Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biodiesel production from candlenut oil using a non-catalytic supercritical methanol transesterification process: optimization, kinetics, and thermodynamic studies
Marwan Abdulhakim Shaaha,
Md Sohrab Hossain *a,
Faisal Allafia,
Mohd Omar Ab Kadirb and
Mardiana Idayu Ahmad
*a,
Faisal Allafia,
Mohd Omar Ab Kadirb and
Mardiana Idayu Ahmad *a
*a
aEnvironmental Technology Division, School of Industrial Technology, Universiti Sains Malaysia, 11800 Penang, Malaysia. E-mail: sohrab@usm.my; mardianaidayu@usm.my; Fax: +6046533678; Tel: +6046532216 Tel: +6046532214
bPultex Sdn Bhd, Jalan Kampung Jawa, Bayan Baru, 11950 Bayan Lepas, Penang, Malaysia
First published on 29th March 2022
Abstract
The present study was conducted to determine the feasibility of biodiesel production from candlenut oil using supercritical methanol (scMeOH) as a non-catalytic transesterification process. The influence of the scMeOH transesterification process was determined with varying pressure (85–145 bar), temperature (260–300 °C), methanol to oil (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O) ratio (15
O) ratio (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–35
1–35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and reaction time (15–25 min). The experimental conditions of the scMeOH transesterification process were designed using central composite design (CCD) of experiments, and the process was optimized using response surface methodology (RSM). It was found that scMeOH temperature, pressure, M
1), and reaction time (15–25 min). The experimental conditions of the scMeOH transesterification process were designed using central composite design (CCD) of experiments, and the process was optimized using response surface methodology (RSM). It was found that scMeOH temperature, pressure, M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio, and reaction time substantially influenced the transesterification process. The maximum biodiesel yield of 96.35% was obtained at an optimized scMeOH transesterification process at the pressure of 115 bar, the temperature of 285 °C, M
O ratio, and reaction time substantially influenced the transesterification process. The maximum biodiesel yield of 96.35% was obtained at an optimized scMeOH transesterification process at the pressure of 115 bar, the temperature of 285 °C, M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 30
O ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and reaction time of 22 min. A second-order kinetics model and Eyring equations were utilized to determine the kinetics and thermodynamics of biodiesel production from candlenut oil. The activation energy value was determined to be 28.35 KJ mol−1. Analyses of the thermodynamic properties of biodiesel revealed that the transesterification process was non-spontaneous and endothermic. The physicochemical properties of produced candlenut biodiesel via scMeOH complied with most of the biodiesel properties as per ASTM D6751 and EN14214, thereby referring to good quality biodiesel production. The findings of the present study reveal that the scMeOH is an effective non-catalytic transesterification process for biodiesel production from candlenut oil.
1, and reaction time of 22 min. A second-order kinetics model and Eyring equations were utilized to determine the kinetics and thermodynamics of biodiesel production from candlenut oil. The activation energy value was determined to be 28.35 KJ mol−1. Analyses of the thermodynamic properties of biodiesel revealed that the transesterification process was non-spontaneous and endothermic. The physicochemical properties of produced candlenut biodiesel via scMeOH complied with most of the biodiesel properties as per ASTM D6751 and EN14214, thereby referring to good quality biodiesel production. The findings of the present study reveal that the scMeOH is an effective non-catalytic transesterification process for biodiesel production from candlenut oil.
1. Introduction
Energy is one of the essential elements of our daily life, and it plays a prominent role in all commercial sectors, including transportation, power generation, agriculture, and various other industries.1 It has been reported that global energy consumption will increase rapidly by 50% in 2035 compared to that in 2012.2,3 Currently, fossil fuels such as coal, natural gas, and petroleum-derived products are the primary energy source. Nevertheless, these are finite energy sources and are rapidly depleting. One of the current global problems is the instability of petroleum production. Besides, there is increasing public concern worldwide to determine alternative energy sources to mitigate the growing demand for fossil fuels.1,4 Furthermore, many agreements have been made to reduce greenhouse gas emissions and develop new alternative energy sources to replace fossil sources.5–7Biodiesel is a broad term used for the fuel produced from plant feedstock and biological sources such as vegetable plants, forestry by-products, waste, and animal fats.8 Generally, biodiesel is renewable, non-toxic, and it has a lower degradation time and lower emission rate than conventional petro-diesel.9–12 It has similar properties to petro-diesel, and it can be directly used in the existing engines without any need for engine modification.11 In the past, edible oils were the primary feedstock for biodiesel production.12–14 Several studies have been reported on the production of biodiesel from edible feedstocks such as canola, corn, sunflower, soybean, and palm oil.12–15 However, the use of edible oil as a biodiesel feedstock has some limitations, as it has a high production cost owing to the high prices of vegetable oils.15 Moreover, biodiesel production from edible oils has a significant influence on human food security, resulting in a reduction in food resources.16 To overcome the existing energy problems, more focus and emphasis have shifted towards non-edible oil crops as a feedstock for biodiesel.16,17 Numerous studies have focused on the production of biofuel from non-edible oils, including mahua (Madhuca longifolia), ratanjyot (Jatropha curcas), neem (Azadirachta indica), moringa (Moringa oleifera), and karanja (Pongamia pinnata).14–18 These feedstocks are available in abundance and do not affect the food chain.9,18
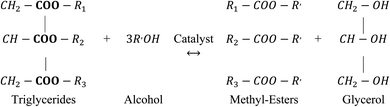
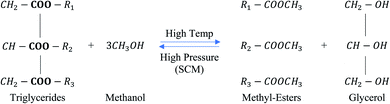
Among all the potential non-edible oils, candlenut is considered as a promising feedstock for biodiesel production, because of its high oil content and it can plant in non-fertile land like sandy, saline, and gravely soils that are not suitable for food production.18–21 Aleurites moluccana L. Wild, known as candlenut or kemiri, is a flowering tropical tree belonging to the Euphorbiaceae family. Even though the candlenut tree is native to Indo-Malaysia, it is now one of the world's major domesticated multipurpose trees.21 It has a medium size with a crown shape, irregular branches with large green leaves, and a maximum length of 20 m. It grows rapidly in the semi-tropical and tropical climate under the following ideal conditions: 0–1200 m height, soils with pH 5–8, 18–30 °C temperature, and 600–4300 mm rainfall. Candlenut is also known as candleberry, Indian walnut, varnish tree, buah keras (Malaysia), or kukui nut (Hawaii).21 Its seed oil content varies from 50–60 wt%, and the average annual oil yield is 3000 kg ha−1.20 The extracted oil is used in paints, varnishes, and the production of high-quality biofuels.19 The main processes for synthesizing biodiesel are dilution, microemulsion formation, pyrolysis, and transesterification.6,16 Among these, catalytic transesterification is the most commonly used process for biodiesel production.20,22 The basic catalytic transesterification reaction is shown in Fig. 1. Two types of catalysts, homogeneous (acidic or basic) and heterogeneous (acidic, basic, or enzymatic), are used for the transesterification of vegetable oils.13,23 However, candlenut oil cannot be used in conventional base-transesterification methods without pre-treatment because of the high proportion of free fatty acids (FFAs). It has been reported that the FFA content of candlenut oil is more than 7%.20 To overcome this problem, a two-step reaction (acid catalytic esterification and base catalytic transesterification) was developed to reduce FFA content in the feedstock. In this process, the oil is pre-treated with an acid catalyst to reduce FFA content to less than 1%, followed by a base catalytic transesterification reaction to produce biodiesel. The biodiesel production pathway using the two-step transesterification method is shown in Fig. 2. The main disadvantages of the two-step process include the long reaction time and low recovery of the catalyst.6,24–26 In the literature, few studies have reported the use of a two-step transesterification technique for candlenut biodiesel production. Pham et al.26 produced candlenut biodiesel via a two-step transesterification method. Sulistyo et al.27 carried out a two-step transesterification of high FFA-containing candlenut oil to produce biodiesel.
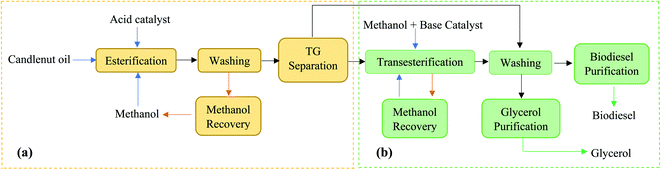 | ||
| Fig. 2 Biodiesel production pathway using two-step transesterification method: (a) esterification, (b) transesterification. | ||
Saka and Kusdiana28 developed the supercritical methanol (scMeOH) transesterification technology to overcome the problems associated with the catalytic transesterification method. This technology can produce biodiesel in a single step without the requirement of any catalyst. Biodiesel production from high FFA-containing feedstock and high biodiesel conversion are the main advantages of the scMeOH transesterification process.28,29 Moreover, the absence of a catalyst allows for simple purification and separation steps to be carried out during biodiesel production.29 Furthermore, as the temperature increases, the solubility of oil and methanol also increased, as the dielectric constant of the polar components decreases.30 The scMeOH transesterification of triglycerides is shown in Fig. 3. Supercritical methanol (scMeOH) transesterification reaction not only decreases the capital cost by leaving out soap separation and production of wastewater but is also able to produce high purity biodiesel from feedstocks with high FFA content.30 The separation of biodiesel is also easier since no catalyst is used, and the reaction can be completed in a shorter time compared to the two-step process.28,31 However, it should be noted that the process requires the elevation of pressure to a supercritical stage. The selected pressure should also be high enough to ensure solubility between the oil and methanol to enable high conversion efficiency in a short time frame.31
Although studies have been conducted on biodiesel production from candlenut oil, a knowledge gap exists in the optimization of candlenut biodiesel production method parameters that give the maximum biodiesel yield using RSM. The optimal biodiesel production parameters have never been investigated. In large-scale biodiesel production methods, it is very important to maximize the biodiesel yield at the lowest production cost. Optimizing each variable of the operational process would contribute to the improvement of the process and make it more efficient.14,19 This study aimed to produce biodiesel via a scMeOH transesterification process using candlenut oil. Additionally, the effects of temperature, pressure, time and methanol to oil (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O) ratio on scMeOH were determined. The response surface methodology was used for the optimization of the scMeOH technology for biodiesel production. A kinetic and thermodynamic model was employed to analyze the scMeOH transesterification process. Finally, the physicochemical properties and fatty acid composition of the oil extracted from candlenut seeds and the resultant biodiesel were determined and compared with the ASTM and European standards. The findings of the present study will be applicable in producing biodiesel from non-edible oil containing high FFAs and moisture.
O) ratio on scMeOH were determined. The response surface methodology was used for the optimization of the scMeOH technology for biodiesel production. A kinetic and thermodynamic model was employed to analyze the scMeOH transesterification process. Finally, the physicochemical properties and fatty acid composition of the oil extracted from candlenut seeds and the resultant biodiesel were determined and compared with the ASTM and European standards. The findings of the present study will be applicable in producing biodiesel from non-edible oil containing high FFAs and moisture.
