm−1, related to the presence of a 2,5-disubstituted thiophene moiety,49,50 are still observable, indicating the thiophene ring is maintained as such in the macromolecular architecture obtained upon IV reaction. It may be useful to recall here the typical signal of C
C stretching, and (iii) of the strong band at 874 cm−1, upon their reaction with sulfur during the IV process. On the other hand, the S-r-DIT samples show spectra which retain the features of the thiophene ring and methyl groups of the starting DIT. Indeed, the vibration so-called of ring breathing and that due to the out-of-plane heteroaromatic = C–H wagging at, respectively, 1453 and 799 cm−1, related to the presence of a 2,5-disubstituted thiophene moiety,49,50 are still observable, indicating the thiophene ring is maintained as such in the macromolecular architecture obtained upon IV reaction. It may be useful to recall here the typical signal of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C olefinic double bond does not appear as such in thiophene moiety.49
C olefinic double bond does not appear as such in thiophene moiety.49
Thermogravimetric analysis carried out on the synthesized S-r-DIT copolymers revealed a relatively high thermal stability (Table 5 and Fig. 2). Indeed, increasing the sulfur content, the temperature of the degradation onset (TD) ranges from about 220 up to 245 °C, while the temperature of the maximum rate of mass loss (TVmax) increases from 245 up to 288 °C passing from 60 to 90 wt% sulfur content. In the same conditions, degradation of pure sulfur takes place in a single step, with maximum degradation rate at 287 °C, and is practically complete slightly above 300 °C. Thus, in the TGA curves of the inverse vulcanized copolymers the first degradation step, with maximum degradation rate around 280 °C, is primarily assigned to the loss, in the form of volatile products, of the polysulfide chains present in their macromolecular architecture, and secondly to the partially superimposed degradation of the organic portion of the samples. Indeed, the thermograms from 300–320 up to 700 °C show a gently sloping plateau essentially due to the carbonaceous residue originated from the presence of DIT comonomer cross-links in the macromolecular structure. TGA measurements also showed a good agreement between the residual mass (wt%) measured at 700 °C (that is prior of introducing oxygen atmosphere into the instrument furnace) and the amount of DIT monomer used in the reaction feed.
The DSC analysis revealed an essentially amorphous behavior of the copolymers, as expected for the random macromolecular structures resulting from the IV reaction.13,47 In fact, the DSC thermograms collected on heating (here not shown) evidenced the mere presence of a glass transition for the copolymers with up to 80 wt% sulfur content. For samples with higher content of sulfur a broad (faint in the case of S/DIT = 85/15) exotherm followed by an endotherm, centered at 100 °C (ΔHm of 2.5 J g−1) and 103 °C (ΔHm of 25 J g−1) for S/DIT 85/15 and 90/10, respectively, were observed. In analogous conditions, the DSC heating curve of elemental sulfur showed an endotherm centered at 120.5 °C with ΔHm of 45 J g−1, in line with the complex melting behavior of pure sulfur.52 Such phenomena can be ascribed to the cold crystallization and successive melting of the relatively long polysulphide chains present in the poorly crosslinked macromolecular architectures of copolymers at very high sulfur content, in accordance with what proposed by Jung et al.,13 although the concomitant presence of unreacted sulfur and/or its formation by copolymer decomposition at high temperatures cannot be completely ruled out. The Tg values (Table 4) of the S-r-DIT copolymers appear quite low, ranging from −27 to about 0 °C when passing from 90 to 60 wt% sulfur content. Indeed, by increasing the DIT comonomer percentage, the points of reticulation increased, reducing segmental chain mobility.
