Open Access Article
Open Access ArticleEnhanced total flavonoid accumulation and alleviated growth inhibition of germinating soybeans by GABA under UV-B stress†
Minglang Gu a,
Jia Yangb,
Xin Tian
a,
Jia Yangb,
Xin Tian a,
Weiming Fanga,
Jinpeng Xua and
Yongqi Yin
a,
Weiming Fanga,
Jinpeng Xua and
Yongqi Yin *a
*a
aCollege of Food Science and Engerning, Yangzhou University, Yangzhou, Jiangsu 225127, People's Republic of China. E-mail: yqyin@yzu.edu.cn; Fax: +86-514-89786551; Tel: +86-514-89786551
bYangzhou Center for Food and Drug Control, Yangzhou, Jiangsu 225009, People's Republic of China
First published on 25th February 2022
Abstract
Germination of soybeans under ultraviolet-B (UV-B) treatment is a simple and effective way to enrich soybean isoflavones, but its mechanism of action is not yet clear. G-Aminobutyric acid (GABA) is a signaling molecule that is involved in the accumulation of secondary metabolites as well as the regulation of plant development and metabolism. In this study, the effects of exogenous GABA and its inhibitors on the physiological and biochemical, antioxidant systems, total flavonoid content, activity and gene expression of isoflavone metabolism related enzyme in germinating soybeans under UV-B treatment were investigated. Compared to UV-B treatment alone, soybean treated with GABA (5 mM) in combination with UV-B significantly increased sprout length, fresh weight, Ca2+ inward flow and peroxidase and catalase activities, and decreased malondialdehyde and H2O2 and O2˙− fluorescence intensity, while soybean treated with GABA inhibitor showed the opposite trend. Meanwhile, total flavonoid content increased by 11.2% and 6.7%, respectively, in 2- and 4 day-old soybeans under UV-B treatment, compared to UV-B treatment alone. Moreover, the application of GABA under UV treatment significantly increased the activity of phenylalanine ammonia-lyase and cinnamic acid-4-hydroxylase, with values increasing by 43.6% and 18.5%, respectively, in four-day-old soybean compared to UV treatment alone, which also increased the relative expression of key genes involved in isoflavone metabolism. The GABA inhibitor 3-mercaptopropionic acid blocked these occurrences. According to this research, GABA could operate as a signaling molecule to mediate isoflavone accumulation in soybean sprouts under UV radiation and stimulate soybean sprout growth.
1. Introduction
Soybean (Glycine max L.) is a significant source of vegetable protein and one of the most important food and oil crops for the global food sector.1,2 Isoflavones, the major phenolic molecule in soybeans, are a secondary metabolite that can prevent cardiovascular disease, menopausal syndrome, reduce osteoporosis and certain cancers,3–5 making the development of isoflavone-rich meals popular all over the world. According to research, germination under abiotic stress is a simple and effective approach to enrich isoflavones as internal enzymes like protease and amylase are activated during the germination process,6 leading to the breakdown of macromolecules and inducing the synthesis of life-giving substances such as isoflavones.7 Simultaneously, ultraviolet-B (UV-B) radiation has been repeatedly shown to promote the production of secondary metabolites in various plants8–12 such as soybean, barley and tomato, including some reports on soy isoflavones.13–17 Soybean sprouts have been observed to accumulate large quantities of phenolic chemicals18 when exposed to UV-B radiation.G-Aminobutyric acid (GABA) is a four-carbon non-protein amino acid19 that plays various physiological roles in plants and animals, including pH regulation, development and growth, carbon and nitrogen nutritional balance, and stress response.20,21 In recent years, its dual physiological roles as a signaling molecule for secondary metabolite accumulation22 and plant resistance to abiotic stresses23 have been widely described. It has been demonstrated that GABA can be involved in abiotic stresses as a plant stress tolerance agent to scavenge free radicals produced in the plant and that GABA treatment increases the phenolic acid content of tomato plants under both stress and non-stress conditions.24 Previously, research revealed that GABA is a key signaling molecule in the metabolism of phenolic buildup in sprouting soybean seedlings22 and sprouting barley25 under salt stress. GABA mediated the accumulation of phenolic compounds and strengthened the antioxidant system in sprouting barley under salt stress according to Ma et al.,25 and similar conclusions were obtained by Xie et al.26 Although GABA has been proven to be a signaling molecule for phenolic component enrichment in growing soybeans when exposed to salt, it is unknown if GABA has the same impact when exposed to UV-B radiation. It is therefore necessary to explore whether GABA can act as a signaling molecule for isoflavone enrichment in germinating soybean under UV-B radiation and its exact mechanism of action.
To address this issue, the effects of GABA and its inhibitor 3-mercaptopropionic acid (3-MP) on total flavonoid content, key enzyme activities, antioxidant enzyme activities and their gene expression in germinating soybean seedlings under UV-B radiation treatment were investigated in this study, thus exploring the effects of GABA on total flavonoid accumulation and growth metabolism in germinating soybean seedlings under UV-B radiation.