2. Material and methods
2.1 Sample collection and preparation
The candlenut seeds used in this study were collected from a local market in Penang Island, Malaysia. The seeds were dried at 104 °C for 4 h to remove the water content and then ground to the desired particle size for oil extraction. Candlenut oil was extracted using supercritical carbon dioxide technology. A 3 L supercritical reactor was loaded with 500 g of ground candlenut seeds, and the CO2 gas was delivered to the reactor using a high-pressure pump. The extraction was carried out at a pressure of 30 MPa and temperature of 60 °C for a duration of 90 min. Oil yield was measured using eqn (1) to calculate the oil content of candlenut seeds. Then, the physicochemical properties and fatty acid composition of the extracted candlenut oil were determined.
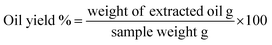 | (1) |
2.2 Transesterification of candlenut oil using scMeOH
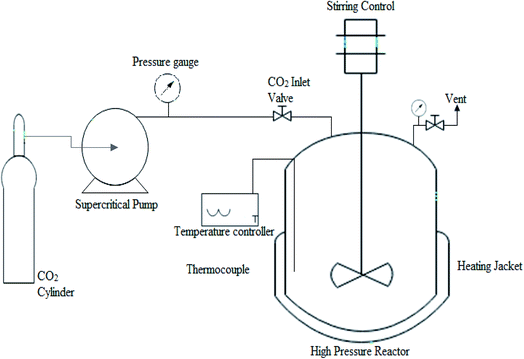
The experimental setup for transesterification of candlenut oil under scMeOH conditions is shown in Fig. 4. Crude candlenut oil was used for non-catalytic transesterification in a 300 ml modified autoclave batch reactor made of stainless steel (model 4520, PARR instrument, USA). A thermocouple was connected to a temperature controller to control the temperature in the reactor at a variance of error of less than 4 °C. Two pressure gauges, used to monitor the pressure, were placed next to the pump and inside the reactor. In a conical flask, candlenut oil and methanol were mixed at 60 °C for 10 min, before being placed in the vessel, and continuously stirred at 300 rpm. A high stirring speed above 500 rpm negligibly affected the ester content, as reported by Ryu et al.32 The pressure inside the vessel was maintained by purging with the N2 gas. After the target temperature was reached, CO2 was pumped into the reactor using a supercritical pump to increase the pressure inside the vessel to the target pressure. After achieving the desired conditions, the reaction was maintained for a specific period that had been set in advance. When the reaction was complete, the reactor was rapidly transferred to an ice bath to quench the reaction. The reactor was then opened, and the liquid content was transferred to a round flask for vacuum distillation. Later, the oil was weighed to determine biodiesel yield, which was calculated using the following equation:
 | (2) |
The CCD matrix was used for determining the conditions for the reaction experiment based on laboratory-located preliminary experiments. The process parameters that were chosen in this study were the reaction time (min), reaction temperature (oC), reaction pressure (bar), and M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio. The variables and their levels were carefully selected following an examination of the parameters. Studies have indicated that increasing the temperature to more than 300 °C affects fatty acid composition. Thus, the maximum and minimum temperature ranges were chosen as 260 and 300 °C, respectively. Being a rapid method, the scMeOH reaction is normally completed within 15–25 min (observed in previous experiments). Thus, a minimum of 10 min is considered the shortest time required to complete the transesterification reaction under scMeOH conditions. Given this, the longer duration of the reaction (35 min) indicates that the efficiency of conversion is low. It has been suggested that the most likely cause for a low efficiency of conversion is the decomposition of esters over a longer reaction period. Therefore, the experiment reaction times were maintained between 10 and 30 min. Furthermore, the larger increase in temperature when scMeOH transesterification occurred could be impacted by the high M
O ratio. The variables and their levels were carefully selected following an examination of the parameters. Studies have indicated that increasing the temperature to more than 300 °C affects fatty acid composition. Thus, the maximum and minimum temperature ranges were chosen as 260 and 300 °C, respectively. Being a rapid method, the scMeOH reaction is normally completed within 15–25 min (observed in previous experiments). Thus, a minimum of 10 min is considered the shortest time required to complete the transesterification reaction under scMeOH conditions. Given this, the longer duration of the reaction (35 min) indicates that the efficiency of conversion is low. It has been suggested that the most likely cause for a low efficiency of conversion is the decomposition of esters over a longer reaction period. Therefore, the experiment reaction times were maintained between 10 and 30 min. Furthermore, the larger increase in temperature when scMeOH transesterification occurred could be impacted by the high M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio. Hence, in practical terms, it is not cost-effective to use a substantial volume of methanol. Accordingly, it is preferable to reduce the amount of methanol as much as possible to minimize downstream separation and purification expenditure. Therefore, the M
O ratio. Hence, in practical terms, it is not cost-effective to use a substantial volume of methanol. Accordingly, it is preferable to reduce the amount of methanol as much as possible to minimize downstream separation and purification expenditure. Therefore, the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O proportion was screened from the lowest ratio of 15
O proportion was screened from the lowest ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to the highest ratio of 35
1 to the highest ratio of 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The pressure directly affects the scMeOH transesterification reaction. To ensure that the working conditions in the reactor were above the critical pressure of methanol (above 80 bar), and since temperature is directly related to pressure, the reaction pressure was optimized. In summary, the reaction parameters selected were as follows: reaction time of 10, 15, 20,25, and 30 min; reaction temperatures of 260, 270, 280, 290, and 300 °C; M
1. The pressure directly affects the scMeOH transesterification reaction. To ensure that the working conditions in the reactor were above the critical pressure of methanol (above 80 bar), and since temperature is directly related to pressure, the reaction pressure was optimized. In summary, the reaction parameters selected were as follows: reaction time of 10, 15, 20,25, and 30 min; reaction temperatures of 260, 270, 280, 290, and 300 °C; M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratios of 15
O ratios of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20
1, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 25
1, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 30
1, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 35
1, and 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; and reaction pressures of 85, 100, 115, 130, and 145 bar.
1; and reaction pressures of 85, 100, 115, 130, and 145 bar.
2.3 Experimental design
The experimental design (DOE) used RSM to optimize the reaction parameters for a greater yield of biodiesel. By utilizing RSM according to four factors and five levels of CCD, an investigation was conducted on the impact of four independent variables and their interactivity on the outcomes of the reaction (yield of biodiesel). RSM using the CCD method is the most popular among all optimization tools for reaction conditions. Full or fractional designs with center points are included; these integrate with a set of axial points, thereby allowing curvatures in the modeled results to be more successfully predicted. This study involved a range of independent variables examined through five levels. Coding for these was as follows: −α, −1, 0, +1, and +α, as shown in Table 1. The CCD design was observed, resulting in a 4-factor and 5-level design being introduced. A total of 30 experiments were performed, as shown in Table 2. Calculations for the total number of experiments were performed according to eqn (3).| T = 2n + 2n + m | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O molar ratio (A), temperature (B, oC), pressure (C, bar), and time (D, min).
O molar ratio (A), temperature (B, oC), pressure (C, bar), and time (D, min).
| Symbol | factor | Unit | Levels | ||||
|---|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |||
| A | Methanol to oil ratio | M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O (%) O (%) |
15 | 20 | 25 | 30 | 35 |
| B | Reaction temperature | oC | 260 | 270 | 280 | 290 | 300 |
| C | Reaction pressure | Bar | 85 | 100 | 115 | 130 | 145 |
| D | Reaction time | Min | 10 | 15 | 20 | 25 | 30 |
| Run No. | Coded process variables | Biodiesel yield% | ||||
|---|---|---|---|---|---|---|
| A | B | C | D | Actual | Predicted | |
| 1 | 0 | 0 | 0 | 0 | 89.60 | 89.58 |
| 2 | 0 | 0 | −α | 0 | 82.55 | 82.32 |
| 3 | 0 | α | 0 | 0 | 93.55 | 93.83 |
| 4 | 1 | 1 | −1 | 1 | 93.90 | 94.18 |
| 5 | 0 | 0 | 0 | 0 | 89.20 | 89.58 |
| 6 | 1 | 1 | 1 | −1 | 86.60 | 87.028 |
| 7 | −1 | −1 | 1 | −1 | 77.20 | 76.93 |
| 8 | 1 | −1 | −1 | 1 | 83.90 | 84.37 |
| 9 | 0 | 0 | 0 | 0 | 89.70 | 89.58 |
| 10 | 0 | 0 | α | 0 | 90.50 | 91.01 |
| 11 | −α | 0 | 0 | 0 | 73.60 | 74.22 |
| 12 | α | 0 | 0 | 0 | 81.15 | 80.82 |
| 13 | 1 | 1 | −1 | −1 | 81.90 | 81.44 |
| 14 | −1 | 1 | 1 | 1 | 93.90 | 93.67 |
| 15 | 0 | 0 | 0 | α | 96.10 | 95.99 |
| 16 | 0 | 0 | 0 | 0 | 89.30 | 89.58 |
| 17 | −1 | 1 | −1 | −1 | 78.45 | 78.54 |
| 18 | 1 | −1 | 1 | −1 | 80.15 | 79.83 |
| 19 | 0 | 0 | 0 | 0 | 89.75 | 89.58 |
| 20 | 1 | 1 | 1 | 1 | 96.90 | 96.68 |
| 21 | −1 | 1 | 1 | −1 | 85.60 | 84.81 |
| 22 | 1 | −1 | 1 | 1 | 86.90 | 86.79 |
| 23 | −1 | −1 | −1 | −1 | 70.850 | 70.75 |
| 24 | −1 | −1 | −1 | 1 | 80.40 | 79.98 |
| 25 | −1 | 1 | −1 | 1 | 90.50 | 90.47 |
| 26 | −1 | −1 | 1 | 1 | 82.95 | 83.09 |
| 27 | 0 | −α | 0 | 0 | 76.15 | 76.15 |
| 28 | 0 | 0 | 0 | 0 | 89.94 | 89.58 |
| 29 | 1 | −1 | −1 | −1 | 74.10 | 74.34 |
| 30 | 0 | 0 | 0 | −α | 76.75 | 77.11 |
2.4 Statistical analysis
A quadratic polynomial regression model was developed to describe the relationships between the reaction parameters and repetitions and to enable predictions of biodiesel yields as functions of the independent variables. The general quadratic polynomial eqn (4) is given as follows:
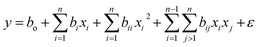 | (4) |
2.5 Kinetic and thermodynamic model of biodiesel production
Although the second-order rate constant is commonly used for adsorption studies, the second-order rate equation has also been employed to describe the kinetic behavior of liquid–liquid conversion.25 The present study used a second-order rate model to describe the kinetic behavior of biodiesel production from candlenut oil using scMeOH transesterification. The production of biodiesel from candlenut oil can be expressed as follows:
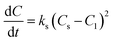 | (5) |
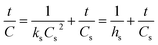 | (6) |
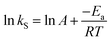 | (7) |
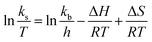 | (8) |
| ΔG = ΔH − TΔS | (9) |
2.6 Physicochemical properties of candlenut lipids and biodiesel
The viscosities of the extracted candlenut oil and biodiesel were determined using a viscometer. The densities of oil and biodiesel were determined following the procedure described by the ASTM D1298 for density measurements. The saponification values of candlenut oil and biodiesel were determined following the AOAC920.160 standard test method. Briefly, approximately 1 g of the sample was mixed with 25 ml of 0.5 N alcoholic KOH, followed by titration with 0.5 HCl. The acid values of both candlenut oil and biodiesel were determined using the AOAC 940.28 standard method. A phenolphthalein indicator (1 ml) was used to quantify the oil and biodiesel titrated using a 0.1 N KOH solution. The acid values of candlenut oil and biodiesel were estimated using the following equation:
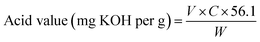 | (10) |
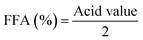 | (11) |
The iodine numbers of candlenut oil and Biodiesel were determined using the AOAC 920.159 test method. The peroxide values of the samples were determined following the standard AOAC 965.33 method. The cloud point (CP) and pour point (PP) were determined using the jar test method according to the steps described in ASTM D2500 and ASTM D7683, respectively. The moisture content of lipids and biodiesel was determined following the AOAC 930.15 test method. The calorific values of both lipids and biodiesel were measured using the bomb calorimeter method following the ASTM D5865 standard using an Isoperibol Calorimeter (6200, Parr Instruments, USA). Approximately 0.05 g of liquid samples was placed in a crucible, and the bomb was filled with oxygen gas (O2) up to 25 atm. The test was started after placing the crucible in the calorimeter. The calorific value was recorded after burning was completed. The cetane number was determined following the ASTM D613 test method.