S-r-DIT copolymers optical properties
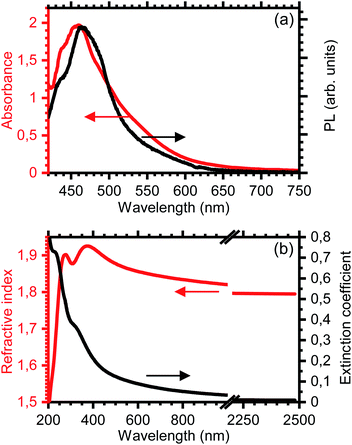
Even though the S-r-DIT copolymers are reticulated, they can be dissolved in toluene up to concentrations as high as 10% v/v. Fig. 3a shows the absorbance of a solution of the S-r-DIT copolymer containing 70 wt% of sulfur. The spectrum exhibits a weakly structured broad peak at 460 nm with a long tail extending up to 700 nm, which indicates strong inhomogeneous broadening with a possible scattering contribution. Remarkably, even though the high concentration of the solution allows self-absorption of photoluminescence, it is possible to detect a broad emission peak at 464 nm, which almost extends along the overall visible spectrum up to 650 nm.The real (n) and imaginary (k) part of the refractive index (n = n + ik) of the copolymer are displayed in Fig. 3b, as retrieved from spectroscopic ellipsometry for a thin film fabricated by spin-coating of the previously mentioned solution on a fused silica substrate. The possibility to obtain films with good optical quality prove the processability of such copolymer, which is of fundamental importance for the fabrication of functional photonic structures.10 Fig. 3b shows that the peak in the extinction coefficient spectrum is shifted towards the ultraviolet part of the spectrum with respect to the absorption peak of the solution. It also displays a very broad tail extending up to the near-infrared region of the spectrum (k = 0.015 at 1500 nm) and two shoulders at 229 and 310 nm assigned to the absorption of the polysulfide chains.53–55
The refractive index, as expected, is very high in an extended spectral region approaching the lower value of 1.795 at 2500 nm, in the transparency spectral region. In the visible range it shows the typical dispersive behavior reaching 1.84 at 780 nm and a maximum value of 1.93 at 375 nm. An additional peak is observed in the dispersion at 270 nm (n = 1.9). Below such wavelengths, the anomalous dispersion is clearly observed with a reduction of n down to 1.5 at 250 nm. Analysing the spectral dispersion properties of the refractive index and comparing them with other optical polymers can be useful to identify the potentiality of the new IV copolymers in applications related to optics and photonics. The properties can be roughly estimates employing the Abbe number (VD),56 which decreases upon increasing the dispersion effect. Fig. 4 reports the refractive index versus the Abbe number for the S-r-DIT copolymer and for polymers used in photonics and in packaging. S-r-DIT possesses a low Abbe number with values comparable to those of highly dispersive polyvinylcarbazole (PVK) and polyvinylalcohol–titania hybrids (PVA-Hy TiO2), indicating a stronger dispersion with respect to standard transparent polymers like polystyrene (PS), polycarbonate (PC), polyethyleneteraphthalate (PET), cellulose acetate (CA), polypropylene (low molecular mass, PP), polyvinylalcohol (PVA) and perfluorinated Aquivion® [see caption of Fig. 4]. On the other hand, the refractive index value is larger than that of PVK, which has the largest index for commercial polymer employed in the field. Then, notwithstanding the low Abbe number, the high refractive index promises potentiality in photonic applications. Indeed, larger index are currently available only for hybrid and composites materials, which are much more dispersive and complex to process.
 | ||
| Fig. 4 Refractive index of polymers used for photonics as a function of the Abbe number. The red dot is for the data of IV S-r-DIT copolymer reported in Fig. 3. PC, PS, PET and PMMA from ref. 57; Aquivion® from ref. 58; all other materials from ref. 59 and references therein reported. | ||
The possibility to fabricate high refractive index thin films from solution with the S-r-DIT IVPs is very stimulating, especially for applications related to the near infrared spectral region.58 Indeed, despite the low glass transition temperatures of DIT-based IVPs, a preliminary report shows that similar systems can be blended with a suitable polymer to fabricate good quality all-polymer dielectric mirrors, which are the fundamental bricks for photonic structures for applications including lasing and improvement of light harvesting in photovoltaic devices.10 Chemical engineering of Tg for the IV copolymers and their processing as thin films of optical quality will be a promising challenge to exploit these materials in photonics.