2. Materials and methods
2.1. Materials and chemicals
Soybean seeds (Glycine max L. cultivar Dongsheng No. 1) were provided by the Nanjing Agricultural University (Nanjing, China) in 2018, and stored at −20 °C until usage. GABA, 3-MP were procured from Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemicals and reagents used (analytical grade) were bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).2.2. Seeds germination and experimental design
100 g soybean seeds were selected without damage and disinfected in 1% (v/v) sodium hypochlorite solution for 15 min. The disinfected seeds were washed with deionized water to neutral pH and then immersed for 6 h in distilled water at 30 °C. Soaked soybeans were divided into numerous germination trays, then placed in a soybean germinator and germinated for four days at 30 °C in a dark incubator. (1) CK: The soybeans were sprayed with deionized water; (2) GABA: the germination was conducted according to (1), and sprayed with 5 mM GABA; (3) 3-MP: the germination was conducted according to (1), and sprayed with 0.2 mM 3 MP; (4) UV-B: during germination, a 15 W UV-B light bulb was put 1 ft above the sprouter in the chamber. This was under 9/15 h light/dark photoperiod, and sprayed with distilled water; (5) UV-B + GABA: the germination was conducted according to (4), and sprayed with 5 mM GABA; (6) UV-B + 3 MP: the germination was conducted according to (4), and sprayed with 0.2 mM 3-MP. The (1)–(6) were marked as CK, G, M, UV, UG, UM respectively. A narrowband UV-B lamp tube with a 313 nm central wavelength (15 W, ZW20S19Y, Beijing Electronic Resource, Inc., China) was utilized for UV-B treatment. To prevent wavelengths less than 280 nm from passing through the UV-B tube, 0.13 mm thick cellulose diacetate filters were used. Every 12 h during germination, 40 mL of different culture solution was sprayed. Biochemical studies were performed on samples obtained at 2 and 4 days after germination (ESI Fig. S1†). The pre-experiment determined the optimal UV irradiation time and GABA concentration (ESI Fig. S2 and S3†).2.3. Determination of sprout length, fresh weight, dry weight and malondialdehyde
Sprout length of 30 randomly selected samples were measured with vernier calipers, in mm. Fresh weight and dry weight were established by properly weighing 30 fresh soybean sprouts (fresh weight) and then baking them in an oven (100°C ± 5 °C, 1 MPa) to a constant weight before measuring (dry weight), constant weight being defined as a difference of no more than 2 mg between the first and second weighing. The content of malondialdehyde (MDA) was determined using the method of Zhuang et al.27 1.0 g of fresh sprouts were added to 5.0 mL 5% trichloroacetic acid and ground into homogenate and centrifuged for 8000g for 10 min. Add 2.0 mL 0.76% thiobarbituric acid solution to the supernatant and stir for 30 min in a boiling water bath. At last, the supernatant was taken and the absorbance values at 450 nm, 532 nm and 600 nm were measured.2.4. Determination of total flavonoid content
Determination of the total flavonoid content using the method of Djeridane et al.28 A standard curve was made with rutin. The diluted samples were mixed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with 2% ethanolic solution of aluminium chloride respectively. Measure the absorbance of the reaction mixture at OD430nm.
1 with 2% ethanolic solution of aluminium chloride respectively. Measure the absorbance of the reaction mixture at OD430nm.
2.5. Determination of intracellular free calcium, H2O2 and O2˙−
The intracellular free calcium was measured using the method of Cheng et al.29 The H2O2 and O2˙− fluorescence staining we referred to the method of Zhang et al.30 Soybean root tips were washed with deionized water and cut to 3 mm length in 300 μL of HBSS staining solution (containing 0.02% Pluronic F-127, 10 μM Fluo-4AM, pH 7.2) and incubate in the dark at 4 °C for 2 h, then wash three times with hanks balanced salt buffer solution (HBSS) and incubate in the dark at 25 °C for 2 h. The incubated samples were placed on dry slides and observed using an LSM 880 NLO confocal laser scanning microscope (Leica Excitation Technology Co., Ltd., Germany).2.6. Determination of antioxidant enzyme activity
Peroxidase (POD) and Catalase (CAT) activities were measured as described by Wang et al.31 1.0 g of fresh sprout were added to 5 mL of acetate buffer (pH 7.0, 50 mM) containing soluble polyvinyl chloride pyrrolidine (4%) and Triton X-100 (1%), ground on ice and homogenized at 4 °C. Centrifuge at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g and centrifuge for 25 min. A unit of POD activity (U) is defined as a change of 0.01 per min in OD470nm. 1.0 g of fresh sprouts were added to 5 mL of phosphate buffer solution (pH 7.0, 50 mM) including soluble polyvinyl pyrrolidine (1%) and ground on ice and homogenized at 4 °C. Centrifuge at 12
000g and centrifuge for 25 min. A unit of POD activity (U) is defined as a change of 0.01 per min in OD470nm. 1.0 g of fresh sprouts were added to 5 mL of phosphate buffer solution (pH 7.0, 50 mM) including soluble polyvinyl pyrrolidine (1%) and ground on ice and homogenized at 4 °C. Centrifuge at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g and centrifuge for 25 min. A unit of CAT activity (U) was defined as an increase of 0.01 per min in OD240nm.
000g and centrifuge for 25 min. A unit of CAT activity (U) was defined as an increase of 0.01 per min in OD240nm.
2.7. Determination of isoflavone metabolism-related enzyme activity
To determine phenylalanine ammonia-lyase (PAL) activities, we referred to the method by Assis et al.32 1.0 g of fresh sprouts were added to 5 mL of boric acid–borax buffer solution (pH 7.0, 50 mM) containing soluble polyvinyl pyrrolidine (0.5%) and mercaptoethanol (5 mM) and ground on ice and homogenized at 4 °C. Centrifuge at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g and centrifuge for 25 min. The unit (U) of PAL activity was defined as 0.01 change in absorbance per gram of bean sprouts (fresh weight) enzymatic reaction system per hour at OD290nm.
000g and centrifuge for 25 min. The unit (U) of PAL activity was defined as 0.01 change in absorbance per gram of bean sprouts (fresh weight) enzymatic reaction system per hour at OD290nm.
Cinnamic acid 4-hydroxylase (C4H) activities were measured according to Lamb and Rubery.33 1.0 g of fresh sprouts were added to 5 mL of Tris–HCl buffer (pH 8.9, 0.1 M) and ground on ice and homogenised by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 25 min at 4 °C. The unit (U) of C4H activity was defined as 0.01 change in absorbance per gram of bean sprouts (fresh weight) enzymatic reaction system per hour at OD340nm.
000g for 25 min at 4 °C. The unit (U) of C4H activity was defined as 0.01 change in absorbance per gram of bean sprouts (fresh weight) enzymatic reaction system per hour at OD340nm.