2.7 GC-FID and FTIR analyses
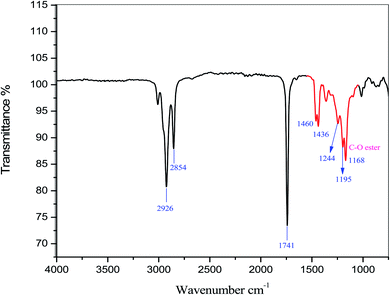
The fatty acid compositions of lipids and biodiesel were determined via gas chromatography (GC), and the fatty acids were analyzed by converting them to their respective fatty acid methyl esters (FAMEs). Approximately, 0.1 μg of biodiesel was injected into the fused silica capillary column (30 m × 0.25 mm, id, 0.25 μm). The temperature of the detector and injector was 250 °C, and pure helium was used as the carrier gas. Initially, the temperature of the oven was kept at 100 °C for 10 min, before being raised at 10 °C per min until a final temperature of 240 °C was achieved, which was held for 15 min. A standard FAME mixture (Supelco 37-component FAME Mix, Sigma Chemicals, USA) was used to estimate fatty acids. The percentage of fatty acids in the lipids and biodiesel was determined using an internal normalization technique.The characteristics of the bBiodiesel from candlenut were determined using a Fourier transform infrared (FTIR) spectrometer (PerkinElmer System 2000 FTIR), which comprised a detection device with a spectral range of 4000–600 cm−1 with a resolution of 4 cm−1. FTIR spectra were analyzed using the Nicolet OMNIC 5.01 software provided with the apparatus. The fatty acid esters present in the biodiesel from candlenut were identified from FTIR spectrum absorption bands.
3. Results and discussion
3.1 scMeOH transesterification of candlenut oil
The effect of scMeOH on the production of biodiesel from candlenut oil was determined at various temperatures (250–310 °C), pressures (85–150 bar), and M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratios (10
O ratios (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–40
1–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
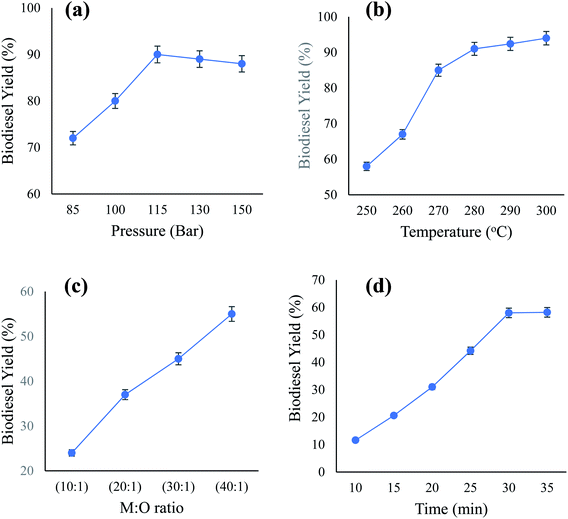
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The reaction time (10–35 min) is shown in Fig. 5. It was found that the scMeOH pressure, temperature, ratio, and conversion time substantially influenced biodiesel production from candlenut oil. The percentage of biodiesel increased with an increase in pressure from 85 to 115 bar (Fig. 5(a)). However, the amount of biodiesel produced was negligible with an excessive increase in pressure to 150 bar. The highest biodiesel yield obtained was 90% at a pressure of 115 bar, temperature of 250 °C, M
1). The reaction time (10–35 min) is shown in Fig. 5. It was found that the scMeOH pressure, temperature, ratio, and conversion time substantially influenced biodiesel production from candlenut oil. The percentage of biodiesel increased with an increase in pressure from 85 to 115 bar (Fig. 5(a)). However, the amount of biodiesel produced was negligible with an excessive increase in pressure to 150 bar. The highest biodiesel yield obtained was 90% at a pressure of 115 bar, temperature of 250 °C, M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 30
O ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and conversion time of 30 min. The reaction speed increased with increasing pressure, as higher levels of methanol solubility and molecular activity were derived from reactions wherein the pressure was increased. This explains the increase in the rate of the reaction and the relative likelihood of particle collision. When the reaction pressure increases, it allows greater splitting of the triglyceride and methyl combination bonds and becomes FAME.
1, and conversion time of 30 min. The reaction speed increased with increasing pressure, as higher levels of methanol solubility and molecular activity were derived from reactions wherein the pressure was increased. This explains the increase in the rate of the reaction and the relative likelihood of particle collision. When the reaction pressure increases, it allows greater splitting of the triglyceride and methyl combination bonds and becomes FAME.
The biodiesel production from candlenut oil using scMeOH transesterification increased with an increase in temperature from 250 °C to 280 °C at a pressure of 85 bar, M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 30
O ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and reaction time of 30 min (Fig. 5(b)). The amount of biodiesel increased gradually with a further increase in temperature from 280 °C to 310 °C. Approximately 55% of biodiesel was produced at a temperature of 250 °C; the percentage of biodiesel production increased to 91.4% at a temperature of 290 °C and reached a maximum of 92.6% at 300 °C. Similarly, Rahimi et al.34 also found that an increase in temperature increases biodiesel yield, and the highest yield is obtained at 290 °C. Their study demonstrated that at a higher temperature, biodiesel yield starts to decrease, as the extremely high temperature leads to the degradation of methyl esters. Furthermore, Ghoreishi and Moein35 reported that an excessive increase in temperature above 300 °C causes the decomposition of FAMEs and results in a reduction in biodiesel yield. Fig. 5(c) shows that the amount of biodiesel increased with an increase in the M
1, and reaction time of 30 min (Fig. 5(b)). The amount of biodiesel increased gradually with a further increase in temperature from 280 °C to 310 °C. Approximately 55% of biodiesel was produced at a temperature of 250 °C; the percentage of biodiesel production increased to 91.4% at a temperature of 290 °C and reached a maximum of 92.6% at 300 °C. Similarly, Rahimi et al.34 also found that an increase in temperature increases biodiesel yield, and the highest yield is obtained at 290 °C. Their study demonstrated that at a higher temperature, biodiesel yield starts to decrease, as the extremely high temperature leads to the degradation of methyl esters. Furthermore, Ghoreishi and Moein35 reported that an excessive increase in temperature above 300 °C causes the decomposition of FAMEs and results in a reduction in biodiesel yield. Fig. 5(c) shows that the amount of biodiesel increased with an increase in the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio. In the transesterification process, methanol is responsible for moving the equilibrium toward the product (biodiesel), and the subsequent maximum possible conversion occurs using extra methanol. The higher M
O ratio. In the transesterification process, methanol is responsible for moving the equilibrium toward the product (biodiesel), and the subsequent maximum possible conversion occurs using extra methanol. The higher M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O molar ratios resulted in a transesterification process with greater efficiency because the contact of methanol with triglycerides increased. An increase in the M
O molar ratios resulted in a transesterification process with greater efficiency because the contact of methanol with triglycerides increased. An increase in the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio from 10
O ratio from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 20
1 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 increased biodiesel yield, and the maximum biodiesel yield was 55% at a M
1 increased biodiesel yield, and the maximum biodiesel yield was 55% at a M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 40
O ratio of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, pressure of 85 bar, temperature of 250 °C, and reaction time of 30 min. However, biodiesel yield decreased at a higher M
1, pressure of 85 bar, temperature of 250 °C, and reaction time of 30 min. However, biodiesel yield decreased at a higher M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio (40
O ratio (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Aboelazayem et al.6 reported similar results for the transesterification of cooking oil at an M
1). Aboelazayem et al.6 reported similar results for the transesterification of cooking oil at an M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 37
O ratio of 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; Ghoreishi and Moein35 reported that 33
1; Ghoreishi and Moein35 reported that 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is the optimum M
1 is the optimum M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio for producing biodiesel from waste vegetable oil. Furthermore, the findings of this study also agree with the results reported by Santana et al.,36 who demonstrated that a high M
O ratio for producing biodiesel from waste vegetable oil. Furthermore, the findings of this study also agree with the results reported by Santana et al.,36 who demonstrated that a high M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio leads to a reduction in biodiesel production. At higher levels, excess methanol interferes with the separation of glycerin due to increased solubility. Fig. 5(d) shows that the amount of biodiesel increased with an increase in reaction time from 10 to 20 min at a pressure of 85 bar, M
O ratio leads to a reduction in biodiesel production. At higher levels, excess methanol interferes with the separation of glycerin due to increased solubility. Fig. 5(d) shows that the amount of biodiesel increased with an increase in reaction time from 10 to 20 min at a pressure of 85 bar, M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 30
O ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and temperature of 250 °C. The highest, about 91.4% of lipids, was separated at a separation time of 30 min, pressure 85 MPa, and temperature of 290 °C.
1, and temperature of 250 °C. The highest, about 91.4% of lipids, was separated at a separation time of 30 min, pressure 85 MPa, and temperature of 290 °C.
