Open Access Article
Open Access ArticleNMR analysis of the enantiomeric purity of chiral diols by a new chiral boron agent†
Xuebo Zhangab,
Jing Xuab,
Zhaofeng Sunb,
Guangling Bian *b and
Ling Song
*b and
Ling Song *b
*b
aCollege of Chemistry and Materials Science, Fujian Normal University, Fuzhou, China
bThe Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian, China. E-mail: glb@fjirsm.ac.cn; songling@fjirsm.ac.cn
First published on 8th February 2022
Abstract
A new boric agent with bridged structure, boric acid D, was first synthesized and used as an excellent chiral derivative agent for highly efficient enantiodiscrimination of various diols. The derivatization reaction is fast and complete, easy to operate and has high accuracy in measurement of ee values. The characteristic split NMR signals are well-distinguishable with a large chemical shift nonequivalence (up to 0.39 ppm).
Introduction
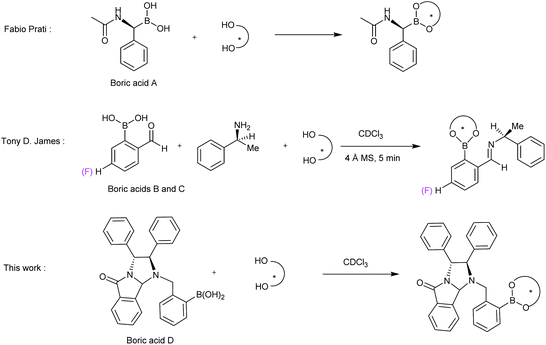
Chiral compounds have been widely used in many fields, such as pharmacy, chemistry, biology and so on.1,2 Since their activities are highly dependent on their optical purity, it is very important to develop accurate, rapid, and convenient methods for the determination of enantiomeric purity of chiral compounds. Various methods, including HPLC,3 GC,4 MS,5 NMR spectroscopy,6–9 CD,10 X-ray crystallography11 and fluorescence spectroscopy,12 have been developed to discriminate enantiomers. Among them, NMR methods have been of wide interest because of their advantages of low requirements for samples, fast and accurate measurement and no need for standard curves.Chiral diols are very important chiral inducers and organic synthons.13 So far, only a few NMR methods,14 including chiral solvating agents (CSAs)15–18 and chiral derivatizing agents (CDAs),19,20 have been reported for the chiral recognition of diols, but the obtained chemical shift nonequivalent (ΔΔδ) values are small in general. Among, chiral boric acids are potential excellent chiral recognition agents for diols, because diols are very easy to be derivatized by boric acids to generate stable cyclic ester compounds, resulting in a relatively large ΔΔδ values.21 In 1988, Snyder et al. developed camphanylboronic acid as a simple, effective CDA for the optical purity determination of 1,2-diols by 13C NMR spectroscopy.22 In 2003, Prati Fabio et al. developed (S)-(+)-N-acetylphenylglycineboronic acid as a CDA for enantiomeric excess (ee) determination of 1,2-diols by 1H NMR (Fig. 1, boric acid A). The derivatized cyclic boric acid ester shows high diastereomeric proton NMR signal.20 In 2006, Tony D. James group reported a three-component recognition system for measuring excess enantiomers of chiral diols (Fig. 1, boric acid B).23 Chiral diols were mixed with 2-formylphenylboronic acid and alpha-methylbenzylamine to form phenylborate ester imines, and then the split signals of the imines were observed for determination of ee of diols. In 2009, they developed another three-component chiral derivatization scheme to accurately determine ee of chiral diols by 19F NMR spectroscopy (Fig. 1, boric acid C).24 1,2-Diphenylethylenediamine is a very important chiral skeleton. In the past, we have developed several agents based on 1,2-diphenylethylenediamine to recognize chiral carboxylic acids, amino acids, amines, alcohols and other compounds with great success.25–28 Based on our experience and inspired by the above chiral boric acid agents, herein, a new boric acid derived from chiral 1,2-diphenylethylenediamine was developed for measuring ee of chiral diols (Fig. 1, boric acid D). This boric acid is simple in synthesis and highly effective and practical for enantiodiscrimination of 1,2- and 1,3-diols.