A more comprehensive characterization of these and similar inverse vulcanized polymer systems will possibly be deepened in further research work, also in view of finding other fields of application.
Conclusions
A novel molecule, namely 2,5-diisopropenylthiophene (DIT), purposely designed as suitable comomomer for inverse vulcanization with elemental sulfur, over-supplied by-product of gas and oil industry, was here successfully achieved through a simple double Suzuki–Miyaura cross-coupling reaction on commercially available 2,5-dibromothiophene substrate. Initial setting of reaction parameters was determined by applying a simple design of experiment based on a multivariate approach to a series of SMCCRs selected from literature by considering the influence of substrate typology, solvent, and reaction temperature on the reaction yield. Optimization of SMCCR conditions in terms not only of mere reaction yield but also -and importantly- of improved compliance with sustainability issues was accomplished by deepening the role of the base as well as of water and phase transfer catalyst in the reaction medium.DIT molecule was then proven as suitable comonomer in the inverse vulcanization process, rapidly risen as hot topic in the recent literature. A series of random S-r-DIT copolymers with sulfur content from 60 up to 90 wt% was prepared and characterized. As envisaged, these sulfur-rich copolymers are endowed with very high refractive index (n = 1.795 at 2500 nm).
On the overall, gathered results can be viewed as a case study where a monomer was purposely designed to suitably react in the simple, solvent-free, easy-to-scale-up inverse vulcanization process and thus achieve inverse vulcanized polymers with noticeably high refractive index and other interesting properties. Both monomer synthesis and polymerization conditions were optimized taking into account as much as possible the green chemistry principles.
Author contributions
Conceptualization, P. S and D. C.; synthesis, C. T., C. D. and G. L.; design of experiment: G. L. and C. T.; characterization, C. T., C. D., G. L., P. L., and M. P.; device fabrication, P. L.; writing, review and editing, P. S., G. L., P. L., M. P. and D. C.; supervision, P. S. and D. C.; project administration, P. S.; funding acquisition P. S. and D. C.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support of Compagnia di San Paolo (Turin, Italy) Research Project “PIVOT – Sulfur-based Polymers from Inverse Vulcanization as high refractive index materials for planar photonic crystals: dielectric mirrors and microcavities” (IDROL 20583) is gratefully acknowledged. Thanks are due also to PRIN 2020 Project “PETALS Polymer mETamateriALs for nanophotonicS” (prot. 2020TS9LXS) funded by the Italian Ministry for Universities and Research (MUR). The authors warmly thank Dr Martina Marsotto for performing DSC measurements.References
- Cross-coupling reactions: a practical guide, Topics in Current Chemistry Series 219, ed. N. Miyaura, Springer-Verlag, New York, USA, 2002 Search PubMed.
- R. Narayanan, Molecules, 2010, 15, 2124–2138 CrossRef CAS PubMed.
- A. Biffis, P. Centomo, A. Del Zotto and M. Zecca, Chem. Rev., 2018, 118, 2249–2295 CrossRef CAS PubMed.
- Nobel Prize in Chemistry, 2010, available online, https://www.nobelprize.org/prizes/chemistry/2010/summary/, accessed on 10 December 2021 Search PubMed.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- N. Miyaura and A. Suzuki, J. Chem. Soc., Chem. Commun., 1979, 19, 866–867 RSC.
- American Chemical Society, Green chemical syntheses and processes, in Green Chemical Syntheses and Processes, American Chemical Society, Washington, DC, USA, 2000, vol. 767, pp. 1–5 Search PubMed.
- S. E. Hooshmand, B. Heidari, R. Sedghi and R. S. Varma, Green Chem., 2019, 21, 381–405 RSC.