2.8. Ribonucleic acid extraction and quantitative real-time polymerase chain reaction analysis
Total Ribonucleic Acid (RNA) was isolated from frozen soybeans powdered with liquid nitrogen using the Plant RNA Extraction Kit (R6827-01, OMEGA, USA) according to the manufacturer's instructions. The PrimeScript™ RT Master Mix Kit (RR036A, Takara, Japan) was used to reverse transcribe RNA samples into cDNA. Three replicates of each cDNA were quantified using SYBRR Premix EX-TaqTM (RR420A, Takara, Japan) and the ABI 7500 Sequence Detection System, according to the manufacturer's procedure (Applied Biosystems, USA). Table S1† listed the oligonucleotide primer sequences utilized in this study for quantitative real-time polymerase chain reaction (qRT-PCR). The 2−ΔΔCt method34 was used to calculate relative gene expression levels.2.9. Data processing and statistical analysis
Three biological and technical replicates were used in the experiment, and the data were expressed as mean ± standard deviation. DPS software was used to statistically analyze the test results, and Tukey's multiple comparisons with a significance test at the 0.05 level (p < 0.05) were used to compare average.3. Results
3.1. Effects of GABA on physiological and biochemical indexes of soybean germination under UV-B treatment
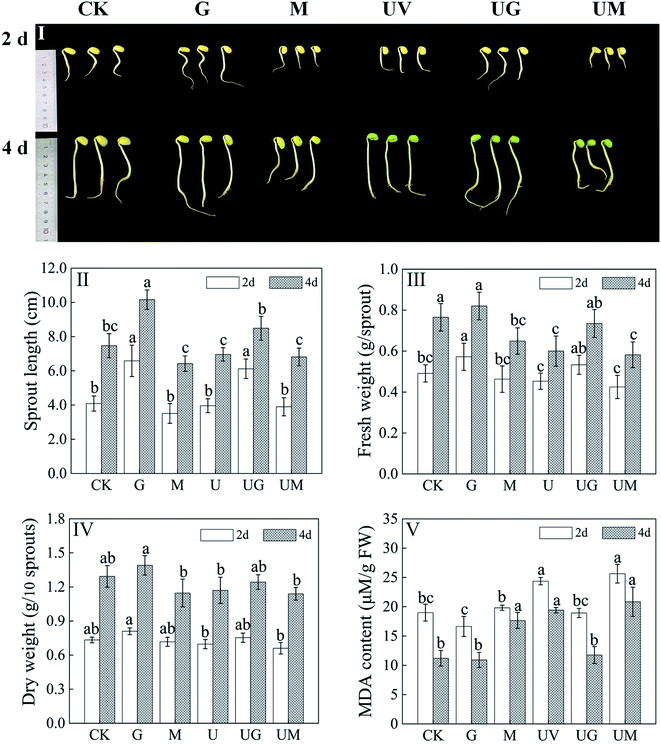
Morphological plots of soybean under different treatments are shown in Fig. 1I. Compared to the control (CK) treatment, 4 day-old soybeans under UV-B treatment showed a significant decrease in fresh weight (Fig. 1III). Compared to the CK treatment, the application of GABA alone resulted in a significant increase in sprout length (p < 0.05), with 61.2% and 36.1% increase in sprout length for 2- and 4 day-old soybeans, respectively (Fig. 1II), while the addition of 3 MP resulted in a significant decrease in sprout length (p < 0.05); similarly, under UV-B radiation, the addition of GABA resulted in a significant increase in sprout length compared to the UV treatment alone (p < 0.05). The addition of GABA and 3 MP also had similar effects on the fresh and dry weights of germinating soybeans (Fig. 1III and IV). In addition, malondialdehyde (MDA) is one of the common indicators of oxidative stress and reflects the extent of membrane lipid peroxidation in plants, as shown in Fig. 1IV, the addition of GABA reduced the extent of membrane damage and significantly reduced the MDA content compared to UV-B treatment (p < 0.05), while the addition of 3-MP increased the MDA content. These results suggest that UV-B treatment has a stressful effect on soybean growth, and that exogenous GABA effectively promotes the growth of sprouts, while the inhibitor further inhibits the growth and development of soybean.3.2. Effects of GABA on total flavonoid content in germinated soybeans under UV-B treatment
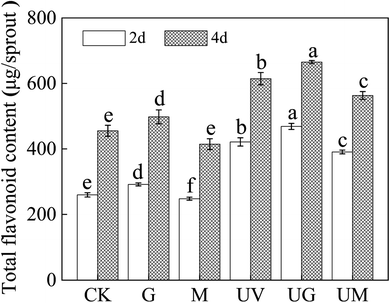
The effect of different treatments on the total flavonoids content of germinating soybeans was investigated. As shown in Fig. 2, the UV-B plus GABA treatment resulted in the highest total flavonoids content, with 1.81 and 1.46 times more total flavonoids in 2- and 4 day-old soybeans than in the CK group. Under non-radiation conditions, the addition of GABA alone increased the total flavonoid content of 2- and 4 day-old soybeans by 12.3% and 9.2%, respectively, compared to CK, whereas the addition of 3-MP decreased the total flavonoid content of 2- and 4 day-old soybeans by 4.9% and 9.9%, respectively, compared to CK. Similarly, under UV-B treatment, the addition of GABA increased the total flavonoid content of 2- and 4 day-old soybean by 11.2% and 6.7%, respectively, while the addition of 3-MP decreased the total flavonoid content of 2- and 4 day-old soybean by 7.9% and 9.3%, respectively, compared to UV-B treatment. We can thus conclude that GABA plays an important role in the accumulation of total flavonoids in the sprouts.3.3. Effect of GABA on intracellular free calcium, H2O2 and O2˙− fluorescence in soybean sprouts under UV-B treatment
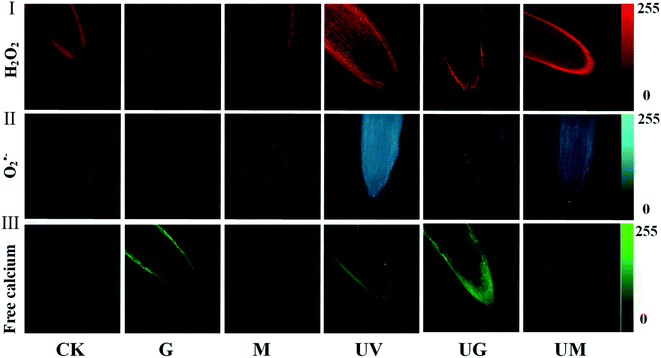