Results and discussion
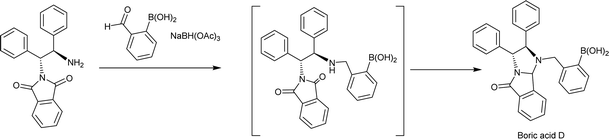
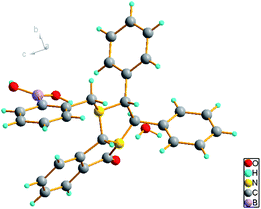
First, boric acid D was synthesized from (R,R)-N-phthalimide-1,2-diphenylethylamine and 2-formylphenyl-boronic acid by one-step reductive amination (Scheme 1). Interestingly, the reductive amination did not stop at the stage of the secondary amine and further intermolecular reaction finally produced a new product with bridged structure in 50% yield, and its structure was confirmed by X-rays diffraction analysis (Fig. 2, CCDC: 2061749†).With boric acid D in hand, we tested its capability as an NMR derivatization agent for chiral discrimination of diols. We first dissolved 10 mmol racemic hydrobenzoin (1) and 30 mmol of boric acid D in 0.6 mL of CDCl3 in an NMR tube. The mixture was mixed for 15 min at 25 °C by ultrasound and then 1H NMR data were collected on a Bruker Avance 400 MHz spectrometer (Fig. 3A). To our delight, the HA signals of boric acid D in the formed two diastereomeric derivatives had a very clear baseline separation with a large ΔΔδ value (0.39 ppm) (Fig. 3B). Moreover, the signal of HA is in the middle of the field, and it is hardly overlapped with other signals and easy to be distinguished, so it is a very practical characteristic peak for chiral analysis. Another, the development of CDAs requires that CDA should be stable enough, and the substrate and derivative cannot be racemized during the derivatization process. To verify whether boric acid D is suitable as a CDA for the determination of the diol's ee, the following experiment was carried out: the reaction of optically pure (R,R)-1 and boric acid D at room temperature in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was continuously monitored by 1H NMR for 12 hours. It was found that the derivatization was completed within 15 min (the benzyl hydrogen or hydroxyl hydrogen NMR signal of free (R,R)-1 disappeared) and no racemization was observed during the whole process (Fig. S5, ESI†). Therefore, boric acid D can be used as a CDA. Subsequently, we explored the effects of other factors on the chiral discrimination ability of boric acid D (Table 1). Decreasing the molar ratio of boric acid D/1 slowed down the reaction (Table 1, entries 1–3). While the molar ratio was 1
3 was continuously monitored by 1H NMR for 12 hours. It was found that the derivatization was completed within 15 min (the benzyl hydrogen or hydroxyl hydrogen NMR signal of free (R,R)-1 disappeared) and no racemization was observed during the whole process (Fig. S5, ESI†). Therefore, boric acid D can be used as a CDA. Subsequently, we explored the effects of other factors on the chiral discrimination ability of boric acid D (Table 1). Decreasing the molar ratio of boric acid D/1 slowed down the reaction (Table 1, entries 1–3). While the molar ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, diol 1 could not react completely. When the reaction temperature was increased to 35 °C, the reaction time was slightly shortened to 12 min. However, lowering the reaction temperature, the reaction rate decreased dramatically, and the reaction time increased to 30 min when the reaction temperature dropped to 0 °C (Table 1, entries 1, 4 and 5). In addition, the reaction solvent was optimized. CD3CN and toluene-d8 showed poor solubility for boric acid D. Although DMSO had good solubility for boric acid D, but the derivatization reaction could not proceed. Compared with them, CDCl3 is the most suitable solvent (Table 1, entries 1 and 6–8). Therefore, in order to complete the derivatization reaction quickly and conveniently, the following best derivatization conditions were adopted: 3
1, diol 1 could not react completely. When the reaction temperature was increased to 35 °C, the reaction time was slightly shortened to 12 min. However, lowering the reaction temperature, the reaction rate decreased dramatically, and the reaction time increased to 30 min when the reaction temperature dropped to 0 °C (Table 1, entries 1, 4 and 5). In addition, the reaction solvent was optimized. CD3CN and toluene-d8 showed poor solubility for boric acid D. Although DMSO had good solubility for boric acid D, but the derivatization reaction could not proceed. Compared with them, CDCl3 is the most suitable solvent (Table 1, entries 1 and 6–8). Therefore, in order to complete the derivatization reaction quickly and conveniently, the following best derivatization conditions were adopted: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of boric acid D/substrates, CDCl3 as solvent, reaction at 25 °C.
1 molar ratio of boric acid D/substrates, CDCl3 as solvent, reaction at 25 °C.