3.2 Development of a regression model
The results obtained from all the variables (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O, T oC, Pbar, and Tmin) employed in this study are summarized in Table 3. The software fitted the linear, quadratic, and cubic outcomes from the factors of the experiment onto a model, and the effects of the interactions among them were also fitted. The yield was predicted using the model as a function of the given factors. The statistical model was chosen according to various statistical assessments, including the adjusted coefficient of determination (Radj2), predicted coefficient of determination (Rpre2), lack of fit, and associated aliased coefficients. Eqn (12) represent the developed quadratic models with empirical relationships between responses and reaction variables within specific levels in terms of experimental factors.
O, T oC, Pbar, and Tmin) employed in this study are summarized in Table 3. The software fitted the linear, quadratic, and cubic outcomes from the factors of the experiment onto a model, and the effects of the interactions among them were also fitted. The yield was predicted using the model as a function of the given factors. The statistical model was chosen according to various statistical assessments, including the adjusted coefficient of determination (Radj2), predicted coefficient of determination (Rpre2), lack of fit, and associated aliased coefficients. Eqn (12) represent the developed quadratic models with empirical relationships between responses and reaction variables within specific levels in terms of experimental factors.| yBD = 89.58 + 1.65A + 4.42B + 2.17C + 4.72D − 0.17AB − 0.17AC + 0.20AD + 0.023BC + 0.68BD − 0.77CD − 3.02A2 − 1.15B2 − 0.73C2 − 0.76D2 | (12) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio, heat level, pressure, and duration. The impact of each reaction variable on the response is illustrated by regression equations. A synergetic impact is indicated by the positive sign of each term, whereas a negative sign indicates an antagonistic effect.6,32 The impact of the reaction variable on the response is represented by a linear coefficient. Meanwhile, the interactive effect on the response of the process variables is represented by the coefficient of variable interaction. Finally, the impact on the response of variable excess is represented by a quadratic coefficient.
O ratio, heat level, pressure, and duration. The impact of each reaction variable on the response is illustrated by regression equations. A synergetic impact is indicated by the positive sign of each term, whereas a negative sign indicates an antagonistic effect.6,32 The impact of the reaction variable on the response is represented by a linear coefficient. Meanwhile, the interactive effect on the response of the process variables is represented by the coefficient of variable interaction. Finally, the impact on the response of variable excess is represented by a quadratic coefficient.
| Source | Sum of square | DF | Mean square | F value | P value |
|---|---|---|---|---|---|
| Biodiesel | Biodiesel | Biodiesel | Biodiesel | Biodiesel | |
| Model | 1467.13 | 14 | 104.79 | 477.58 | <0.0001 |
| A | 65.34 | 1 | 65.34 | 297.77 | <0.0001 |
| B | 469.05 | 1 | 469.05 | 2137.60 | <0.0001 |
| C | 113.10 | 1 | 113.10 | 515.43 | <0.0001 |
| D | 535.05 | 1 | 535.05 | 2438.42 | <0.0001 |
| AB | 0.46 | 1 | 0.46 | 2.13 | 0.1643 |
| AC | 0.47 | 1 | 0.47 | 2.16 | 0.1614 |
| AD | 0.64 | 1 | 0.64 | 2.91 | 0.1083 |
| BC | 0.00 | 1 | 0.00 | 0.03 | 0.8502 |
| BD | 7.29 | 1 | 7.29 | 33.22 | <0.0001 |
| CD | 9.45 | 1 | 9.45 | 43.09 | <0.0001 |
| A2 | 249.36 | 1 | 249.36 | 1136.43 | <0.0001 |
| B2 | 36.05 | 1 | 36.05 | 164.29 | <0.0001 |
| C2 | 14.52 | 1 | 14.52 | 66.19 | <0.0001 |
| D2 | 15.69 | 1 | 15.69 | 71.52 | <0.0001 |
| Residual | 3.29 | 15 | 0.21 | ||
| Lack of fit | 2.89 | 10 | 0.28 | 3.65 | 0.0827 |
| Pure error | 0.39 | 5 | 0.07 | ||
| Cor total | 1470.42 | 29 |
As eqn (6) shows, all linear coefficients have positive signs, indicating that biodiesel yield increases when any one process variable (O ratio, T oC, Pbar, and Tmin) increases. Eqn (6) and (7) evidently show that varying factor A, the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio, produced the most substantial impact on the yields of biodiesel and glycerol, since, amongst the variables, its coefficient is the largest. Furthermore, the adequacy of the model in the prediction has been assessed; hence, all errors linked to normality assumptions can be reported. Biodiesel yield ranged from 70.85–96.9%, depending on the conditions of each experiment. As all the linear terms and quadratic terms, except the interaction terms AB, AC, AD, and BC, were found to be significant model terms in maximizing the biodiesel yield, the adequacy of the models in the predictions was checked using several analyses. The predicted model accuracy was evaluated using the coefficient of correlation (R2). The closer the R2 approaches unity, the better the indication of similarity between the actual and predicted values. The R2, Rpre2, and Radj2 values for the biodiesel predicted model were 0.9978, 0.9883, and 0.9985, respectively. These results indicate that almost 99.7% of the sum of the variation is qualified by the variables in the experiment for biodiesel yield.
O ratio, produced the most substantial impact on the yields of biodiesel and glycerol, since, amongst the variables, its coefficient is the largest. Furthermore, the adequacy of the model in the prediction has been assessed; hence, all errors linked to normality assumptions can be reported. Biodiesel yield ranged from 70.85–96.9%, depending on the conditions of each experiment. As all the linear terms and quadratic terms, except the interaction terms AB, AC, AD, and BC, were found to be significant model terms in maximizing the biodiesel yield, the adequacy of the models in the predictions was checked using several analyses. The predicted model accuracy was evaluated using the coefficient of correlation (R2). The closer the R2 approaches unity, the better the indication of similarity between the actual and predicted values. The R2, Rpre2, and Radj2 values for the biodiesel predicted model were 0.9978, 0.9883, and 0.9985, respectively. These results indicate that almost 99.7% of the sum of the variation is qualified by the variables in the experiment for biodiesel yield.
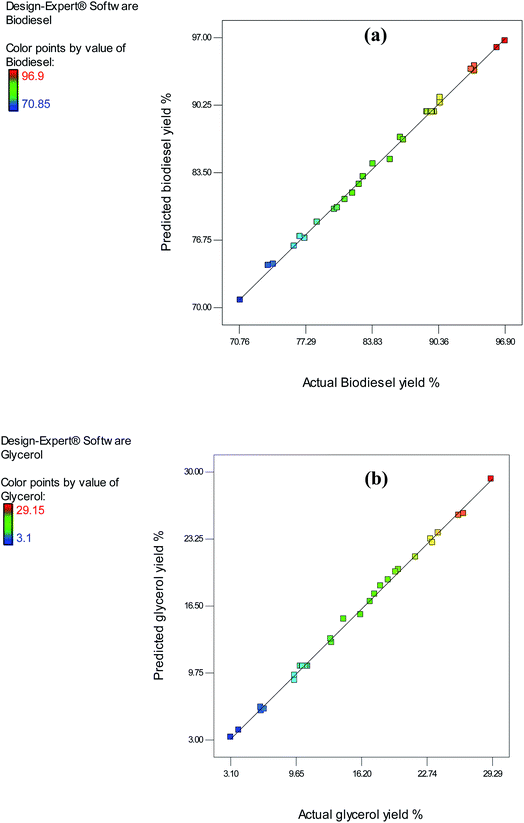
The significance of the predicted models was determined using statistical data obtained from variance analysis. Furthermore, the interactions between the reaction parameters and the significance of their impacts were examined. ANOVA produced the parameter values for the yield of biodiesel as presented in Table 3. The model of biodiesel was assessed for their significance according to p-values and F-tests at a confidence level of 95%. The lesser the p-value than 0.05, the greater is the significance of the correlated parameter. Our observations showed the high significance of both models for biodiesel, where the p-value was <0.0001. Such measurements ensure that the model is significant in representing the outcomes of real experiments. Additionally, one type of ANOVA analysis, lack of fit, measures the extent to which the model failed in its representation of the data points in the experiment. A non-significant value for lack of fit indicates a model with a high fit. The biodiesel lack fit value was 0.0827. The test's non-significance illustrates the successful representation of the data from the experiment in the models. Furthermore, the similar nature of the predicted and actual data ensures that the model accurately predicts the response variables. Fig. 6(a) and (b) illustrate the actual and predicted data from the experiment, using a model created to depict the yield of biodiesel.
The significance of each parameter was determined using ANOVA. As shown in Table 3, all the examined factors had a significant linear impact on the yield of biodiesel. The p-values for the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
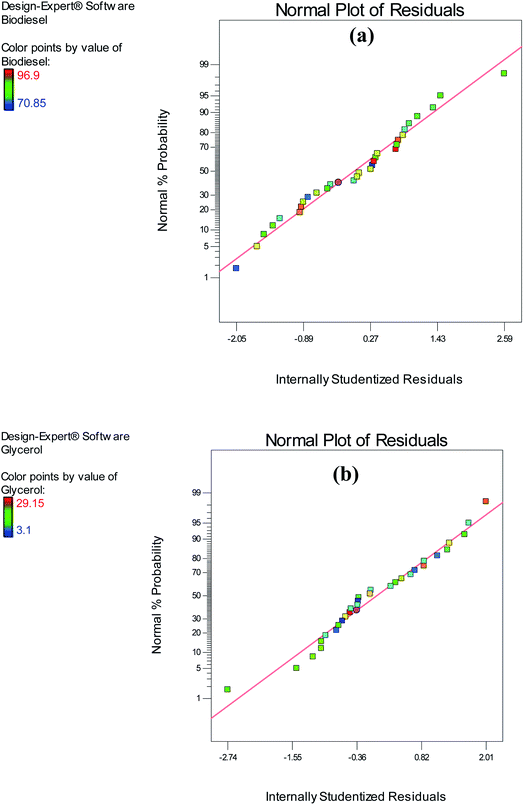
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio (A), temperature (B), pressure (C), and reaction time (D) were less than 0.05, for biodiesel. Furthermore, in each quadratic model, the corresponding coefficients were positive for all the factors associated with biodiesel yield. As shown in the analyses, the interaction between the variables temperature – duration (BD) and pressure – duration (CD) had a significant impact on the yield of biodiesel. Conversely, the interactivity of the variables with other factors had a non-significant impact on the yield of biodiesel. Furthermore, verifying the assumptions of ANOVA was vital because it was used in the predicted model validation.6 An investigation of the normality of residuals was conducted utilizing a normal plot, and relatively straight lines were formed, as shown in Fig. 7(a) and (b). The validity of the first assumption was ensured by this test, as a normal distribution of residuals was obtained for the model of biodiesel.