In each treatment, the root tips of 4 day-old germinating soybeans were stained with free calcium, H2O2, and O2˙− fluorescence as shown in Fig. 3. The more red, blue and green areas were stained in root tips, the more H2O2, O2˙− and intracellular free calcium were present. Under UV-B radiation, compared with the UV-B treatment, the UV-B plus GABA treatment increased the fluorescence intensity of green (intracellular free calcium), but UV-B plus 3-MP treatment decreased it. A similar phenomenon was observed under non-radiation conditions. The strongest fluorescence intensity was observed under the GABA plus UV-B treatment (Fig. 3III). In this study, ROS were characterised by measuring the distribution of superoxide anion radicals and hydrogen peroxide in the root tips of soybean sprouts., UV-B treatment caused the accumulation of H2O2 and O2˙− in the root tips of germinating soybeans, the addition of GABA alleviated this effect, while 3 MP treatment made the situation worse (Fig. 3I and II). These results showed that UV-B with GABA treatment lowered H2O2 content and O2˙− generation rate, as well as reduced damage to sprouting soybeans.3.4. Effect of GABA on antioxidant enzyme in soybean sprouts under UV-B treatment
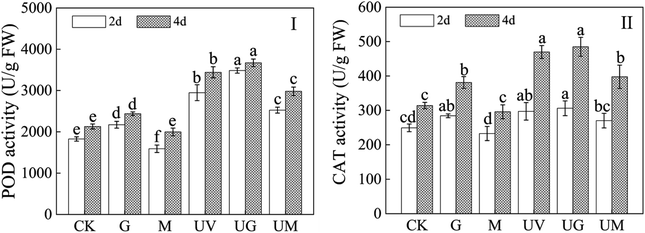
Measurement of antioxidant enzymes can respond to changes in metabolism in soybeans during a certain period of time. Compared to the CK, UV-B treatment significantly increased POD and CAT activity in soybean sprouts during germination (Fig. 4I and II). Meanwhile, compared to the UV-B treatment, the addition of GABA significantly increased POD activity (p < 0.05), but had no significant effect on CAT activity (p > 0.05). But the addition of 3-MP significantly reduced POD and CAT activities (p < 0.05). The POD activity of 2- and 4 day-old soybean sprouts treated with GABA plus UV-B treatment was 1.18 and 1.07 times higher than that of UV-B radiation-treated alone soybean sprouts, respectively (Fig. 4I).3.5. Effect of GABA on isoflavone metabolism-related enzyme activity in soybean sprouts under UV-B treatment
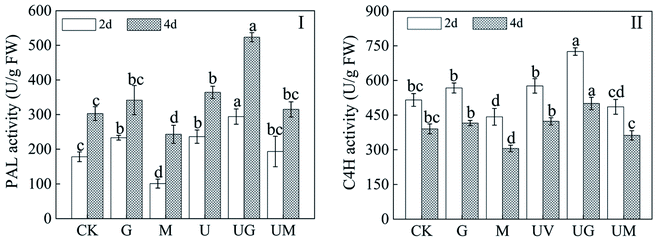
PAL and 4CL are the rate-limiting enzymes for isoflavone biosynthesis. In soybean sprouts treated with UV-B radiation, the activity levels of isoflavone metabolism-related enzymes (PAL, C4H) were significantly higher than CK (Fig. 5). When compared to the UV-B treatment, the use of GABA under UV-B radiation had a significant increase in PAL and C4H activity during germination. However, 3-MP could have the opposite effect (Fig. 5I and II). Meanwhile, under 3-MP treatment alone, PAL and C4H activity in germinated soybeans decreased significantly (Fig. 5I and II) compared with CK.3.6. Effect of GABA on the relative expression of antioxidant enzyme genes in soybean sprouts under UV-B treatment
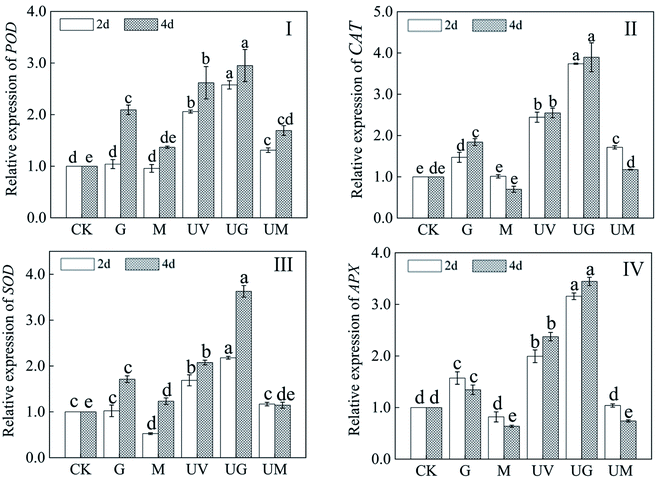
To evaluate the impact of GABA treatment on the antioxidant enzyme system, the antioxidant enzyme genes (POD, CAT, SOD and APX) were chosen (Fig. 6). Under UV-B radiation during germination, the relative expression levels of POD, CAT, SOD, and APX were considerably up-regulated compared to the control (p < 0.05). Meanwhile, after 2 and 4 days of UV-B radiation, the addition of GABA considerably boosted relative expression of the four genes, whereas the addition of 3-MP greatly decreased them (Fig. 6). In 4 day-old sprouts added with GABA and UV-B, relative expression of POD, CAT, SOD, and APX increased by 2.93, 3.83, 3.66, and 3.42 times, respectively, compared to CK. It should be observed that in 4 day-old sprouts treated with GABA, the relative expression levels of practically all identified genes were considerably higher (p < 0.05) than in CK.3.7. Effect of GABA on the relative expression of isoflavone metabolism-related genes in soybean sprouts under UV-B treatment
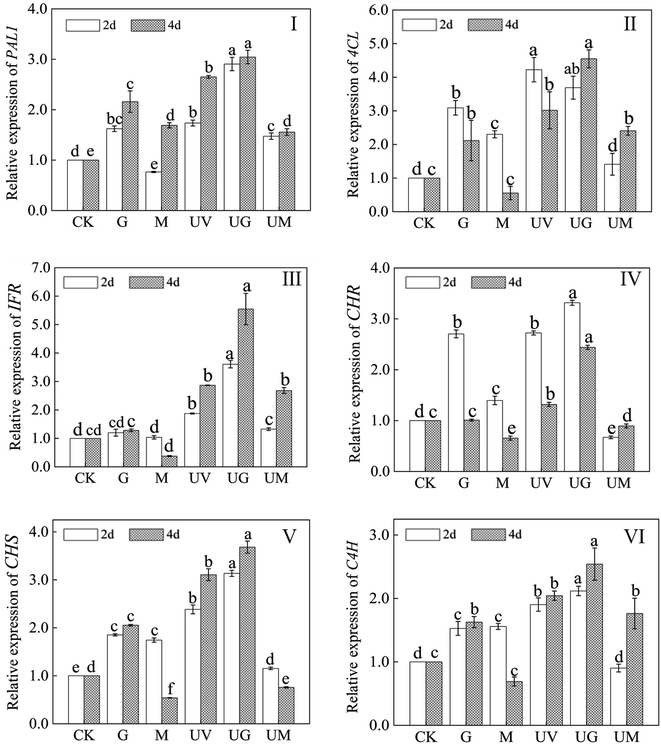
The effects of GABA treatment on germinated soybean were further studied by isoflavone biosynthesis genes (PAL1, 4CL, IFR, CHR, CHS, and C4H). The relative expression levels of PAL1, 4CL, IFR, CHR, CHS, and C4H were significantly up-regulated under UV-B radiation compared to the control (p < 0.05) (Fig. 7). Meanwhile, following 2 and 4 days of UV-B exposure, GABA significantly increased relative expression of these genes, with the exception of 4CL (p < 0.05). Except for IFR and 4CL, 3 MP significantly reduced relative expression of these genes (p < 0.05). After 4 days of germination, GABA plus UV-B treatment increased the expression of PAL1, IFR, CHR, CHS, and C4H by 1.15, 1.94, 1.86, 1.19, and 1.25 times, respectively, compared to UV-B treatment alone. UV-B treatment alone enhanced the relative expression of 4CL on day 2 of germination (p < 0.05), but GABA plus UV-B treatment decreased its gene expression. Furthermore, in 2- and 4 day-old soybeans, GABA treatment alone significantly elevated gene expression of PAL1, 4CL, CHS, and C4H in non-radiation conditions (p < 0.05).4. Discussion