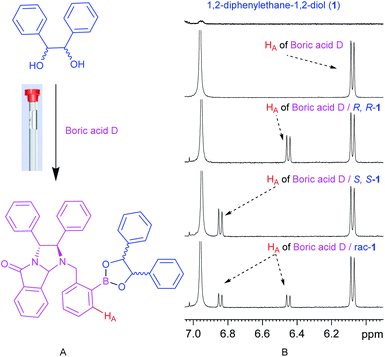 | ||
| Fig. 3 (A) The structures of rac-1,2-diphenylethane-1,2-diols (1) and boric acid D/rac-1. (B) 1H NMR spectra of 1, boric acid D, boric acid D/R-1, boric acid D/S-1 and boric acid D/rac-1 in CDCl3. | ||
| Entry | Solvent | Molar ratio (boric acid D/1) | T (°C) | t b(min) |
|---|---|---|---|---|
| a Boric acid D and 1 (10 mM) were mixed in the specified solvent (0.6 mL) and 1H NMR data were collected on a Bruker Advance 400 MHz spectrometer.b Time from the beginning of the reaction to the disappearance of the benzyl hydrogen or hydroxyl hydrogen NMR signal of free 1.c Diol 1 cannot react completely after 12 hours. | ||||
| 1 | CDCl3 | 3/1 | 25 | 15 |
| 2 | CDCl3 | 2/1 | 25 | 25 |
| 3 | CDCl3 | 1/1 | 25 | —c |
| 4 | CDCl3 | 3/1 | 35 | 12 |
| 5 | CDCl3 | 3/1 | 0 | 30 |
| 6 | CD3CN | 3/1 | 25 | — |
| 7 | Toluene-d8 | 3/1 | 25 | — |
| 8 | DMSO | 3/1 | 25 | — |
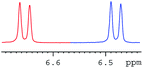
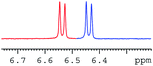
In order to study the scope and limitations of boric acid D, we investigated the derivatization reactions of 12 racemic chiral diols with different structural types including alkyl, aryl, large sterically hindered and cyclic alkanes (Table 2). All the substrates were measured under the optimal conditions, and clear baseline separations were obtained (ΔΔδ = 0.06–0.39 ppm). In entries 1–5 and 7–8, the configurations were determined by comparing with spectra of nonracemic samples of known configurations. The other five diols have no chiral samples for comparison, so the corresponding configuration was not determined. Diol 6 exists in the form of three stereoisomers, including a pair of chiral enantiomers and meso isomers. They could be well separated after derivatization with boric acid D (entry 6, the peak of 6.6 ppm represents meso isomers). The results showed that diols containing large hindered groups or aromatic groups could produce the larger ΔΔδ values. Derivatives of diols containing primary alcohols had relatively small ΔΔδ values, and ΔΔδ values of 1,3-diol derivatives were smaller than those of 1,2-diol derivatives. The proposed reason is that the substrates with large steric hindrance and aromatic groups generate more rigidity difference between the epimers (a pair of derivatives), which makes the chemical environments of HA more different resulting in larger ΔΔδ values of HA.
| Entry | Chiral diols | Spectrab | ΔΔδc (ppm) |
|---|---|---|---|
| a All samples were prepared by mixing boric acid D and chiral diols in NMR tubes (10 mM of the chiral diols and 30 mM of boric acid D in 0.6 mL of CDCl3). 1H NMR were collected on a Bruker Avance 400 MHz. Spectrometer at 25 °C.b The configurations were determined by comparing with spectra of nonracemic sample of known configurations; the red and blue lines represent the S and R enantiomers of analytes, respectively (entry 1 red is (S,S) and blue is (R,R), entry 8 red is (−) and blue is (+)).c ΔΔδ values of the proton HA of the derivatives. | |||
| 1 |  |
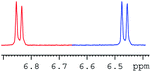 |
0.39 |
| 2 |  |
 |
0.18 |
| 3 | 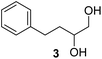 |
 |
0.10 |
| 4 | 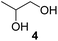 |
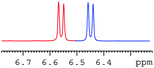 |
0.11 |
| 5 | 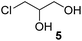 |
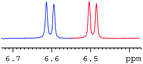 |
0.11 |
| 6 | 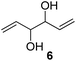 |
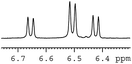 |
0.23 |
| 7 |  |
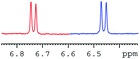 |
0.27 |
| 8 |  |
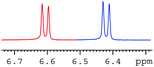 |
0.18 |
| 9 |  |
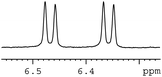 |
0.11 |
| 10 |  |
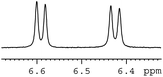 |
0.17 |
| 11 |  |
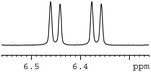 |
0.08 |
| 12 |  |
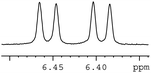 |
0.06 |
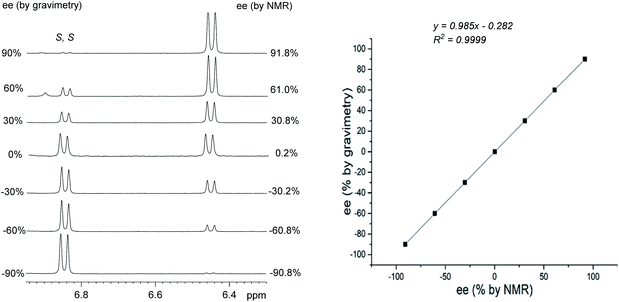
In order to further prove the practicality of boric acid D as a CDA for determination of ee, the substrate 1 with different ee values was determined by integration of HA signal of derivative. Fig. 4 shows that boric acid D maintains analytic resolution of 1 over a wide range of ee values. The linear relationship between gravimetry determined values and those NMR-determined values is good with R2 = 0.9999. The absolute errors are within 2% in the ee measurements between NMR integrations and the gravity calibration.