O ratio (A), temperature (B), pressure (C), and reaction time (D) were less than 0.05, for biodiesel. Furthermore, in each quadratic model, the corresponding coefficients were positive for all the factors associated with biodiesel yield. As shown in the analyses, the interaction between the variables temperature – duration (BD) and pressure – duration (CD) had a significant impact on the yield of biodiesel. Conversely, the interactivity of the variables with other factors had a non-significant impact on the yield of biodiesel. Furthermore, verifying the assumptions of ANOVA was vital because it was used in the predicted model validation.6 An investigation of the normality of residuals was conducted utilizing a normal plot, and relatively straight lines were formed, as shown in Fig. 7(a) and (b). The validity of the first assumption was ensured by this test, as a normal distribution of residuals was obtained for the model of biodiesel.
3.3 Effects of process variables and their interactions
The impact of individual parameters and their interactions was studied to analyze and investigate the influence of parameter variations on the responses.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio results in a reduction in biodiesel yield. This negative impact is shown in Fig. 8(a). A previous study by Aboelazayem et al.6 on high fatty acid waste cooking oil, where he reported a negative influence of the M
O ratio results in a reduction in biodiesel yield. This negative impact is shown in Fig. 8(a). A previous study by Aboelazayem et al.6 on high fatty acid waste cooking oil, where he reported a negative influence of the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio on biodiesel yield, agrees with the present findings. According to Nan et al.,37 increasing the M
O ratio on biodiesel yield, agrees with the present findings. According to Nan et al.,37 increasing the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio during biofuel synthesis using non-catalytic scMeOH has a significant impact on biodiesel yield. The authors provided an explanation for their results, suggesting that the homogenous reaction phase required only reduced molar ratios. Accordingly, an increased M
O ratio during biofuel synthesis using non-catalytic scMeOH has a significant impact on biodiesel yield. The authors provided an explanation for their results, suggesting that the homogenous reaction phase required only reduced molar ratios. Accordingly, an increased M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio had no significant impact on solution homogeneity in the present study. Conversely, the M
O ratio had no significant impact on solution homogeneity in the present study. Conversely, the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio positively affected the yield of glycerol. This outcome was anticipated, since previous reports suggested that the M
O ratio positively affected the yield of glycerol. This outcome was anticipated, since previous reports suggested that the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio improves the transesterification reaction used to produce glycerol.36
O ratio improves the transesterification reaction used to produce glycerol.36
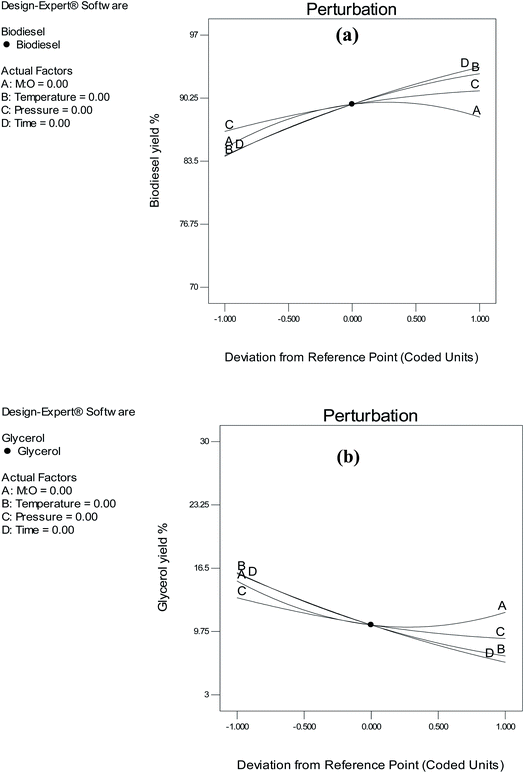 | ||
| Fig. 8 Perturbation plot showing the effect of individual variables on (a) biodiesel yield and (b) glycerol yield. | ||
The temperature of the reaction is the primary parameter when using scMeOH for biodiesel production. A high reaction temperature leads to the thermal degradation of biodiesel. According to Imahara et al.,38 the yield of biodiesel is negatively affected by reaction temperatures above 300 °C. Furthermore, the yield of biodiesel is negatively affected by low reaction temperatures. As the critical methanol temperature is 239 °C, the range of temperature selected in this study was 260–300 °C. Our findings showed that the yield of biodiesel was positively affected by the reaction temperature. As shown in Fig. 8(a), the yield of biodiesel increased with an increase in reaction temperature, which is in agreement with the results of a previous study, which reported that the yield of biodiesel is positively impacted by a rise in temperature.37 Conversely, lower reaction temperatures had a positive effect on the yield of glycerol.
Another important parameter in scMeOH transesterification is the reaction pressure. It has a substantial effect on solution properties, such as the intensity of hydrogen bonds and density.6 It has been reported that the effect of reaction pressure on biodiesel yield is not highly significant. Nevertheless, in this study, the reaction pressure had a significant effect on the biodiesel yield. As the supercritical pressure of methanol is 80 bar, the pressure range selected was 85–145 bar. The results of this study agree with those of Tsai et al.,39 who indicated that the yield of biodiesel varies by approximately 10% when the pressure is increased from 100 to 250 bar. Nonetheless, the yield of glycerol was not significantly affected by the reaction pressure, as illustrated in Fig. 8(b). Increasing the reaction pressure to 130 bar led to a reduction in glycerol yield, yet a drop in reaction pressure slightly affected the yield of glycerol. Furthermore, the shorter duration of the scMeOH transesterification reaction is one of its advantages over the catalyzed methods. In this study, the duration was selected as 15–30 min, as recommended in previous studies.6 Results showed that the reaction time had the greatest impact on the yield of biodiesel. Biodiesel yield increased with an increase in the reaction time from 15 to 25 min. Conversely, the reaction duration showed an inverse relationship with glycerol yield. An increase in the reaction time led to a reduction in glycerol yield.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 40
O ratio of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Their explanation for this is the increase in the rate of thermal degradation of methyl esters.
1. Their explanation for this is the increase in the rate of thermal degradation of methyl esters.
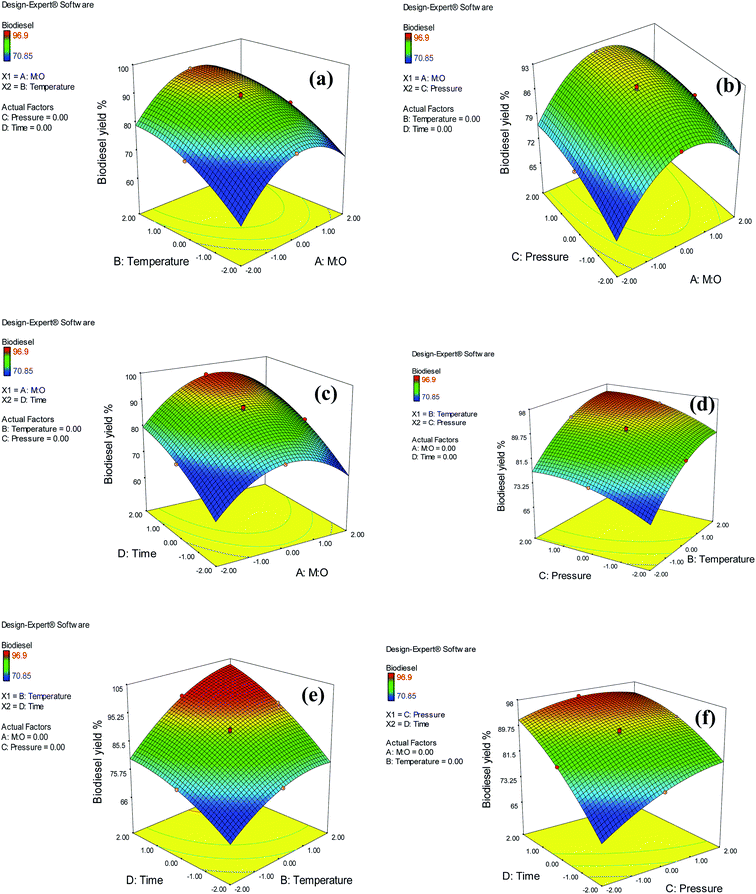
The interactions between the reaction pressure and reaction time had a significant impact on the yield of biodiesel. The pressure in the reactor was increased by pressurizing the CO2 gas, which was used as a co-solvent to raise the reaction pressure to the desired pressure using a supercritical pump. Furthermore, CO2 enhances the solubility of methanol in oil. The 3D response surface plot for the reaction pressure and time for biodiesel yield is shown in Fig. 9(f). As assessed from ANOVA (Table 3), there was a significant interaction effect between reaction pressure and time, which is also confirmed by the graph in Fig. 9(f). The reaction pressure had a negligible effect on biodiesel yield at shorter reaction periods. However, a slightly negative effect of the reaction pressure was observed at longer reaction durations. Ong et al.41 reported that the impact of increasing pressure is not crucial, as it exceeds the critical pressure of methanol. They explained that both transesterification and esterification involve the same number of moles of reactants and products. Hence, a change in pressure does not affect the chemical equilibrium of the reaction, according to Le Chatelier's principle. The negative effect of an increasing pressure might result from FAME degradation, as CO2 reduces the critical point of the system and hence requires a milder temperature.6 The 3D response surface plots of the insignificant effects of the interactions between (a) M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O and temperature, (b) M
O and temperature, (b) M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O and pressure, (c) M
O and pressure, (c) M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O and reaction time, (d) temperature and pressure, and (e) temperature and reaction time are shown in Fig. 9.
O and reaction time, (d) temperature and pressure, and (e) temperature and reaction time are shown in Fig. 9.