As the functional food industry has grown worldwide in recent years, soy isoflavones have become increasingly popular because of their disease prevention and health promoting properties. Several studies have demonstrated that UV-B treatment is an effective method for enhancing soy isoflavones. The significant increase in total flavonoid content under UV-B treatment compared to the CK group (Fig. 2) confirms the feasibility of treating germinated soybeans with UV-B radiation for isoflavone production. However, UV-B radiation in this study significantly reduced the fresh weight of germinating soybeans, increased MDA content (Fig. 1) and increased H2O2 and O2˙− fluorescence intensity (Fig. 3), these results suggest that UV-B radiation has an inhibiting effect on the growth and biomass of germinating soybeans although the total flavonoid content increased significantly. Therefore, ensuring the biomass of soybean sprouts while accumulating isoflavones during soybean germination is a critical issue to be addressed.Recently, the role of GABA as a signaling molecule regulating the accumulation of phenolic substances in higher plants has been repeatedly demonstrated.22,23,26 Furthermore, it has also been reported that GABA is a signaling molecule that regulates gene expression in plants under salt stress.35 In the pre-experiment (Fig. S3†), it was found that low concentrations of GABA (5 mM) increased soybean sprout biomass and stimulated the synthesis of isoflavones, while high concentrations of GABA (>5 mM) had the opposite effect. In order to further increase the accumulation of isoflavones, it is essential to understand the mechanism of the effect of GABA on isoflavones synthesis under UV-B radiation, so this experiment added an inhibitor of GABA synthesis (3-MP) under UV-B radiation. It was found that the addition of GABA increased the antioxidant properties and total flavonoid content of soybean sprouts under UV-B radiation, while 3-MP treatment had the opposite effect. Therefore, we can deduce that GABA is engaged in the growth metabolism and production of isoflavones in soybean sprouts exposed to UV-B radiation. This study showed that under UV-B radiation, The application of GABA increased sprout length and biomass, decreased MDA content (Fig. 1) and reduced H2O2 and O2˙− fluorescence intensity (Fig. 3) in germinating soybean. The addition of the GABA synthesis inhibitor 3-MP made things worse. These findings imply that GABA eased the inhibitory effect of UV-B radiation on sprouting soybean growth and biomass, which is consistent with the findings that exogenous GABA regulates the growth and development of germinating soybean under salt stress.22 Moreover, GABA modulates the growth and development of barley shoot seedlings under stress, according to Ma et al.,36 and they argued that GABA treatment in the root tip cells of barley seedlings alleviates stress by causing a substantial and persistent net Ca2+ influx into the root tip cells of barley seedlings, which is similar with the root tip free calcium results in this experiment (Fig. 3III).
In the present study, the intracellular free calcium and Ca2+ migration fluorescence intensity was the strongest in GABA plus UV-B treatment compared to CK and UV-B treatments (Fig. 3III). Ca2+, as an essential nutrient, is not only involved in regulating physiological metabolic processes during plant growth and development, but it also acts as a second messenger in plant signal transduction, coupling extracellular signals with intracellular physiological responses, while it is also involved in the response of higher plants to abiotic stresses. Under abiotic stress, the antioxidant enzyme activities of various plants such as broccoli37 and soybean38 were significantly increased by exogenous calcium treatment, while the antioxidant system was weakened in calcium-deficient plants, which is consistent with the findings of this paper that the addition of exogenous GABA increased the content of endogenous Ca2+ and thus enhanced the antioxidant enzyme activities. In addition, several studies39 have shown that the increase in Ca2+ level facilitates the accumulation of secondary metabolites in higher plants, which is consistent with the increased total flavonoid content in the present study. In future studies, we will measure changes in endogenous Ca2+ levels and distribution to further explore the association between GABA and Ca2+ and the possible role on antioxidant systems and total flavonoid accumulation.
This study also investigated the effect of GABA on the activity of some antioxidant enzymes (POD, CAT) and the expression of antioxidant enzyme genes (POD, CAT, SOD, APX). In agreement with Rezaei et al.,40 exogenous GABA significantly increased the CAT activity of black cumin under water deficit stress and alleviated the stress to some extent. And interestingly, trends in antioxidant enzyme activity and related gene expression were generally consistent with the total flavonoid content. As soybean sprouts acquire substantial amounts of phenolic compounds to protect themselves from oxidative damage produced by high levels of reactive oxygen species (ROS),18 we can expect that the antioxidant capacity of UV-B treated soybean sprouts is positively linked with total flavonoid content.
PAL and C4H are two enzymes required for the metabolic core reactions of isoflavone biosynthesis.41 The activities of PAL and C4H in soybean sprouts under UV-B radiation were significantly increased by GABA treatment, whereas 3-MP markedly inhibited their activities, suggesting that GABA participates in the regulation of key enzyme activities for isoflavones in soybean sprouts under UV-B radiation. Furthermore, in terms of isoflavone metabolism-related genes' relative expression, the relative expression of PAL1, 4CL, IFR, CHR, CHS and C4H involved in the flavonoid biosynthesis pathway were significantly up-regulated during seed germination under UV-B radiation compared to the CK, which is consistent with previous findings. Wei42 suggested that UV-B radiation boosted the formation of CHS, F4H, FLS in the phenolic acid, flavonoid and anthocyanin biosynthetic pathways. The formation of transcripts for enzymes such as CHS, F4H and FLS in the phenolic acid, flavonoid and anthocyanin biochemical activities were stimulated by UV-B radiation. The relative expression of PAL1, 4CL, CHS and C4H was significantly up-regulated by the addition of GABA compared to the CK, while the addition of GABA inhibitors had the opposite effect. In this study, GABA plus UV-B treatment significantly induced a net inward flow of Ca2+ in soybean root cells (Fig. 3), resulting in a significant increase in CHS expression. This is consistent with the study that increased Ca2+ in Arabidopsis cell culture under UV-B treatment increased CHS expression.43 Meanwhile, the addition of GABA under UV-B treatment resulted in the highest relative expression of all genes, and the relative expression levels of these genes were consistent with the total flavonoid content. These results suggest that GABA promotes isoflavone biosynthesis by up-regulating the expression of these genes, which is consistent with previous findings.
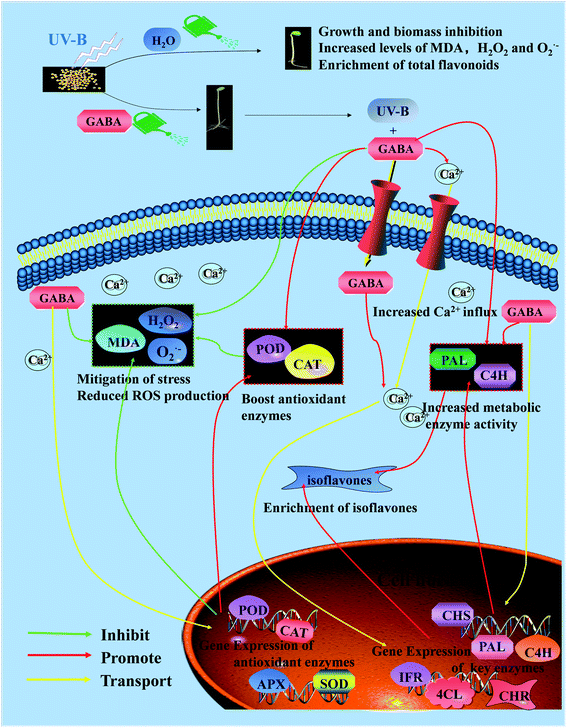
As a result, we can identify GABA as the signaling molecule that mediates the synthesis of isoflavone in the presence of UV-B. Exogenous GABA enhanced endogenous GABA signaling,44 which stimulated the activity of key enzymes engaged in isoflavone synthesis in soybean sprouts (Fig. 5) and promoted isoflavone synthesis. GABA was signaling positively regulated the synthesis of isoflavone in soybean sprouts during UV-B exposure. GABA metabolism is required for isoflavone production in soybean sprouts, and exogenous GABA increases isoflavone levels by increasing the activity and gene expression of critical phenylpropane pathway enzymes.45,46 Based on these results, we obtained a schematic diagram of the molecular mechanism of a hypothetical GABA as a signaling molecule to stimulate isoflavone synthesis in soybean during germination under UV-B radiation (Fig. 8).
5. Conclusion
This study show that soybean germinating under GABA combined with UV-B treatment would be an effective way to accumulate total flavonoids. The application of exogenous GABA increased the total flavonoids content in 2- and 4 day-old soybean sprouts under UV-B stress, with 80.7% and 46.1% increase compared to CK treatment, respectively.GABA (5 mM) could further increase the growth of soybean sprouts under UV-B radiation by increasing the activity of antioxidant enzymes, minimizing the formation of reactive oxygen species and maintaining membrane integrity during soybean germination. Meanwhile, the application of exogenous GABA under UV-B stress induced total flavonoids accumulation by increasing activity and relative gene expressions of the flavonoid biosynthesis-relate enzymes. These findings add to our new knowledge of how isoflavones accumulate in soybean sprouts when exposed to UV-B radiation and will allow us to better understand the processes of isoflavones accumulation in soybean seedlings under abiotic stress, paving the way for further studies on their mechanisms of action. In addition, this study will also provide technical support for the industrial production of isoflavone-rich soybean sprouts.
Author contributions
Minglang Gu, Xin Tian and Yongqi Yin are responsible for experimental related work, writing – original manuscript preparation, and writing – review and editing preparation. Jia Yang, Jinpeng Xu and Weiming Fang are responsible for methodological investigation and analysis of results.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by China Postdoctoral Science Foundation funded project (2019M651978) and Innovation & Entrepreneurship Training Program for College Students of Yangzhou University (X20210893).References
- Y. Q. Hu, Z. Q. Sheng, J. Y. Liu, L. Qi, S. Qiang, X. L. Song and L. Biao, Sexual compatibility of transgenic soybean and different wild soybean populations, J. Integr. Agric., 2022, 21, 36–48 CrossRef
.