Experimental
General
All commercially available compounds were used without further purification. Melting point was obtained on an SGW-4 micro melting point apparatus. Optical rotations were measured on SGW-1 automatic polarimeter. Boric acid D and diol were mixed by a 200 W KQ5200DE ultrasound. 1H and 13C NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer using TMS (δH or δC = 0) or CDCl3 (δH = 7.26, δC = 77.16) as internal standards.The preparation procedure of boric acid D
(R,R)-N-Phthalimide-1,2-diphenylethylamine (1 g, 2.92 mmol), 2-formylphenylboronic acid (526 mg, 3.51 mmol) anhydrous sodium sulfate (3 g, 20.46 mmol) and dichloromethane (25 mL) were added to around-bottom flask. And then being stirred for 3 h at room temperature under the protection of nitrogen atmosphere, sodium triacetoxyborohydride (1.859 g, 8.77 mmol) was added in multiple batches. The mixture was further stirred for 8 h at room temperature. The reaction solution was added to water and the organic layer was separated. The aqueous layer was extracted three times with chloroform. The organic layers were combined, dried with anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluting with dichloromethane/acetic acid = 100/5) to give 0.672 g boric acid D, a colorless solid, yield 50%. The boric acid D was further recrystallized in ethyl acetate to obtain colorless crystals for single crystal analysis. Mp 217–218 °C. [α]22D = +50.0 (c = 0.1 in CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 7.6 Hz, 1H, ArH), 7.67–7.65 (m, 1H, ArH), 7.51 (t, J = 7.3 Hz, 1H, ArH), 7.42–7.38 (m, 1H, ArH), 7.36–7.19 (m, 13H, ArH), 7.02 (s, 2H, OH) 6.05 (d, J = 7.7 Hz, 1H, HA), 5.47 (s, 1H, HC) 5.34 (d, J = 8.0 Hz, 1H, HE), 4.14 (d, J = 8.0 Hz, 1H, HD), 4.05 (q, J = 12.6 Hz, 2H, HB) (Fig. S4, ESI†). 13C NMR (100 MHz, CDCl3): δ 171.9, 142.3, 140.2, 139.8, 136.5, 136.4, 133.1, 132.2, 130.8, 130.7, 130.1, 129.0, 128.8, 128.7, 128.1, 127.8, 126.7, 125.8, 125.0, 124.3, 84.2, 82.7, 64.4, 60.5. HRMS(ESI) m/z calcd for C29H26BN2O3[M + H]+: 461.2036, found 461.2038.General methods for the chiral discrimination of diols by boric acid D
10 mmol of the diols and 30 mmol of boric acid D and 0.6 mL of CDCl3 were added to the NMR tube and the mixture was mixed for 15 min at 25 °C by ultrasound. The 1H NMR data were collected on a Bruker Avance 400 MHz spectrometer.Conclusions
A boric acid bearing a novel bridged structure has been synthesized to serve as an excellent CDA for enantiodiscrimination of various diols. The aryl hydrogen signal of CDA can be used as the characteristic signal for ee determination, yielding a large split value up to 0.39 ppm that is very easily discernible. The derivatization reaction is thorough, fast, easy to operate, and the process does not undergo racemization. The measurement of ee value is accurate. So, it is especially suitable for enantiodiscrimination and ee determination of chiral diols.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial supports from The Key Laboratory of Coal to Ethylene Glycol and its Related Technology, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences.Notes and references
- N. M. Maier, P. Franco and W. Lindner, J. Chromatogr. A, 2001, 906, 3–33 CrossRef CAS PubMed.