3.4 Process optimization and experimental validation
Optimization of the reaction variables affecting biodiesel yield from high fatty acid candlenut oil using RSM has not been studied before. To optimize the reaction responses (the yield of biodiesel), the optimal conditions for achieving the desired objective were evaluated by implementing the numerical features of the Design expert 7.0.0 software. Subsequently, the software-generated 30 solutions for optimal conditions, from which the most desirable solution was selected. The resulting optimum conditions resulted in 96.35% yields for biodiesel at a 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M
1 M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O molar ratio, 285 °C, and 115 bar pressure in 22 min of reaction time. To validate the predicted optimum conditions, three experiments were conducted under these conditions, and the average result was considered as the experimental outcome. The experimental validation resulted in a biodiesel yield of 96.13%, which demonstrated the adequacy of the predicted optimum conditions within a relative error of 0.22% from the experimental results. The candlenut biodiesel yield and the optimal experimental conditions of scMeOH transesterification were almost similar, as reported by Singh et al.31 The study reported that the maximum biodiesel production of 95.67% from jojoba oil using the supercritical methanol transesterification process at the optimum conditions of reaction temperature 287 °C, reaction pressure of 123 bar, methanol to oil ratio of 30
O molar ratio, 285 °C, and 115 bar pressure in 22 min of reaction time. To validate the predicted optimum conditions, three experiments were conducted under these conditions, and the average result was considered as the experimental outcome. The experimental validation resulted in a biodiesel yield of 96.13%, which demonstrated the adequacy of the predicted optimum conditions within a relative error of 0.22% from the experimental results. The candlenut biodiesel yield and the optimal experimental conditions of scMeOH transesterification were almost similar, as reported by Singh et al.31 The study reported that the maximum biodiesel production of 95.67% from jojoba oil using the supercritical methanol transesterification process at the optimum conditions of reaction temperature 287 °C, reaction pressure of 123 bar, methanol to oil ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and reaction time of 23 min.31
1, and reaction time of 23 min.31
3.5 Kinetic and thermodynamic modeling
As shown in Fig. 5(a), the optimal scMeOH pressure was 110 MPa at a temperature of 250 °C, an M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 30
O ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and a reaction time of 25 min to produce biodiesel from candlenut oil. Fig. 10 shows biodiesel production from candlenut oil at 110 bar with varying temperatures (250–300 °C) and reaction times (5–30 min). It was found that biodiesel yield increased with increasing temperature and reaction time and reached equilibrium at 30 min conversion time. Accordingly, kinetic and thermodynamic studies were conducted for biodiesel production from candlenut oil via the scMeOH reaction at 110 bar and varying temperatures (250–300 °C) and reaction time (5–30 min). A second-order kinetic model equation was used to express the overall conversion curves of biodiesel from candlenut oil at 110 bar, as shown in Fig. 11. Based on the second-order rate model shown in eqn (5) and (6), the estimated correlation results are listed in Table 4. It was found that the hs, ks, and Cs values significantly increased with an increase in temperature from 250 °C to 300 °C. This result suggests that rapid biodiesel production from candlenut oil can be achieved using the scMeOH reaction with the parameters reported in the current study.42,43 Furthermore, the high correlation coefficient (R2), greater than 0.90, indicates that the proposed second-order kinetics model adequately describes the scMeOH conversion behavior of biodiesel from candlenut oil at temperatures in the range of 250–300 °C.43
1, and a reaction time of 25 min to produce biodiesel from candlenut oil. Fig. 10 shows biodiesel production from candlenut oil at 110 bar with varying temperatures (250–300 °C) and reaction times (5–30 min). It was found that biodiesel yield increased with increasing temperature and reaction time and reached equilibrium at 30 min conversion time. Accordingly, kinetic and thermodynamic studies were conducted for biodiesel production from candlenut oil via the scMeOH reaction at 110 bar and varying temperatures (250–300 °C) and reaction time (5–30 min). A second-order kinetic model equation was used to express the overall conversion curves of biodiesel from candlenut oil at 110 bar, as shown in Fig. 11. Based on the second-order rate model shown in eqn (5) and (6), the estimated correlation results are listed in Table 4. It was found that the hs, ks, and Cs values significantly increased with an increase in temperature from 250 °C to 300 °C. This result suggests that rapid biodiesel production from candlenut oil can be achieved using the scMeOH reaction with the parameters reported in the current study.42,43 Furthermore, the high correlation coefficient (R2), greater than 0.90, indicates that the proposed second-order kinetics model adequately describes the scMeOH conversion behavior of biodiesel from candlenut oil at temperatures in the range of 250–300 °C.43
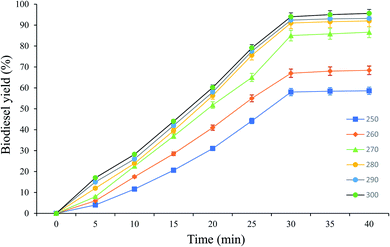 | ||
| Fig. 10 Biodiesel production from candlenut oil using scMeOH transesterification reaction at pressure 110 bar with varying temperature and reaction time. | ||
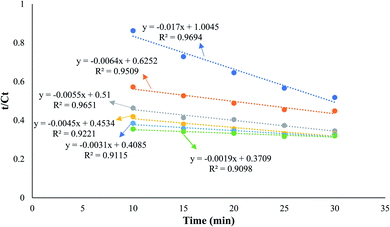 | ||
| Fig. 11 Second-order kinetic model for the biodiesel production from candlenut oil using scMeOH transesterification reaction with varying temperatures, ranging from 250 to 300 °C. | ||
| Temperature (°C) | Second-order kinetic model | R2 | Thermodynamic quantities of activation | ||||
|---|---|---|---|---|---|---|---|
| hs (mg g−1 min−1) | ks (mg mg−1 min−1) | Cs (mg g−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | ΔG (kJ mol−1) | ||
| 250 | 0.995 | 3.44 | 58.82 | 0.9694 | 231.22 | 26.57 | 164.79 |
| 260 | 1.59 | 39.05 | 156.25 | 0.9509 | 162.13 | ||
| 270 | 2.105 | 69.61 | 181.81 | 0.9651 | 159.48 | ||
| 280 | 2.205 | 108.91 | 222.22 | 0.9221 | 156.82 | ||
| 290 | 2.44 | 254.73 | 322.58 | 0.9115 | 154.16 | ||
| 300 | 2.69 | 746.85 | 526.31 | 0.9098 | 151.51 | ||
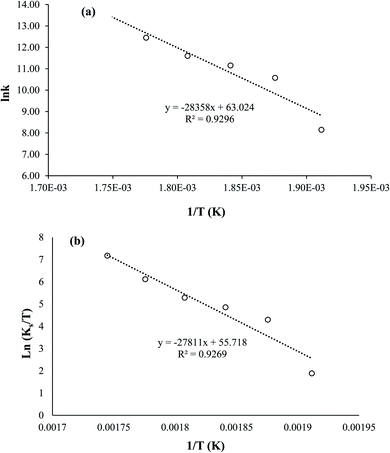
Fig. 5 shows the relationship between the absolute temperature and second-order rate constant for estimating the activation energy (Fig. 12(a)) and thermodynamic properties (Fig. 12(b)) for the production of biodiesel from candlenut oil. The estimated activation energy for biodiesel production from candlenut oil using the scMeOH reaction was 28.35 kJ mol−1. The positive activation energy reveals that the scMeOH transesterification process is a temperature-dependent process, indicating the production of biodiesel increased with increasing temperature.38 The kinetics behavior of the scMeOH transesterification process varies with the supercritical CO2 (ref. 42) extraction process. For instance, Mohammad Ilias et al. [2022] observed that the lipids extraction from chicken by-product waste using scCO2 was not temperature-dependent. However, the estimated activation energy value for the scMeOH transesterification process was lower than other non-catalytic transesterification processes like the microwave-assisted transesterification process.43 The estimated ΔH and ΔS values were 231.22 kJ mol−1 and 26.57 kJ mol−1 K−1, respectively. The positive ΔH value indicated that biodiesel production from candlenut oil was endothermic. The ΔG of activation decreased with an increase in temperature. The estimated positive ΔG values revealed that biodiesel production from candlenut oil using the scMeOH reaction was unspontaneous. Moreover, the high correlation coefficient (0.9269) for estimating the thermodynamic properties of activation reveals that the transition state theory reliably describes the biodiesel production from candlenut oi using scMeOH reaction between 250 °C and 300 °C.
3.6 Physicochemical properties of candlenut biodiesel
In the present study, oil was extracted from candlenut seeds using the scCO2 extraction method. Approximately 63% of the oil was separated from candlenut seeds at a pressure of 35 MPa, a temperature of 60 °C, and an extraction time of 90 min. The extracted oil was used as a feedstock for biodiesel production using the scMeOH transesterification reaction. The physicochemical properties of the separated candlenut oil and the biodiesel produced from candlenut oil, using optimized experimental conditions of scMeOH transesterification (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M
1 M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O, 285 °C, 125 bar, and 22 min), were analyzed and compared with a biodiesel standard, and other biodiesel feedstocks as shown in Table 5. Viscosity is an important parameter of biodiesel because it affects the fuel injection system, causes poor fuel combustion, and increases exhaust emissions. Here, the viscosity decreased with an increase in temperature. The viscosities of the extracted candlenut oil and biodiesel were found to be approximately 25.80 and 4.8 mm2 s−1, respectively. The viscosity of candlenut oil was found to be lower than that of wild radish oil (36.32 mm2 s−1) and higher than that of Jatropha oil, (19.77 mm2 s−1).20,43,44 However, the viscosity of candlenut biodiesel was found to be lower than the biodiesel produced from Jatropha, jojoba and castor as they were 4.84 mm2 s−1, 15.4 mm2 s−1, and 5.86 mm2 s−1, respectively. Lower viscosity will improve the fuel flow through the ignition system, which resulting in a good fuel combustion and lower the exhaust emission. Moreover, the viscosity of candlenut biodiesel was found to comply with the biodiesel international standards (EN14214 and ASTM D 6751). Density is one of the important properties of biodiesel fuel; it has a direct effect on the engine performance because the motor fuel injection system operates on a volume metering system. The densities of candlenut oil and biodiesel were found to be approximately 914 and 871 kg m−3, respectively. The density of the extracted candlenut oil was slightly lower than the densities of jatropha oil and radish oil (918 and 918.8 kg m−3).20 The candlenut biodiesel has a lower density than Jatropha biodiesel and castor biodiesel which are 879 kg m−3, and 946 kg m−3, while the density of castor biodiesel was the same as the density of candlenut biodiesel. Furthermore, the density of the synthesized biodiesel was found to comply with the biodiesel standard limits (800–900 kg m−3) according to the EN14214 standard.