- S. G. Jo, Y. I. Kang, K. S. Om, Y. H. Cha and S. Y. Ri, Growth, photosynthesis and yield of soybean in ridge-furrow intercropping system of soybean and flax, Field Crops Res., 2022, 275, 108329 CrossRef
.
- Y. H. Chan, K. K. Lau, K. H. Yiu, S. W. Li, H. T. Chan, S. Tam, X. O. Shu, C. P. Lau and H. F. Tse, Isoflavone intake in persons at high risk of cardiovascular events: implications for vascular endothelial function and the carotid atherosclerotic burden, Am. J. Clin. Nutr., 2007, 86, 938–945 CrossRef CAS PubMed
.
- P. S. Williamson-Hughes, B. D. Flickinger, M. J. Messina and M. W. Empie, Isoflavone supplements containing predominantly genistein reduce hot flash symptoms: a critical review of published studies, Menopause, 2006, 13, 831–839 CrossRef PubMed
.
- X. Dong, W. Xu, R. A. Sikes and C. Wu, Combination of low dose of genistein and daidzein has synergistic preventive effects on isogenic human prostate cancer cells when compared with individual soy isoflavone, Food Chem., 2013, 141, 1923–1933 CrossRef CAS PubMed
.
- P. Joshi and K. Varma, Effect of germination and dehulling on the nutritive value of soybean, Nutr. Food Sci., 2016, 46, 595–603 CrossRef
.
- S. Kumari and S. K. Chang, Effect of cooking on isoflavones, phenolic acids, and antioxidant activity in sprouts of prosoy soybean (Glycine max L.), J. Food Sci., 2016, 81, 1679–1691 CrossRef PubMed
.
- M. Schreiner, I. Mewis, S. Huyskens-Keil, M. A. K. Jansen, R. Zrenner, J. B. Winkler, N. O'Brien and A. Krumbein, UV-B-induced secondary plant metabolites-potential benefits for plant and human health, CRC Crit. Rev. Plant Sci., 2012, 31, 229–240 CrossRef CAS
.
- Apoorva, D. Jaiswal, S. Pandey-Rai and S. B. Agrawal, Untangling the UV-B radiation-induced transcriptional network regulating plant morphogenesis and secondary metabolite production, Environ. Exp. Bot., 2021, 192 DOI:10.1016/j.envexpbot.2021.104655
.
- H. J. Xue and M. Yue, Effects of enhanced UV-B radiation on terrestrial plant secondary metabolite, Acta Bot., 2004, 24, 1131–1137 CAS
.
- V. K. Gupta, I. Ali, T. A. Saleh, A. Nayak and S. Agarwal, Chemical treatment technologies for waste-water recycling—an overview, RSC Adv., 2012, 2, 6380–6388 RSC
.
- S. Ullah, S. U. Khan, T. A. Saleh and S. Fahad, Mad honey: uses, intoxicating/poisoning effects, diagnosis, and treatment, RSC Adv., 2018, 8, 18635–18646 RSC
.
- J. Jiao, Q. Y. Gai, W. Wang, M. Luo, C. B. Gu, Y. J. Fu and W. Ma, Ultraviolet radiation-elicited enhancement of isoflavonoid accumulation, biosynthetic gene expression, and antioxidant activity in Astragalus membranaceus hairy root cultures, J. Agric. Food Chem., 2015, 63, 8216–8224 CrossRef CAS PubMed
.
- M. Kaducová, A. Eliašová, K. Trush, M. Bačovčinová, K. Sklenková and P. Pal’ove-Balang, Accumulation of isoflavonoids in Lotus corniculatus after UV-B irradiation, Theor. Exp. Plant Physiol., 2021, 49, 1–10 Search PubMed
.
- R. Soury, M. Jabli, T. A. Saleh, W. S. Abdul-Hassan, E. Saint-Aman, F. Loiseau, C. Philouze and H. Nasri, Tetrakis(ethyl-4(4-butyryl)oxyphenyl) porphyrinato zinc complexes with 4,4′-bpyridin: synthesis, characterization, and its catalytic degradation of Calmagite, RSC Adv., 2018, 8, 20143–20156 RSC
.
- D. G. Song, A. Tariq, K. W. Pan, S. U. Khan, T. A. Saleh, S. X. Gong, A. P. Zhang and X. G. Wu, Influence of planting distance and density on the yield and photosynthetic traits of sweet potato (Ipomoea balatas L.) under an intercropping system with walnut (Juglans regia) saplings, Soil Tillage Res., 2020, 196, 104484 CrossRef
.
- T. A. Saleh, Protocols for synthesis of nanomaterials, polymers, and green materials as adsorbents for water treatment technologies, Environ. Technol. Innovation, 2021, 24, 101821 CrossRef CAS
.
- E. Hideg, M. Jansen and A. Strid, UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates?, Trends Plant Sci., 2013, 18, 107–115 CrossRef CAS PubMed
.
- D. Majumdar and S. Guha, Conformation, electrostatic potential and pharmacophoric pattern of GABA (gamma-aminobutyric acid) and several GABA inhibitors, J. Mol. Struct., 1988, 180, 125–140 CrossRef
.
- A. M. Kinnersley and F. J. Turano, Gamma Aminobutyric Acid (GABA) and Plant Responses to Stress, CRC Crit. Rev. Plant Sci., 2000, 19, 479–509 CrossRef CAS
.
- C. Jiao, γ-Aminobutyric acid boosts chilling tolerance by promoting the methionine sulfoxide reductase-thioredoxin reductase system in peach fruit, Hortic., Environ. Biotechnol., 2022, 23, 1–9 Search PubMed
.
- Y. Zhao, C. Xie, P. Wang, Z. Gu and R. Yang, GABA Regulates Phenolics Accumulation in Soybean Sprouts under NaCl Stress, Antioxidants, 2021, 10(6), 990 CrossRef CAS PubMed
.
- Y. Ma, P. Wang, M. Wang, M. Sun, Z. Gu and R. Yang, GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress, Food Chem., 2019, 270, 593–601 CrossRef CAS PubMed
.
- K. Vijayakumari, K. C. Jisha and J. T. Puthur, GABA/BABA priming: a means for enhancing abiotic stress tolerance potential of plants with less energy investments on defence cache, Acta Physiol. Plant., 2016, 38(9), 230 CrossRef
.
- Y. Ma, P. Wang, Z. Chen, Z. Gu and R. Yang, GABA enhances physio-biochemical metabolism and antioxidant capacity of germinated hulless barley under NaCl stress, J. Plant Physiol., 2018, 231, 192–201 CrossRef CAS
.
- C. Xie, P. Wang, M. Sun, Z. Gu and R. Yang, Nitric oxide mediates γ-aminobutyric acid signaling to regulate phenolic compounds biosynthesis in soybean sprouts under NaCl stress, Food Biosci., 2021, 44, 101–113 Search PubMed
.
- L. Zhuang, K. Xu, Y. Zhu, F. Wang and L. Guo, Calcium affects glucoraphanin metabolism in broccoli sprouts under ZnSO4 stress, Food Chem., 2020, 334 DOI:10.1016/j.foodchem.2020.127520
.
- A. Djeridane, M. Yousfi, B. Nadjemi, D. Boutassouna, P. Stocker and N. Vidal, Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds, Food Chem., 2006, 97, 654–660 CrossRef CAS
.
- C. Cheng, Y. Liu, W. Fang, J. Tao, Z. Yang and Y. Yin, iTRAQ-based proteomic and physiological analyses of mustard sprouts in response to heat stress, RSC Adv., 2020, 10, 6052–6062 RSC
.
- M. Zhang, S. He, B. Qin, X. Jin, M. Wang, C. Ren, L. Cao and Y. Zhang, Exogenous melatonin reduces the inhibitory effect of osmotic stress on antioxidant properties and cell ultrastructure at germination stage of soybean, PLoS One, 2020, 15 DOI:10.1371/journal.pone.0243537
.
- Y. S. Wang, S. P. Tian and Y. Xu, Effects of high oxygen concentration on pro-and anti-oxidant enzymes in peach fruits during postharvest periods, Food Chem., 2005, 91, 99–104 CrossRef CAS
.
- J. S. Assis, R. Maldonado, T. Muoz, M. I. Escribano and C. Merodio, Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit, Postharvest Biol. Technol., 2001, 23, 33–39 CrossRef CAS
.
- C. J. Lamb and P. H. Rubery, A spectrophotometric assay for trans-cinnamic acid 4-hydroxylase activity, Anal. Biochem., 1975, 68, 554–561 CrossRef CAS PubMed
.
- K. J. Livak and T. D. Schmittgen, Analysis of Relative Gene Expression Data using Real-Time Quantitative PCR, Methods, 2002, 25, 402–408 CrossRef PubMed
.
- S. Q. Shi, Z. Shi, Z. P. Jiang, Q. I. Li-Wang, X. M. Sun, L. I. Chun-Xiu, J. F. Liu, W. F. Xiao and S. G. Zhang, Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: regulatory roles for H2O2 and ethylene production, Plant, Cell Environ., 2010, 33, 149–162 CrossRef CAS PubMed
.
- Y. Ma, P. Wang, Z. Gu, Y. Tao, C. Shen, Y. Zhou, Y. Han and R. Yang, Ca2+ involved in GABA signal transduction for phenolics accumulation in germinated hulless barley under NaCl stress, Food Chem.: X, 2019, 2, 100023 CAS
.
- V. Ling, W. A. Snedden, B. J. Shelp and S. M. Assmann, Analysis of a soluble calmodulin binding protein from fava bean roots: identification of glutamate decarboxylase as a calmodulin-activated enzyme, Plant Cell, 1994, 6, 1135–1143 CAS
.
- W. A. Snedden, T. Arazi, H. Fromm and B. J. Shelp, Calcium/calmodulin activation of soybean glutamate decarboxylase, Plant Physiol., 1995, 108, 543–549 CrossRef CAS PubMed
.
- V. Martins, M. Unlubayir, A. Teixeira, H. Gerós and A. Lanoue, Calcium and methyl jasmonate cross-talk in the secondary metabolism of grape cells, Plant Physiol. Biochem., 2021, 165, 228–238 CrossRef CAS PubMed
.
- E. Rezaei-Chiyaneh, S. M. Seyyedi, E. Ebrahimian, S. S. Moghaddam and C. A. Damalas, Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa L.), Ind. Crops Prod., 2018, 112, 741–748 CrossRef CAS
.
- E. W. Ward, D. M. Cahill and M. K. Bhattacharyya, Abscisic acid suppression of phenylalanine ammonia-lyase activity and mRNA, and resistance of soybeans to Phytophthora megasperma f. sp. glycinea, Plant Physiol., 1989, 91, 23–27 CrossRef CAS PubMed
.
- Z. Wei, C. Li, T. Gao, Z. Zhang, B. Liang, Z. Lv, Y. Zou and F. Ma, Melatonin increases the performance of Malus hupehensis after UV-B exposure, Plant Physiol. Biochem., 2019, 139, 630–641 CrossRef CAS PubMed
.
- H. Frohnmeyer, L. Loyall, M. R. Blatt and A. Grabov, Millisecond UV-B irradiation evokes prolonged elevation of cytosolic-free Ca2+ and stimulates gene expression in transgenic parsley cell cultures, Plant J., 1999, 20, 109–117 CrossRef CAS PubMed
.
- F. Ö. Çekiç, Exogenous GABA stimulates endogenous GABA and phenolic acid contents in tomato plants under salt stress, CBUJOS, 2018, 14, 61–64 Search PubMed
.
- D. H. Cho and S. T. Lim, Changes in phenolic acid composition and associated enzyme activity in shoot and kernel fractions of brown rice during germination, Food Chem., 2018, 256, 163–170 CrossRef CAS PubMed
.
- D. Ma, Y. Li, J. Zhang, C. Wang, H. Qin, H. Ding, Y. Xie and T. Guo, Accumulation of phenolic compounds and expression profiles of phenolic acid biosynthesis-related genes in developing grains of white, purple, and red wheat, Front. Plant Sci., 2016, 7, 528 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00523a |
| This journal is © The Royal Society of Chemistry 2022 |