- T. S. Kleine and N. A. Nguyen, et al., ACS Macro Lett., 2016, 5, 1152–1156 CrossRef CAS.
- C. Tavella, P. Lova, M. Marsotto, G. Luciano, M. Patrini, P. Stagnaro and D. Comoretto, Crystals, 2020, 10, 154 CrossRef CAS.
- Reaxys ID 35700134, Reaxys search engine, https://www.reaxys.com/.
- M. M. Coleman, J. R. Shelton and J. L. Koenig, Ind. Eng. Chem. Prod. Res. Dev., 1974, 13, 154–166 CrossRef CAS.
- W. J. Chung and J. J. Griebel, et al., Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed.
- Z. J. Sun and M. Xiao, et al., J. Mater. Chem., 2014, 2, 9280–9286 RSC.
- J. Lim, J. Pyun and K. Char, Angew. Chem., Int. Ed., 2015, 54, 3249–3258 CrossRef CAS PubMed.
- J. J. Griebel and N. A. Nguyen, et al., ACS Macro Lett., 2015, 4, 862–866 CrossRef CAS.
- J. J. Griebel, R. S. Glass, K. Char and J. Pyun, Prog. Polym. Sci., 2016, 58, 90–125 CrossRef CAS.
- J. J. Griebel, G. Li, R. S. Glass, K. Char and J. Pyun, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 173–177 CrossRef CAS.
- D. Gomez and J. A. Mecerreyes, et al., J. Power Sources, 2016, 329, 72–78 CrossRef.
- M. K. Salman, B. Karabay, L. C. Karabay and A. Cihaner, J. Appl. Polym. Sci., 2016, 133, 43655 CrossRef.
- M. P. Crockett and A. M. Evans, et al., Angew. Chem., Int. Ed., 2016, 55, 1714–1718 CrossRef CAS PubMed.
- L. E. Anderson and T. S. Kleine, et al., ACS Macro Lett., 2017, 6, 500–504 CrossRef CAS.
- J. M. Chalker, M. J. H. Worthington, N. A. Lundquist and L. J. Esdaile, Top. Curr. Chem., 2019, 377, 16 CrossRef PubMed.
- D. A. Boyd, Angew. Chem., Int. Ed., 2016, 55, 15486–15502 CrossRef CAS PubMed.
- J.-G. Wagenfeld, K. Al-Ali, S. Almheirri, A. F. Slavens and N. Calvet, Waste Manage., 2019, 95, 78–89 CrossRef PubMed.
- P. T. Dirlam and A. G. Simmonds, et al., RSC Adv., 2015, 5, 24718–24722 RSC.
- D. J. Parker, H. A. Jones, S. Petcher, L. Cervini, J. M. Griffin, R. Akhtar and T. Hasell, J. Mater. Chem. A, 2017, 5, 11682–11692 RSC.
- J. A. Smith, X. Wu, N. G. Berry and T. Hasell, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 1777–1781 CrossRef CAS PubMed.
- T. S. Sahu, S. Choi, P. Jaumaux, J. Zhang, C. Wang, D. Zhou and G. Wang, Polyhedron, 2019, 162, 147–154 CrossRef CAS.
- A. D. Tikoalu, N. A. Lundquist and J. M. Chalker, Adv. Sustainable Syst., 2020, 4, 1900111 CrossRef CAS.
- J.-G. Liu and M. Ueda, J. Mater. Chem., 2009, 19, 8907–8919 RSC.
- G. E. P. Box, W. G. Hunter and J. S. Hunter, Statistics for experimenters: an introduction to design, data analysis, and model building, Wiley, New York, 1978 Search PubMed.
- R. Carlson, Design and optimization in organic synthesis, Elsevier, New York, 1992 Search PubMed.
- M. R. Owen, C. Luscombe, L. W. Lai, S. Godbert, D. L. Crookes and D. Emiabata-Smith, Org. Process Res. Dev., 2001, 5, 308–323 CrossRef CAS.
- S. A. Weissman and N. G. Anderson, Org. Process Res. Dev., 2015, 19, 1605–1633 CrossRef CAS.
- Reaxys ID 35700134, Reaxys search engine, https://www.reaxys.com/.
- N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437–3440 CrossRef.
- N. Miyaura, K. Yamada, H. Suginome and A. Suzuki, J. Am. Chem. Soc., 1985, 107, 972–980 CrossRef CAS.
- T. Kitanosono, K. Masuda, P. Xu and S. Kobayashi, Chem. Rev., 2018, 118, 679–746 CrossRef CAS PubMed.
- A. Sanzone, S. Mattiello, G. M. Garavaglia, A. M. Calascibetta, C. Ceriani, M. Sassi and L. Beverina, Green Chem., 2019, 21, 4400–4405 RSC.
- M. N. Khan, Micellar catalysis, CRC Press, 2006 Search PubMed.
- R. H. Myers and D. C. Montgomery, Response surface methodology: process and product optimization using designed experiments, Wiley, New York, 2nd edn, 2002 Search PubMed.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- C. N. R. Rao, R. Venkataraghavan and T. R. Kastu, Can. J. Chem., 1964, 42, 36–42 CrossRef CAS.
- R. M. Silverstein, Spectrometric Identification of Organic Compounds, Wiley, VII edn, 2005 Search PubMed.
- A. S. M. Ghumman, M. M. Nasef, M. R. Shamsuddin and A. Abbasi, Polym. Polym. Compos., 2020, 1–20 Search PubMed.
- J. Coates, Interpretation of Infrared Spectra, A Practical Approach. Encyclopedia of Analytical Chemistry, ed. R.A. Meyers, John Wiley & Sons Ltd, 2006 Search PubMed.
- H. D. Hartough, Thiophene and its derivatives, in The Chemistry of Heterocyclic Compounds Series, Wiley, 1952, vol. 3, pp. 106−132 Search PubMed.
- U. Kodithuwakku, C. de Alwis, M. A. B. Prashantha and D. R. Ratnaweera, Int. J. Chem., 2016, 8, 1–14 CrossRef CAS.
- A. V. Tobolsky, W. MacKnight, R. B. Beevers and V. D. Gupta, Polymer, 1963, 4, 423–427 CrossRef CAS.
- B. Meyer, Chem. Rev., 1976, 76, 367–388 CrossRef CAS.
- H. E. Van Wart, A. Lewis, H. A. Scheraga and F. D. Saeva, Proc. Natl. Acad. Sci. U. S. A., 1973, 70, 2619–2623 CrossRef CAS PubMed.
- E. A. Fehnel and M. Carmack, J. Am. Chem. Soc., 1949, 71, 84–93 CrossRef CAS PubMed.
- B. Meyer, M. Gouterman, D. Jensen, T. V. Oommen, K. Spitzer and T. Stroyer-Hansen, The Spectrum of Sulfur and its Allotropes, Advances in Chemistry, 1972, vol. 110, ch. 4, pp. 53–72 Search PubMed.
- E. Hecht and A. Zajac, Optics by E, Hecht, Addison Wesley, 2nd edn, 1987 Search PubMed.
- https://www.mcanac.co.jp/en/service/detail/6b001.html?c1n=by+Chemical+Structure&c1s=structure%26c2n=Polymers%26c2s=01, accessed on 10 December 2021.
- H. Megahd, C. Oldani, S. Radice, A. Lanfranchi, M. Patrini, P. Lova and D. Comoretto, Aquivion–Poly(N-vinylcarbazole) Holistic Flory–Huggins Photonic Vapor Sensors, Adv. Opt. Mater., 2021, 9, 2002006 CrossRef CAS.
- P. Lova, H. Megahd, P. Stagnaro, M. Alloisio, M. Patrini and D. Comoretto, Appl. Sci., 2020, 10, 4122 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00654e |
| This journal is © The Royal Society of Chemistry 2022 |