O, 285 °C, 125 bar, and 22 min), were analyzed and compared with a biodiesel standard, and other biodiesel feedstocks as shown in Table 5. Viscosity is an important parameter of biodiesel because it affects the fuel injection system, causes poor fuel combustion, and increases exhaust emissions. Here, the viscosity decreased with an increase in temperature. The viscosities of the extracted candlenut oil and biodiesel were found to be approximately 25.80 and 4.8 mm2 s−1, respectively. The viscosity of candlenut oil was found to be lower than that of wild radish oil (36.32 mm2 s−1) and higher than that of Jatropha oil, (19.77 mm2 s−1).20,43,44 However, the viscosity of candlenut biodiesel was found to be lower than the biodiesel produced from Jatropha, jojoba and castor as they were 4.84 mm2 s−1, 15.4 mm2 s−1, and 5.86 mm2 s−1, respectively. Lower viscosity will improve the fuel flow through the ignition system, which resulting in a good fuel combustion and lower the exhaust emission. Moreover, the viscosity of candlenut biodiesel was found to comply with the biodiesel international standards (EN14214 and ASTM D 6751). Density is one of the important properties of biodiesel fuel; it has a direct effect on the engine performance because the motor fuel injection system operates on a volume metering system. The densities of candlenut oil and biodiesel were found to be approximately 914 and 871 kg m−3, respectively. The density of the extracted candlenut oil was slightly lower than the densities of jatropha oil and radish oil (918 and 918.8 kg m−3).20 The candlenut biodiesel has a lower density than Jatropha biodiesel and castor biodiesel which are 879 kg m−3, and 946 kg m−3, while the density of castor biodiesel was the same as the density of candlenut biodiesel. Furthermore, the density of the synthesized biodiesel was found to comply with the biodiesel standard limits (800–900 kg m−3) according to the EN14214 standard.
| Property | scCO2 extracted candlenut oil | Candlenut biodiesel | Jatropha biodiesel4 | Caster biodiesel46 | Jojoba biodiesel47 | ASTM 6751 | EN14214 |
|---|---|---|---|---|---|---|---|
| Density (kg m−3) @ 15 °C | 914 ± 5 | 871 ± 6 | 879 | 946 | 871 | — | 860–900 |
| Viscosity (mm2 s−1) | 25.8 ± 3 | 4.8 ± 2 | 4.84 | 15.4 | 5.86 | 1.9–6 | 3.5–5 |
| Pour point (oC) | 2 ± 0.08 | −2.3 ± 0.1 | 3 | −30 | −6 | −15 to 16 | — |
| Cloud point (oC) | 10 ± 1 | 3 ± 1 | 2.8 | −18 | −2 | −3 to 12 | — |
| Moisture content (%) | 0.1 ± 0.01 | 0.01 ± 0.01 | 0.02 | 0.04 | 0.053 | >0.03 | >0.05 |
| Iodine number gI2/100 g | 122 ± 2 | 130 ± 2 | — | 78.21 | 74.7 | — | 120 max |
| Cetane number | — | 51 ± 1 | 51 | 43.7 | — | >47 | 51 |
| Acid value (mg KOH per g) oil | 15.8 ± 1.2 | 0.38 ± 0.02 | 0.3 | 2.8 | 0.22 | 0.5 max | 0.5 max |
| FFAs (%) | 7.9 ± 0.6 | 0.19 ± 0.01 | 0.15 | 1.4 | 0.11 | ||
| Saponification value (mg KOH per g) | 172 ± 2 | 187 ± 1.5 | — | ||||
| Peroxide value (meq kg−1) | 8.6 ± 0.8 | 5.8 ± 0.5 | — | ||||
| Calorific value (MJ kg−1) | 42 ± 2 | 42.3 ± 1.2 | 38.5 | 38.3 | 42.8 | — | 35 |
The acid value and FFAs are critical parameters for biodiesel production.44 The quality of biodiesel improves with a reduction in the AC and FFA content.20 The estimated acid value and FFAs for the extracted candlenut oil were 15.8 mg KOH per g and 7.9%, respectively. In the two-step transesterification process for biodiesel production, the FFAs must be first subjected to an esterification process to reduce the FFA content to less than 1% to facilitate the occurrence of transesterification reaction and biodiesel production. However, the scMeOH transesterification method produces biodiesel without the need for a pre-treatment step (esterification).29 The acid value and FFAs of the produced biodiesel were found to be 0.38 mg KOH per g and 0.19%, respectively, which are similar to the Jatropha biodiesel and slightly higher than the acid value of jojoba biodiesel and castor biodiesel. The acid value of the produced candlenut biodiesel is desirable because it complies with the biodiesel standard ranges described in ASTM D6751 and EN 14214.
The presence of peroxides in oil and biodiesel indicates its rancidity and oxidation stability. Research has shown that the desired peroxide value for essential oils is less than 10 meq kg−1, and the rancidity of biodiesel over the peroxide value is 50 meq kg−1.39,43 The peroxide values in candlenut lipids and the produced biodiesel were 8.6 and 5.8 meq kg−1, respectively. Thus, it can be presumed that the oxidation stability of the biodiesel produced from candlenut oil is good. The saponification number indicates the presence of fatty acid chains in the oil [Sohrab]. The saponification values of candlenut oil and biodiesel were 172 and 187 meg KOH per g oil. The saponification value of the produced biodiesel was below the saponification value limit described in the ASTM D6751 standard. The iodine number is related to the degree of unsaturation and the stability of oils and biodiesel. It measures the number of double bonds that react with iodine, resulting in the polymerization of fuel due to epoxide formation via the addition of oxygen in double bonds. As the iodine number increases, the number of unsaturated fatty acids increases, which lowers the stability of biodiesel. In this study, the iodine number values of candlenut oil and biodiesel were 122 and 130 I2/100 g, respectively. The iodine number of candlenut biodiesel was found higher than the iodine value of jojoba and castor biodiesel, which is due to the presence of the unsaturated fatty acids in candlenut biodiesel feedstock. However, the iodine number of the produced biodiesel was found to be above the biodiesel standard limit.
The cetane number is another important property of biodiesel, as it has a direct effect on fuel ignition. A better ignition quality of the fuel is always associated with a higher cetane value. The cetane number affects several engine performance parameters such as drivability, combustion, white smoke, stability, CO and HC emissions, and noise.40 The cetane number of candlenut biodiesel was 51, which was higher than the castor biodiesel and similar to Jatropha biodiesel. It was found that the cetane number of the produced biodiesel complied with the EN14214 standard limit.
The CP and PP were used to measure fuel usability at cold temperatures. The higher CP and PP values indicate poor cloud flow properties of biodiesel. The CP and PP values increase with an increase in the levels of saturated compounds in the biofuel;39,44 in this study, the CP and PP values for biodiesel were found to be within the ASTM D6751 standard range. The calorific value indicates the energy content of the oil and biofuel. The calorific value has a direct effect on the engine performance in terms of torque and maximum horsepower. A higher calorific value results in high temperatures and improves engine performance during the combustion of biodiesel. The calorific values of candlenut oil and biodiesel were 42 and 42.3 MJ kg−1, respectively. The calorific value of the produced biodiesel was found to be slightly lower than that of the biodiesel produced from jojoba oil (42.82 MJ kg−1) and higher than the calorific values of jatropha oil, caster oil, and jojoba oil, which were 38.5 MJ kg−1, 38.34 MJ kg−1, and 38.33 MJ kg−1, respectively.20,41 The calorific value of candlenut biodiesel complied with the EN14214 standard range. These findings indicated that candlenut oil can be used as a potential feedstock for biodiesel production.
As shown in Table 5, the candlenut biodiesel properties comply with both international standards (ASTM D6751 and EN14214) which makes it a good biodiesel fuel that can be used directly in the existing engines. Moreover, comparing the main properties of the candlenut biodiesel with other biodiesel like Jatropha biodiesel, jojoba biodiesel, and castor biodiesel showed that the candlenut biodiesel has better values with the biodiesel standard range. The viscosity and density of candlenut biodiesel were found lower than other biodiesel, which is more desirable for biodiesel fuel to improve fuel combustion, avoid engine knocking and decrease exhaust emission. Furthermore, the cetane number and the calorific value of candlenut biodiesel were found higher than other biodiesels and within the international biodiesel standards. FAME composition of the scCO2-extracted candlenut oil and biodiesel was determined using GC-FID analyses, as shown in Table 6. It was found that candlenut oil contains 10.98% saturated fatty acids, 24.7% mono-unsaturated fatty acids, and 64.20% polyunsaturated fatty acids. Linoleic acid (C18:2) was the most abundant (40.1%), followed by oleic acid (C18:1) (24.7%) and linolenic acid (C18:3) (24.1%). Furthermore, candlenut biodiesel showed a higher proportion (89.92%) of the unsaturated fatty acid methyl esters. The degree of unsaturation of fatty acid methyl esters significantly affects the cold flow properties and oxidation stability.45 Thus, candlenut biodiesel is presumed to have low oxidation stability.
| Fatty acid | Carbon number | Fatty acid groups | Fatty acid (%) | |
|---|---|---|---|---|
| Oil | Biodiesel | |||
| a SFA: saturated fatty acids, MUFA: mono-unsaturated fatty acids, PUFA: poly-unsaturated fatty acids. | ||||
| Caprylic acid | C8:0 | SFA | 0.04 | 0 |
| Capric acid | C10:0 | SFA | 0.07 | 0 |
| Lauric acid | C12:0 | SFA | 0.19 | 0.07 |
| Myristic acid | C14:0 | SFA | 0.3 | 0.1 |
| Palmitic acid | C16:0 | SFA | 6.9 | 6.6 |
| Stearic acid | C18:0 | SFA | 2.9 | 3.6 |
| Oleic acid | C18:1 | MUFA | 24.7 | 27.2 |
| Linoleic acid | C18:2 | PUFA | 40.1 | 40.4 |
| Linolenic acid | C18:3 | PUFA | 24.1 | 23.2 |
| Arachidic acid | C20:0 | SFA | 0.58 | 0.5 |
| ΣSFA | 10.98 | 10.87 | ||
| ΣMUFA | 24.7 | 27.2 | ||
| ΣPUFA | 64.20 | 63.60 | ||
Fig. 13 shows the FTIR spectra of candlenut biodiesel. It can be clearly seen that the biodiesel derived from candlenut comprises long-chain fatty acid esters. In the range of 1800–1700 cm−1, the strong peak observed at 1741 cm-1 is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, which typically belongs to esters. The peaks located at 2926 and 2854 cm−1 confirmed the presence of
O, which typically belongs to esters. The peaks located at 2926 and 2854 cm−1 confirmed the presence of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H asymmetric stretching vibration and –CH2 symmetric stretching vibrations, respectively, in candlenut biodiesel. The major spectral region for biodiesel is in the range of 1500–1000 cm−1. The peaks located at 1460 and 1436 cm−1 indicate bending vibrations of –CH2, and the high point located at 1244 cm−1 indicates the bending vibrations of –CH3. The peaks located at 1244, 1195, and 1168 cm−1 indicate the C–O–C anti-symmetric stretching vibration.
C–H asymmetric stretching vibration and –CH2 symmetric stretching vibrations, respectively, in candlenut biodiesel. The major spectral region for biodiesel is in the range of 1500–1000 cm−1. The peaks located at 1460 and 1436 cm−1 indicate bending vibrations of –CH2, and the high point located at 1244 cm−1 indicates the bending vibrations of –CH3. The peaks located at 1244, 1195, and 1168 cm−1 indicate the C–O–C anti-symmetric stretching vibration.