- R. Xie, L.-Y. Chu and J.-G. Deng, Chem. Soc. Rev., 2008, 37, 1243–1263 RSC.
- C. Payne and S. R. Kass, ChemistrySelect, 2020, 5, 1810–1817 CrossRef CAS.
- V. Schurig and H. P. Nowotny, Angew. Chem., Int. Ed., 1990, 29, 939–957 CrossRef.
- L. Wang, Z. Jin, X. Wang, S. Zeng, C. Sun and Y. Pan, Anal. Chem., 2017, 89, 11902–11907 CrossRef CAS PubMed.
- D. Parker, Chem. Rev., 1991, 91, 1441–1457 CrossRef CAS.
- G. Uccello-Barretta and F. Balzano, in Differentiation of Enantiomers II, ed. V. Schurig, Springer International Publishing, Cham, 2013, pp. 69–131 Search PubMed.
- H. Zhang, H. Zhao, J. Wen, Z. Zhang, P. Stavropoulos, Y. Li, L. Ai and J. Zhang, Org. Biomol. Chem., 2021, 19, 6697–6706 RSC.
- T. J. Wenzel and C. D. Chisholm, Prog. Nucl. Magn. Reson. Spectrosc., 2011, 59, 1–63 CrossRef CAS PubMed.
- F. Riobe, A. P. H. J. Schenning and D. B. Amabilino, Org. Biomol. Chem., 2012, 10, 9152–9157 RSC.
- C. Gropp, T. Husch, N. Trapp, M. Reiher and F. Diederich, Angew. Chem., Int. Ed., 2018, 57, 16296–16301 CrossRef CAS PubMed.
- A. Akdeniz, T. Minami, S. Watanabe, M. Yokoyama, T. Ema and P. Anzenbacher, Jr, Chem. Sci., 2016, 7, 2016–2022 RSC.
- K. C. Bhowmick and N. N. Joshi, Tetrahedron: Asymmetry, 2006, 17, 1901–1929 CrossRef CAS.
- D. A. Tickell, E. V. Lampard, J. P. Lowe, T. D. James and S. D. Bull, J. Org. Chem., 2016, 81, 6795–6799 CrossRef CAS PubMed.
- T. Ema, D. Tanida and T. Sakai, J. Am. Chem. Soc., 2007, 129, 10591–10596 CrossRef CAS PubMed.
- I. Pal, S. R. Chaudhari and N. Suryaprakash, New J. Chem., 2014, 38, 4908–4912 RSC.
- S. Jang and H. Kim, iScience, 2019, 19, 425–435 CrossRef CAS PubMed.
- M. S. Seo, S. Jang and H. Kim, Chem. Commun., 2018, 54, 6804–6807 RSC.
- A. M. Kelly, Y. Perez-Fuertes, J. S. Fossey, S. L. Yeste, S. D. Bull and T. D. James, Nat. Protoc., 2008, 3, 215–219 CrossRef CAS PubMed.
- E. Caselli, C. Danieli, S. Morandi, B. Bonfiglio, A. Forni and F. Prati, Org. Lett., 2003, 5, 4863–4866 CrossRef CAS PubMed.
- S. M. Resnick, D. S. Torok and D. T. Gibson, J. Org. Chem., 1995, 60, 3546–3549 CrossRef CAS.
- M. Tokles and J. K. Snyder, Tetrahedron Lett., 1988, 29, 6063–6065 CrossRef CAS.
- A. M. Kelly, Y. Pérez-Fuertes, S. Arimori, S. D. Bull and T. D. James, Org. Lett., 2006, 8, 1971–1974 CrossRef CAS PubMed.
- S. L. Yeste, M. E. Powell, S. D. Bull and T. D. James, J. Org. Chem., 2009, 74, 427–430 CrossRef CAS PubMed.
- G. Bian, S. Yang, H. Huang, H. Zong, L. Song, H. Fan and X. Sun, Chem. Sci., 2016, 7, 932–938 RSC.
- G. Bian, S. Yang, H. Huang, H. Zong and L. Song, Sens. Actuators, B, 2016, 231, 129–134 CrossRef CAS.
- H. Huang, G. Bian, H. Zong, Y. Wang, S. Yang, H. Yue, L. Song and H. Fan, Org. Lett., 16, 18, 2524–2527 CrossRef PubMed.
- Z. Chen, H. Fan, S. Yang, G. Bian and L. Song, Org. Biomol. Chem., 2018, 16, 6933–6939 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2061749. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d2ra00428c |
| This journal is © The Royal Society of Chemistry 2022 |