4. Conclusion
The present study utilized the scMeoH transesterification process to produce biodiesel from candlenut oil. It was observed that pressure, temperature, M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio, and reaction time of the scMeOH significantly influenced the transesterification process for the biodiesel production. The maximum biodiesel yield obtained was 96.35% at the optimized scMeOH transesterification process, such as the pressure of 115 bar; temperature of 285 °C; M
O ratio, and reaction time of the scMeOH significantly influenced the transesterification process for the biodiesel production. The maximum biodiesel yield obtained was 96.35% at the optimized scMeOH transesterification process, such as the pressure of 115 bar; temperature of 285 °C; M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio of 30
O ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; and reaction time of 22 min. From the kinetics studies, the activation energy was determined to be 28.35 kJ mol−1, indicating the scMeoH transesterification process was temperature dependent. The thermodynamics properties analyses showed that ΔH, ΔS, and ΔG values were 231.22 kJ mol−1, 26.57 J mol−1 K−1, and 164.79–151.51 kJ mol−1,respectively. The estimated thermodynamic property values indicate that the biodiesel production from the candlenut oil using the scMeOH as a non-catalytic transesterification process was endothermic and non-spontaneous. The determination of fatty acids properties in the isolated biodiesel showed that saturated and unsaturated fatty acids content were 10.87% and 63.60%, respectively. The physicochemical properties analyses of the biodiesel showed that density, acid value, cloud point, pour point, saponification values, and iodine values were complied with the international biodiesel standards of ASTM D6751 and EN14214. The findings of the present study indicated that the scMeOH is an effective non-catalytic transesterification process for the biodiesel production from candlenut oil. Therefore, the scMeOH is transesterification process could be applied as an effective alternative of the conventional catalytic transesterification process for the sustainable production of biodiesel from non-edible oil.
1; and reaction time of 22 min. From the kinetics studies, the activation energy was determined to be 28.35 kJ mol−1, indicating the scMeoH transesterification process was temperature dependent. The thermodynamics properties analyses showed that ΔH, ΔS, and ΔG values were 231.22 kJ mol−1, 26.57 J mol−1 K−1, and 164.79–151.51 kJ mol−1,respectively. The estimated thermodynamic property values indicate that the biodiesel production from the candlenut oil using the scMeOH as a non-catalytic transesterification process was endothermic and non-spontaneous. The determination of fatty acids properties in the isolated biodiesel showed that saturated and unsaturated fatty acids content were 10.87% and 63.60%, respectively. The physicochemical properties analyses of the biodiesel showed that density, acid value, cloud point, pour point, saponification values, and iodine values were complied with the international biodiesel standards of ASTM D6751 and EN14214. The findings of the present study indicated that the scMeOH is an effective non-catalytic transesterification process for the biodiesel production from candlenut oil. Therefore, the scMeOH is transesterification process could be applied as an effective alternative of the conventional catalytic transesterification process for the sustainable production of biodiesel from non-edible oil.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank and acknowledge the Ministry of Higher Education (MOHE), Malaysia for financial support through the Fundamental Research Grant Scheme (203/PTEKIND/6711817).References
- S. Dharma, H. H. Masjuki, H. C. Ong, A. H. Sebayang, A. S. Silitonga, F. Kusumo and T. M. I. Mahlia, Energy Convers. Manage., 2016, 115, 178–190 CrossRef CAS.
- T. Ahmad and D. Zhang, Energy Rep., 2020, 6, 1973–1991 CrossRef.
- P. Lisboa, A. R. Rodrigues, J. L. Martín, P. Simões, S. Barreiros and A. Paiva, J. Supercrit. Fluids, 2014, 85, 31–40 CrossRef CAS.
- H. C. Ong, A. S. Silitonga, H. H. Masjuki, T. M. I. Mahlia, W. T. Chong and M. H. Boosroh, Energy Convers. Manage., 2013, 73, 245–255 CrossRef CAS.
- D. Wen, H. Jiang and K. Zhang, Prog. Nat. Sci., 2009, 19, 273–284 CrossRef CAS.
- O. Aboelazayem, M. Gadalla and B. Saha, Energy, 2018, 162, 408–420 CrossRef CAS.
- M. K. Mohammad Ilias, M. S. Hossain, M. H. Zuknik, V. Balkakrishnan, A. Al-Gheethi, A. A. Ghfar, A. Zulkharnain and A. N. A. Yahaya, Biomass Convers. Biorefin., 2022 DOI:10.1007/s13399-022-02454-9.
- G. Knothe and L. F. Razon, Prog. Energy Combust. Sci., 2017, 58, 36–59 CrossRef.
- A. Demirbas, Appl. Energy, 2009, 86, S108–S117 CrossRef CAS.
- K. Hasni, Z. Ilham, S. Dharma and M. Varman, Energy Convers. Manage., 2017, 149, 392–400 CrossRef CAS.
- P. D. Patil and S. Deng, Fuel, 2009, 88, 1302–1306 CrossRef CAS.
- T. Muppaneni, H. K. Reddy, S. Ponnusamy, P. D. Patil, Y. Sun, P. Dailey and S. Deng, Fuel, 2013, 107, 633–640 CrossRef CAS.
- K. Noiroj, P. Intarapong, A. Luengnaruemitchai and S. Jai-In, Renewable Energy, 2009, 34, 1145–1150 CrossRef CAS.
- M. Rahimi, B. Aghel, M. Alitabar, A. Sepahvand and H. R. Ghasempour, Energy Convers. Manage., 2014, 79, 599–605 CrossRef CAS.
- B. Ogaga Ighose, I. A. Adeleke, M. Damos, H. Adeola Junaid, K. Ernest Okpalaeke and E. Betiku, Energy Convers. Manage., 2017, 132, 231–240 CrossRef CAS.
- H. Sanli and M. Canakci, Energy Fuels, 2008, 22, 2713–2719 CrossRef CAS.
- A. E. Atabani, A. S. Silitonga, H. C. Ong, T. M. I. Mahlia, H. H. Masjuki, I. A. Badruddin and H. Fayaz, Renewable Sustainable Energy Rev., 2013, 18, 211–245 CrossRef CAS.
- R. L. Patel and C. D. Sankhavara, Renewable Sustainable Energy Rev., 2017, 71, 464–474 CrossRef CAS.
- A. N. Syimir Fizal, M. S. Hossain, M. Zulkifli, N. A. Khalil, H. Abd Hamid and A. N. Ahmad Yahaya, Int. J. Green Energy, 2022, 19, 72–83 CrossRef CAS.
- M. A. Shaah, F. Allafi, M. S. Hossain, A. Alsaedi, N. Ismail, M. O. A. Kadir and M. I. Ahmad, Int. J. Energy Res., 2021, 45, 17057–17079 CrossRef CAS.
- M. Munir, M. Ahmad, M. Saeed, A. Waseem, M. Rehan, A.-S. Nizami, M. Zafar, M. Arshad and S. Sultana, Renewable Sustainable Energy Rev., 2019, 109, 321–332 CrossRef CAS.
- O. Farobie, Z. Y. M. Leow, T. Samanmulya and Y. Matsumura, Energy Convers. Manage., 2017, 132, 410–417 CrossRef CAS.
- F. Esmi, V. B. Borugadda and A. K. Dalai, Catal. Today, 2022 DOI:10.1016/j.cattod.2022.01.019.
- A. Zhang, H. Wang, J. Miles, J. Shan, A. Sardar and L. Guillen, Ind. Eng. Chem. Res., 2021, 60, 16233–16244 CrossRef CAS.
- M. K. Pasha, L. Dai, D. Liu, W. Du and M. Guo, Biofuels, Bioprod. Biorefin., 2021, 15, 1526–1548 CrossRef CAS.
- L. N. Pham, B. V. Luu, H. D. Phuoc, H. N. T. Le, H. T. Truong, P. D. Luu, M. Furuta, K. Imamura and Y. Maeda, J. Oleo Sci., 2018, 67, 617–626 CrossRef CAS PubMed.
- H. Sulistyo, S. S. Rahayu, I. M. Suardjaja and U. H. Setiadi, World Acad. Eng. Technol., 2008, 48, 485–488 Search PubMed.
- S. Saka and D. Kusdiana, Fuel, 2001, 80, 225–231 CrossRef CAS.
- R. D. Micic, M. D. Tomić, F. E. Kiss, E. B. Nikolić-Djorić and M. Đ. Simikić, J. Supercrit. Fluids, 2015, 103, 90–100 CrossRef CAS.
- S. Zhang, L. Wang, J. Wang, J. Yang, L. Fu, Z. Cai and Y. Fu, Fuel, 2022, 312, 122974 CrossRef CAS.
- N. K. Singh, Y. Singh and A. Sharma, Biomass Bioenergy, 2022, 156, 106332 CrossRef.
- J.-H. Ryu, S.-H. Lee, H.-Y. Shin and S.-Y. Bae, Korean Chem. Eng. Res., 2009, 47, 651–654 CAS.
- S. Yahya, S. K. Muhamad Wahab and F. W. Harun, Renewable Energy, 2020, 157, 164–172 CrossRef CAS.
- M. Rahimi, B. Aghel, M. Alitabar, A. Sepahvand and H. R. Ghasempour, Energy Convers. Manage., 2014, 79, 599–605 CrossRef CAS.
- S. M. Ghoreishi and P. Moein, J. Supercrit. Fluids, 2013, 76, 24–31 CrossRef CAS.
- A. Santana, J. Maçaira and M. A. Larrayoz, Fuel Process. Technol., 2012, 96, 214–219 CrossRef CAS.
- Y. Nan, J. Liu, R. Lin and L. L. Tavlarides, J. Supercrit. Fluids, 2015, 97, 174–182 CrossRef CAS.
- H. Imahara, E. Minami, S. Hari and S. Saka, Fuel, 2008, 87, 1–6 CrossRef CAS.
- Y.-T. Tsai, H.-m. Lin and M.-J. Lee, Bioresour. Technol., 2013, 145, 362–369 CrossRef CAS PubMed.
- H.-Y. Shin, S.-M. Lim, S. C. Kang and S.-Y. Bae, Fuel Process. Technol., 2012, 98, 1–5 CrossRef CAS.
- L. K. Ong, A. Kurniawan, A. C. Suwandi, C. X. Lin, X. S. Zhao and S. Ismadji, J. Supercrit. Fluids, 2013, 75, 11–20 CrossRef CAS.
- M. K. Mohammad Ilias, V. Balakrishnan, M. H. Zuknik, A. Al-Gheethi, A. A. Ghfar and M. S. Hossain, Biomass Convers. Biorefin., 2021 DOI:10.1007/s13399-021-02092-7.
- C. Senthilkumar, C. Krishnaraj, P. Sivakumar and A. Sircar, Chem. Eng. Commun., 2019, 206, 909–918 CrossRef CAS.
- A. K. Agarwal, A. Dhar, J. G. Gupta, W. I. Kim, K. Choi, C. S. Lee and S. Park, Energy Convers. Manage., 2015, 91, 302–314 CrossRef CAS.
- A. B. Fadhil, S. H. Sedeeq and N. M. T. Al-Layla, Energy Sources, Part A, 2019, 41, 892–901 CrossRef CAS.
- S. Rezania, B. Oryani, J. Park, B. Hashemi, K. K. Yadav, E. E. Kwon, J. Hur and J. Cho, Energy Convers. Manage., 2019, 201, 112155 CrossRef CAS.
- G. Perin and P. R. Jones, Curr. Opin. Biotechnol., 2019, 57, 175–182 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |