Open Access Article
Open Access ArticleImpact of purple sweet potato (Ipomoea batatas L.) polysaccharides on the fecal metabolome in a murine colitis model†
Jian Sunab,
Jun Liu *c,
Ge Renc,
Xiaotong Chenc,
Huahao Caic,
Jinhai Hongc,
Juan Kanc,
Changhai Jin
*c,
Ge Renc,
Xiaotong Chenc,
Huahao Caic,
Jinhai Hongc,
Juan Kanc,
Changhai Jin *c,
Fuxiang Niub and
Wenting Zhangb
*c,
Fuxiang Niub and
Wenting Zhangb
aCollege of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002, Jiangsu, China
bXuzhou Institute of Agricultural Sciences in Jiangsu Xuhuai Area, Xuzhou 221131, Jiangsu, China
cCollege of Food Science and Engineering, Yangzhou University, Yangzhou 225127, Jiangsu, China. E-mail: junliu@yzu.edu.cn; chjin@yzu.edu.cn
First published on 12th April 2022
Abstract
Purple sweet potato polysaccharides (PSPP) play an important role in regulating the gut microbiota, modulating intestinal immunity and ameliorating colonic inflammation. In this study, the impact of two PSPPs (PSWP-I and PSAP-I) on the metabolomic profiling of feces from dextran sulfate sodium (DSS)-induced colitis mice was evaluated by ultra-high performance liquid chromatography coupled with triple time-of-flight tandem mass spectrometry (UPLC-Triple-TOF-MS/MS). Results indicated that there were twenty-five metabolites with significant changes and four remarkable metabolic pathways, i.e., cutin, suberine and wax biosynthesis, biosynthesis of unsaturated fatty acids, fatty acid biosynthesis, and steroid hormone biosynthesis. Two key biomarkers of oleic acid and 17-hydroxyprogesterone were screened that responded to PSPPs in colitis mice. The identified metabolites were correlated with the amelioration of intestinal immune function and the modulation of the gut microbiota. Nine pro-inflammatory and eight anti-inflammatory compounds responded to PSPPs, which were related to Bacteroides, norank_f__Clostridiales_vadinBB60_group, unclassified_o__Bacteroidales, Rikenella and Lachnospiraceae_UCG-001. Moreover, PSWP-I and PSAP-I had different regulating effects on intestinal metabolites. Our results revealed a possible metabolomic mechanism of PSPPs to regulate intestinal inflammation function.
1. Introduction
Ulcerative colitis (UC) is a type of inflammatory bowel disease (IBD). UC is a global chronic inflammatory disease and the prevalence of UC is very high in Europe and North America. However, in the past few decades, an unexpected increase in UC incidence has been observed in Asia and the Middle East, and the exact etiology and pathogenesis of UC are still unknown.1,2 In clinical practice, the drugs 5-aminosalicylic acid, corticosteroids and adalimumab are normally adopted for UC therapy.3 However, these drugs are related to several side effects and can even induce cancer.4 Recently, prebiotics derived from natural and healthy foods (e.g. polysaccharides and oligosaccharides) have attracted increasing attention for the intervention or treatment of UC.Natural polysaccharides have been demonstrated to possess a variety of biological activities. It has been demonstrated that the modulation of intestinal flora and immunological regulation are two most important biological activities of polysaccharides, especially for IBD therapy.5 Polysaccharides can alleviate UC, mainly by improving the structure and composition of intestinal flora, enhancing the immune response of the host, and repairing intestinal barrier function and other pathways.6,7 Notably, the consumption of polysaccharides can alter the metabolism of intestinal flora, producing organic acids, short-chain fatty acids, vitamins, nucleic acids, amino acids, and other metabolites. These metabolites act as connecting messengers between intestinal flora and host by regulating host immunity and maintaining epithelial cell barrier function.8 In a recent study, polysaccharides from the mycelia of Ganoderma lucidum were administered to rats. It was found that the content of 2,2-dimethylsuccinic acid, indolelactate and pyridoxal phosphate in the caecum were correlated with the interleukin (IL)-2, IL-4, and interferon gamma (IFN-γ) concentrations in the serum as well as the secretory immunoglobulin A (SIgA) concentration in the ileum.9 Therefore, metabolites can reflect the relationships between gut microbiota and host immunity. In addition, fecal metabolomics can provide useful information on host metabolism. Thus, fecal metabolomics is often employed to investigate intestinal diseases, such as IBD. However, the impact of polysaccharides on the fecal metabolomics of host with UC has not been thoroughly investigated.
A previous study showed the oral administration of purple sweet potato polysaccharides (PSPP) modulated the gut microbiota of mice with dextran sulfate sodium (DSS)-induced colitis.10 The intestinal microorganisms at the phylum, family, and genus levels were significantly changed by PSPP. For example, the relative abundances of Lachnospiraceae, Lactobacillus, and Bifidobacterium increased, while the relative abundances of Bacteroidetes, Erysipelotrichaceae, Parasutterella, and Desulfovibrio decreased. Moreover, PSPP enhanced host immunity by inhibiting the expression of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), IL-1β, and IL-6 in serum and colon.10 However, the effect of PSPP on the metabolites produced by intestinal microorganisms remains unknown.
In this study, ultra-high performance liquid chromatography coupled with triple time-of-flight tandem mass spectrometry (UPLC-Triple-TOF-MS/MS) was applied to dissect the metabolite profiles, metabolic pathways, and correlations among metabolites, gut microbiota and colon immunological factors, in the feces of mice with DSS-induced colitis. The aim of this study was to evaluate the impact of two PSPPs (PSWP-I, PSAP-I) on the fecal metabolome of mice with colitis. Our previous study had documented that PSWP-I and PSAP-I could modulate inflammatory cytokines and gut microbiota in colitis mice.10,11 Both PSWP-I and PSAP-I were purified homogeneous polysaccharides with the molecular weight of 103 kDa and 180 kDa, respectively, as analyzed by high performance gel permeation chromatography (HPGPC).11,12 However, the action mechanisms of PSWP-I and PSAP-I on metabolomics were unknown. The results provide a potential metabolomic mechanism for the use of PSPPs for the intervention therapy of UC and a theoretical foundation for developing PSPPs as functional ingredients.
2. Materials and methods
2.1. Materials and chemicals
Purple sweet potatoes were provided by the Sweet Potato Research Institute of Chinese Academy of Agricultural Sciences (Xuzhou, China). DSS with molecular weight of 40 kDa was purchased from MP Biomedical Co. (Irvine, CA, USA). Methanol, acetonitrile, formic acid, and 2-propanol were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-1β, IL-6, and IL-10 were purchased from Beyotime Institute of Biotechnology (Shanghai, China). All other chemicals were of analytical grade and were obtained from Sinopharm Chemical Reagent Co. (Shanghai, China).2.2. Isolation and characterization of PSPPs
PSPPs were extracted, purified and characterized according to the previous method.13 Briefly, purple sweet potatoes powder was first defatted and decolorized, and then extracted sequentially with deionized water and 0.5 mol L−1 sodium hydroxide. Two crude polysaccharide extracts were obtained and further purified on DEAE-cellulose 52 and Sephadex G-200 columns. The resultant fractions were collected, concentrated and lyophilized to obtain the purified water-soluble polysaccharide and alkali-soluble polysaccharide, namely PSWP-I and PSAP-I, respectively. Methylation reaction, gas chromatography-mass spectrometer (GC-MS) and nuclear magnetic resonance (NMR) were employed to identify the chemical structure of PSWP-I and PSAP-I. PSWP-I was composed of 1,4-α-D-Glcp, 1,6-α-D-Glcp and 1,2-α-D-Manp backbones with β-D-Glcp side chains substituted at the O-6 position. PSAP-I was composed of 1,4-α-D-Glcp backbone with side chains substituted at the O-6 position, consisting of α-D-Manp, α-D-Rhap, β-L-Araf and β-D-Glcp residues.11,122.3. Animals and treatments
Animal experiments were designed and performed according to a previous method.10 4 week-old ICR mice (average body weight 22 ± 2 g) were purchased from the Institute of Comparative Medicine at Yangzhou University, China. The mice were housed in an environment with a 12 h light–dark cycle at 25 ± 1 °C and relative humidity of 60 ± 5%. The mice were free access to standard feed and drinking water during the study. After 1 week of adaptation, the mice were randomly divided into four groups (six mice per group), namely the normal control (NC) group, DSS model control (DSS) group, DSS + PSWP-I group, and DSS + PSAP-I group. Mice in the NC group drank deionized water, while mice in the DSS, DSS + PSWP-I, and DSS + PSAP-I groups drank 2.5% DSS solution for 7 days. The gavage dose of polysaccharides was 400 mg per kg body weight. Mice feces were collected and stored at −80 °C. After consecutive gavage for 28 days, mice were sacrificed and their colon tissues were quickly collected.2.4. Detection of inflammatory cytokines in colon
The colon tissue (100 mg) was dissected, cut into pieces and homogenized in saline at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9. The homogenate was centrifuged at 12
9. The homogenate was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g and 4 °C for 15 min. The supernatant was collected for determining the contents of TNF-α, IL-1β, IL-6, and IL-10 using ELISA Kits.10
000 × g and 4 °C for 15 min. The supernatant was collected for determining the contents of TNF-α, IL-1β, IL-6, and IL-10 using ELISA Kits.10
2.5. Characterization of gut microbiota
Mice feces (200 mg) were used for DNA extraction and PCR analysis. The concentration and purification of DNA were detected by the NanoDrop 2000 apparatus (Thermo Fisher Scientific, Wilmington, DE, USA). The 338-forward primer (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806-reverse primer (5′-GGACTACHVGGGTWTCTAAT-3′) were used for PCR amplification in the V3–V4 hypervariable regions of bacteria 16S rRNA gene. After PCR products were purified, eluted and tested, Illumina Miseq PE300 platform was employed for analyzing the microbial sequences. The construction of related libraries and high-throughput sequencing were performed by Shanghai Majorbio Bio-Pharm Technology (Shanghai, China).102.6. Analysis of fecal metabolomics
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 15 min, the supernatant was collected in a LC-MS glass vial for analysis.
000 × g for 15 min, the supernatant was collected in a LC-MS glass vial for analysis.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing 0.1% formic acid as mobile phase B, gradient elution at 0.4 mL min−1, injection volume of 10 μL, and column temperature of 40 °C. For mass spectroscopy, the scanning modes of positive ion and negative ion were both adopted for collecting the mass spectrum signals. The ionspray voltage at anode (ESI+) was +5000 V, ionspray voltage at cathode (ESI−) was −4000 V, source temperature was 500 °C, MS/MS collusion energy was 20–60 V, and mass range was 50–1000 m/z.
1) containing 0.1% formic acid as mobile phase B, gradient elution at 0.4 mL min−1, injection volume of 10 μL, and column temperature of 40 °C. For mass spectroscopy, the scanning modes of positive ion and negative ion were both adopted for collecting the mass spectrum signals. The ionspray voltage at anode (ESI+) was +5000 V, ionspray voltage at cathode (ESI−) was −4000 V, source temperature was 500 °C, MS/MS collusion energy was 20–60 V, and mass range was 50–1000 m/z.3. Results and discussion
3.1. Alteration of fecal metabolomic profiles
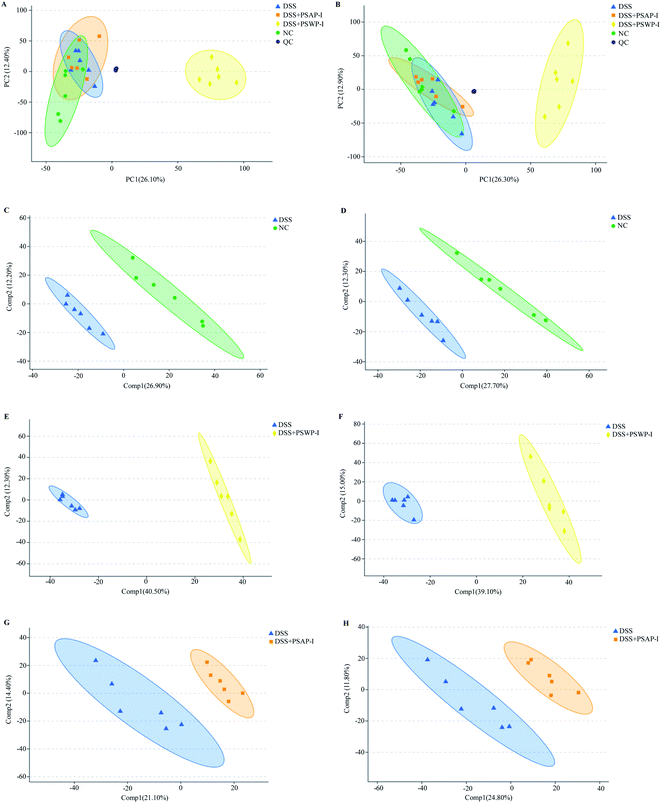
UPLC-Triple-TOF-MS/MS technology was used to analyze mice fecal metabolites. The total ion chromatograms of all fecal samples had good peak shapes and relatively uniform distribution. 9025 and 8344 metabolites were identified in the modes of positive ion and negative ion, respectively (Fig. S1†). Based on the Kyoto encyclopedia of genes and genomes (KEGG) and the human metabolome database (HMDB), all identified metabolites were annotated and classified. In this study, the metabolites in the samples were mainly lipids and amino acids (Fig. S2†).Principal component analysis (PCA) is an unsupervised multivariate statistical analysis that usually shows the overall differences. PCA also highlights outliers and clusters of samples with high similarity. Partial least squares-discriminant analysis (PLS-DA) is a supervised discriminant analysis based on the classic partial least squares regression model. The PLS-DA model is usually used when PCA fails to distinguish the differences between groups of samples. As shown in Fig. 1A and B, in the cation and anion modes, except DSS + PSWP-I group, the differences of metabolomic profiles among NC, DSS and DSS + PSAP-I group in PCA plots were indistinguishable. However, the differences among NC, DSS, DSS + PSWP-I and DSS + PSAP-I group in PLS-DA plots were perfectly displayed (Fig. 1C–H). It was suggested that PLS-DA model was more suitable for identifying the differences between two groups than PCA model. In addition, there were an obvious difference between QC sample and the other treatment groups, indicating that the present results of this experiment were stable and reliable.
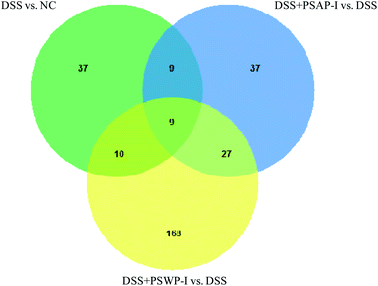
A Venn diagram can be used to visually display the distribution characteristics of metabolites in each metabolite set and specifically clarify the numbers of common and unique metabolites among the metabolite sets. As shown in Fig. 2, the number of total metabolites in the sets of DSS vs. NC, DSS + PSWP-I vs. DSS and DSS + PSAP-I vs. DSS was 65, 214 and 82, respectively. And the number of metabolites in the DSS + PSWP-I vs. DSS set was largest. There were nine common metabolites that were shared by the three sets, and 19 and 18 common metabolites that were shared by the sets of DSS + PSWP-I vs. DSS and DSS vs. NC, DSS + PSWP-I vs. DSS and DSS vs. NC, respectively. The number of unique metabolites in DSS + PSWP-I vs. DSS set was most with 168, followed by DSS vs. NC and DSS + PSAP-I vs. DSS set with 37. Our results indicated that there were similarities and differences in the regulation effects of PSWP-I and PSAP-I on metabolites. In the present study, comprehensive informations about total of 28 common metabolites in sets of DSS + PSWP-I vs. DSS and DSS vs. NC, DSS + PSWP-I vs. DSS and DSS vs. NC were analyzed in following manuscript.
On the basis of the above common metabolites, 25 metabolites with significant changes which responded to PSPPs (PSWP-I, PSAP-I) were listed in Table 1. Compared with DSS vs. NC set, levels of eight metabolites including oleic acid, DL-α-tocopheryl acetate, 17-hydroxyprogesterone, ganolucidic acid C, neocembrene, methyl linolenate, tanacetol A and ganosporeric acid A were significant decreased in DSS + PSWP-I vs. DSS set, while levels of four metabolites including equol, 5′-deoxyadenosine, flavanone and (S)-9-hydroxy-10-undecenoic acid were significant increased (p < 0.05). The results indicated that PSWP-I has a regulatory effect on the 12 compounds. Compared with DSS vs. NC set, levels of ten metabolites (oleic acid, DL-α-tocopheryl acetate, (S)-alpha-amino-4-carboxy-3-furanpropanoic acid, 5-methoxydimethyltryptamine, 17-hydroxyprogesterone, ganolucidic acid C, lysophosphatidyl ethanolamine (lysoPE; 16:1(9Z)/0:0), desipramine, ceramide (Cer; d18:0/15:0), cis-7-hexadecenoic acid) were significant reduced in DSS + PSAP-I vs. DSS set, while levels of eight metabolites (taurochenodeoxycholate-3-sulfate, diosmetin, equol, 24S-OH-7-DHC, retusin 7-methyl ether, 1-hydroxyvitamin D3 3-D-glucopyranoside, mytilin A, 3-hydroxypentadecanoyl carnitine) were significant elevated (p < 0.05). The results revealed that PSAP-I has a regulatory effect on the 18 compounds. The mechanism of PSWP-I and PSAP-I regulating intestinal inflammation by interfering gut microbiota might be related to above compounds produced by intestinal flora.
| Metabolite | Retention time | m/z | Formula | DSS vs. NC | DSS + PSWP-I vs. DSS | DSS + PSAP-I vs. DSS | |||
|---|---|---|---|---|---|---|---|---|---|
| VIP | Change | VIP | Change | VIP | Change | ||||
| a DSS vs. NC represents the DSS model group (DSS) compared with the normal control group (NC); DSS + PSWP-I vs. DSS represents the PSWP-I treatment group (DSS + PSWP-I) compared with the DSS model group (DSS); DSS + PSAP-I vs. DSS represents the PSAP-I treatment group (DSS + PSAP-I) compared with the DSS model group (DSS). m/z: mass-to-charge ratio; VIP: variable importance projection; (↑): up-regulated; (↓): down-regulated; (—): not detected; *: p < 0.05; **: p < 0.01; ***: p < 0.001. | |||||||||
| Oleic acid | 8.44 | 327.25 | C18H34O2 | 2.45 | ↑** | 1.92 | ↓*** | 1.62 | ↓** |
| DL-α-Tocopheryl acetate | 12.09 | 473.40 | C31H52O3 | 1.06 | ↑* | 2.68 | ↓*** | 1.31 | ↓*** |
| 17-Hydroxyprogesterone | 6.20 | 331.23 | C21H30O3 | 2.26 | ↑** | 2.15 | ↓*** | 1.27 | ↓*** |
| Equol | 5.27 | 243.10 | C15H14O3 | 1.60 | ↓* | 1.36 | ↑*** | 1.73 | ↑** |
| Ganolucidic acid C | 6.52 | 563.32 | C30H46O7 | 1.36 | ↑** | 1.38 | ↓*** | 1.00 | ↓*** |
| 5′-Deoxyadenosine | 1.19 | 252.11 | C10H13N5O3 | 2.25 | ↓*** | 1.13 | ↑** | — | — |
| Flavanone | 3.62 | 225.09 | C15H12O2 | 2.24 | ↓* | 1.20 | ↑* | — | — |
| (S)-9-Hydroxy-10-undecenoic acid | 5.06 | 245.14 | C11H20O3 | 1.58 | ↓* | 1.00 | ↑* | — | — |
| Neocembrene | 9.48 | 273.26 | C20H32 | 1.66 | ↑* | 1.04 | ↓** | — | — |
| Methyl linolenate | 9.62 | 293.25 | C19H32O2 | 1.62 | ↑** | 1.26 | ↓** | — | — |
| Tanacetol A | 6.13 | 277.18 | C17H26O4 | 2.34 | ↑** | 1.50 | ↓* | — | — |
| Ganosporeric acid A | 8.73 | 549.25 | C30H38O8 | 1.45 | ↑* | 1.55 | ↓*** | — | — |
| Taurochenodeoxycholate-3-sulfate | 4.71 | 288.62 | C26H45NO9S2 | 1.89 | ↓* | — | — | 1.95 | ↑*** |
| (S)-Alpha-amino-4-carboxy-3-furanpropanoic acid | 1.10 | 198.04 | C8H9NO5 | 1.38 | ↑* | — | — | 1.27 | ↓*** |
| Diosmetin | 5.38 | 299.06 | C16H12O6 | 2.12 | ↓* | — | — | 1.21 | ↑* |
| 5-Methoxydimethyltryptamine | 5.23 | 241.13 | C13H18N2O | 3.32 | ↑** | — | — | 1.77 | ↓*** |
| 24S-OH-7-DHC | 9.18 | 401.34 | C27H44O2 | 2.10 | ↓** | — | — | 2.71 | ↑*** |
| Retusin 7-methyl ether | 5.71 | 299.09 | C17H14O5 | 2.13 | ↓* | — | — | 1.92 | ↑** |
| 1-Hydroxyvitamin D3 3-D-glucopyranoside | 8.07 | 577.37 | C33H52O8 | 1.69 | ↓* | — | — | 1.91 | ↑** |
| LysoPE(16:1(9Z)/0:0) | 8.08 | 452.28 | C21H42NO7P | 1.04 | ↑* | — | — | 1.13 | ↓** |
| Mytilin A | 3.68 | 333.13 | C13H20N2O8 | 1.75 | ↓** | — | — | 2.06 | ↑** |
| Desipramine | 4.98 | 330.20 | C18H22N2 | 2.98 | ↑*** | — | — | 2.33 | ↓*** |
| Cer(d18:0/15:0) | 11.97 | 526.52 | C33H67NO3 | 2.81 | ↑* | — | — | 1.20 | ↓* |
| 3-Hydroxypentadecanoyl carnitine | 6.65 | 424.31 | C22H43NO5 | 1.56 | ↓** | — | — | 1.60 | ↑** |
| cis-7-Hexadecenoic acid | 7.94 | 255.23 | C16H30O2 | 1.82 | ↑** | — | — | 1.69 | ↓* |
Variable importance projection (VIP) is an index that is used to evaluate the importance of different metabolites to the model. The higher VIP value indicated that the importance to the model was higher. The order of VIP values for oleic acid and 17-hydroxyprogesterone in the three metabolite sets was DSS vs. NC > DSS + PSWP-I vs. DSS > DSS + PSAP-I vs. DSS, suggesting DSS had the greatest impact on the two compounds, followed by PSWP-I and PSAP-I. PSPPs (PSWP-I, PSAP-I) significantly affected 25 compounds in mice with colitis, including 14 down-regulated and 11 up-regulated compounds. PSWP-I and PSAP-I could both regulate five compounds of oleic acid, DL-α-tocopheryl acetate, 17-hydroxyprogesterone, equol and ganolucidic acid C. In addition, PSWP-I and PSAP-I also adjusted seven and thirteen different compounds, respectively.
In recent years, some researchers had employed metabolomics to study the pathogenesis of IBD and identified potential biomarkers.14 Based on non-targeted metabolomics using UPLC-Triple-TOF-MS/MS, this study investigated the impact of PSPPs (PSWP-I, PSAP-I) intervention on the fecal metabolites of mice with DSS-induced colitis. PSWP-I and PSAP-I significantly altered levels of 25 compounds in mice with intestinal inflammation, such as oleic acid, 17-hydroxyprogesterone, equol, 5-methoxydimethyltryptamine, Cer(d18:0/15:0) and so on. Oleic acid is a fatty acid compound that induces an oxidative stress response of neutrophils by activating phospholipase A2 (PLA2), causing tissue cell damage in the body.15 17-Hydroxyprogesterone is an intermediate product of corticosteroid metabolism. Abnormal expression of 17-hydroxyprogesterone may be related to autosomal recessive disorders.16 Equol is a non-steroidal isoflavone compound that is usually producted via gut microbiota metabolism and has a strong antioxidant activity.17 5-Methoxydimethyltryptamine is an indole compound, which are catabolites of tryptophan and closely related to many diseases, such as IBD. Indole compounds have toxic effects on the host gut and may induce gene mutations and even cancers.9 Cer(d18:0/15:0) is a phospholipid compound, which is an important component of cell membrane and a precursor of sphingolipin synthesis. Its synthesis is regulated by tumor necrosis factor-α (TNF-α). And Cer(d18:0/15:0) is considered as a secondary lipid transduction messenger in inflammatory diseases, affecting various biological processes such as inflammation, apoptosis and proliferation.18 In DSS-induced colitis mice, levels of oleic acid, 17-hydroxyprogesterone, 5-methoxydimethyltryptamine, Cer(d18:0/15:0) were significantly increased, while an equol level was significantly decreased. However, oral administration of PSPPs (PSWP-I, PSAP-I) could reverse the changes of these compounds, which might affect the development of intestinal inflammation in mice with colitis. A Poria cocos polysaccharide with dose of 300 mg kg−1 significantly altered the levels of 44 compounds in trinitro-benzene-sulfonic acid (TNBS)/ethanol induced acute enteritis mice. Up-regulation of 31 compounds, such as hydroxybutyrate, dihydrotestosterone, glutathione, androsterone, etc., and down-regulation of 13 compounds, including oleic acid, mannose, hexadecane, etc., might play an important role in regulating IBD.19 In present study, PSPPs significantly up-regulated 11 compounds (e.g. equol, 5′-deoxyadenosine, 3-D-glucopyranoside, carnitine) and down-regulated 14 compounds (e.g. oleic acid, 17-hydroxyprogesterone, DL-α-tocopheryl acetate, 5-methoxydimethyltryptamine, ganolucidic acid C). Our results were different, which might be related to the animal model and polysaccharide structure.
3.2. Characterization of significantly metabolic pathways
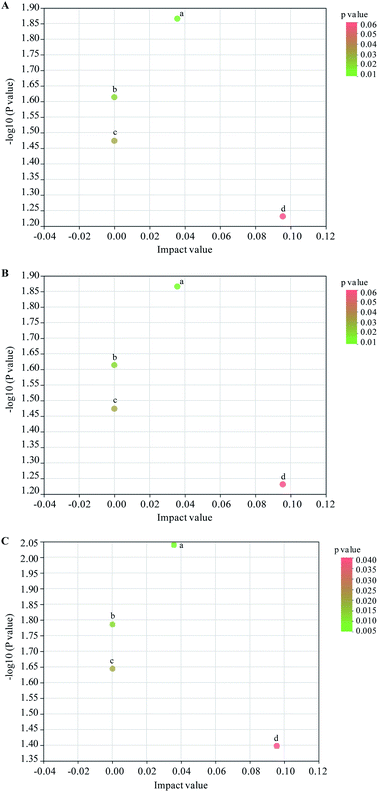
Based on KEGG database, topology analysis was used for analyzing KEGG signal enrichment and significantly metabolic pathways of 25 common compounds. As shown in Fig. 3, four significantly metabolic pathways, including cutin, suberine and wax biosynthesis, biosynthesis of unsaturated fatty acids, fatty acid biosynthesis, steroid hormone biosynthesis, were discovered in sets of DSS vs. NC, DSS + PSWP-I vs. DSS and DSS + PSAP-I vs. DSS. Two metabolites of oleic acid and 17-hydroxyprogesterone were produced through the four metabolic pathways. In sets of DSS vs. NC and DSS + PSWP-I vs. DSS, impact values of cutin, suberine and wax biosynthesis, biosynthesis of unsaturated fatty acids, fatty acid biosynthesis, steroid hormone biosynthesis were 0.0357 (p = 0.0272), 0.0000 (p = 0.0325), 0.0000 (p = 0.0336) and 0.0955 (p = 0.0470), respectively. In the DSS + PSAP-I vs. DSS set, impact values of cutin, suberine and wax biosynthesis, biosynthesis of unsaturated fatty acids, fatty acid biosynthesis, steroid hormone biosynthesis were 0.0357 (p = 0.0182), 0.0000 (p = 0.0218), 0.0000 (p = 0.0226) and 0.0955 (p = 0.0320), respectively (Table S1†). The results showed that PSPPs (PSWP-I, PSAP-I) could significantly change the metabolic pathways of colitis mice, which might be associated with the improvement of gut microbiota disorder.Daniluk et al.14 demonstrated that the main metabolic pathways in IBD (including UC and CD) patients were lipid and amino acid metabolisms. According to our results, the significantly metabolic pathway in DSS, DSS + PSWP-I and DSS + PSAP-I group was lipid metabolism. Normal lipid metabolism pathways, such as cutin, suberine and wax biosynthesis, biosynthesis of unsaturated fatty acids, fatty acid biosynthesis, steroid hormone biosynthesis, were disrupted in DSS-induced mice with colitis. The lipid metabolites (for instance triglycerides) that stored in cells and cell membranes were degraded to release fatty acids to meet the energy needs of the body.20 What's more, large amounts of fatty acids could induce oxidative stress and further aggravate the intestinal inflammation.21 And dysregulated lipid metabolism also affected sugar metabolism. Oral administration of PSPPs (PSWP-I, PSAP-I) significantly down-regulated the metabolic pathways of cutin, suberine and wax biosynthesis, biosynthesis of unsaturated fatty acids, fatty acid biosynthesis and steroid hormone biosynthesis, then ameliorated the lipid metabolism, and thus affected the development progress of intestinal inflammation. Astragalus polysaccharide had a good regulation effect on metabolic pathway in DSS-induced colitis mice.22 The mainly metabolic pathway was also lipid metabolism, including arachidonic acid metabolism, fatty acid synthesis, linoleic acid metabolism and glycerophosphatidic acid metabolism, which was similar to our results.
3.3. Identification of key biomarkers
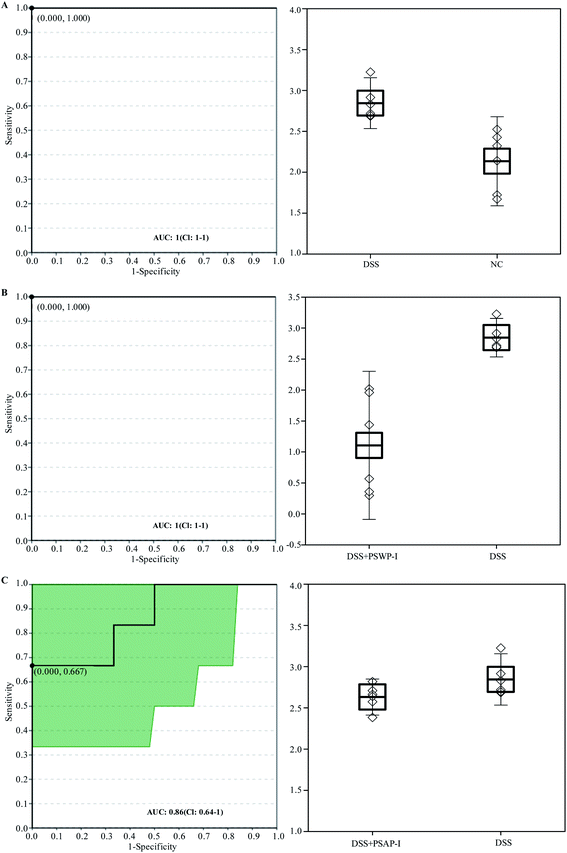
Receiver operator characteristic (ROC) analysis was ofter used to evaluate the potential biomarkers. Two compounds of oleic acid and 17-hydroxyprogesterone were characterized as key biomarkers responded to PSPPs by ROC analysis (Fig. 4 and 5). As shown in Fig. 4, in sets of DSS vs. NC and DSS + PSWP-I vs. DSS, the area under the curve (AUC) and optimal discrimination threshold (ODT) value of oleic acid was 1.00, 1.000, respectively. In the DSS + PSAP-I vs. DSS set, the AUC and ODT value of oleic acid was 0.75, 0.667, respectively. As shown in Fig. 5, in sets of DSS vs. NC and DSS + PSWP-I vs. DSS, the AUC and ODT value of 17-hydroxyprogesterone was 1.00, 1.000, respectively. In the DSS + PSAP-I vs. DSS set, the AUC and ODT value of 17-hydroxyprogesterone was 0.86, 0.667, respectively. As an evaluation index of ROC analysis, the higher AUC value implied the better model. Our results displayed that PSPPs (PSWP-I, PSAP-I) had a significant modulation effect on the key biomarkers of oleic acid and 17-hydroxyprogesterone. And PSWP-I had a greater down-regulation effect on oleic acid and 17-hydroxyprogesterone than PSAP-I.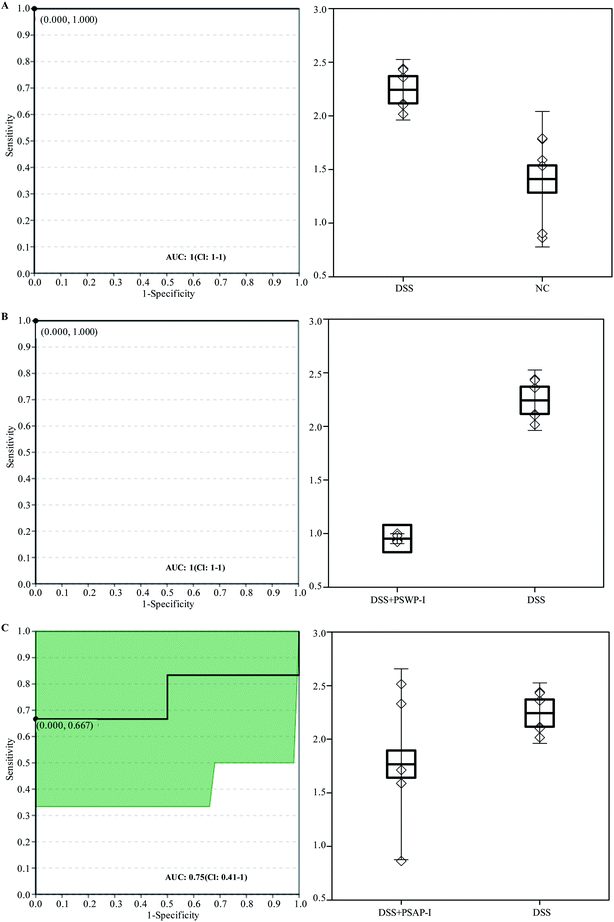 | ||
| Fig. 4 ROC analysis for oleic acid in DSS vs. NC (A), DSS + PSWP-I vs. DSS (B) and DSS + PSAP-I vs. DSS (C). ROC: receiver operator characteristic; AUC: area under the curve. | ||
 | ||
| Fig. 5 ROC analysis for 17-hydroxyprogesterone in DSS vs. NC (A), DSS + PSWP-I vs. DSS (B) and DSS + PSAP-I vs. DSS (C). ROC: receiver operator characteristic; AUC: area under the curve. | ||
ROC is an important statistical analysis method in metabolomics. A supervised learning method is adopted by ROC to conduct linear discrimination analysis (LDA) and classification modeling for the different groups and discover the key biomarkers among groups, which is often used to evaluate the biomarker performance. Chen et al.21 evaluated and predicted the urine and fecal metabolites of obese mice induced by high-fat diet after oral administration of lentinan and Flos Lonicera polysaccharides by ROC analysis. Jin et al.9 studied the effects of Ganoderma lucidum polysaccharide on intestinal metabolites in mice, and identified two key biomarkers of 2,2-dimethylsuccinic acid and indolelactate by ROC analysis. In present study, two key biomarkers (oleic acid, 17-hydroxyprogesterone) responded to PSPPs were characterized. In addition, a recent study performed by Daniluk et al.14 evaluated the differences in serum metabolites between newly diagnosed and untreated patients with Crohn's disease (CD) or UC using untargeted metabolomics. Some inflammatory biomarkers including lactosylceramide, lysophosphatidylcholines, phosphatidylcholines and sphingomyelins were identified for the diagnosis of CD and UC. In contrast to serum, the fecal metabolome could better reflect the host-microbiota interrelation and more closely capture the host metabolism. In our study, two inflammatory markers of oleic acid and 17-hydroxyprogesterone were grabbed from fecal metabolites of colitis mice using non-targeted metabolomics. The investigated results might be helpful to improve the noninvasive diagnosis and to monitor IBD patients based on fecal metabolome.
3.4. Association among fecal metabolites, gut microbiota and immunological factors
In oder to further elucidate the mechanism of gut microbiota regulating colonic inflammation in DSS-induced colitis mice based on metabolites, correlations among fecal metabolites, gut microbiota and immunological factors were elaborated (Fig. 6–8). As shown in Fig. 6, six gut microbiota at genus levels were significantly correlated with more than one inflammatory cytokines. It was exhibited that the abundances of Bacteroide, unclassified_o__Bacteroidales, norank_f__Clostridiales_vadinBB60_group were significantly positively correlated with the levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6), the abundances of norank_f__Clostridiales_vadinBB60_group, Rikenella were significantly negatively correlated with the levels of anti-inflammatory cytokines (IL-10). Lachnospiraceae_UCG-001 and Gemella abundances were significantly negatively correlated with the levels of pro-inflammatory cytokines, while positively correlated with the levels of anti-inflammatory cytokines (p < 0.05). In addition, there were positive correlations between Lactobacillus, Bifidobacterium abundances and anti-inflammatory cytokines levels (p = 0.0590, p = 0.0627). The results indicated that gut microbiota was closely related to immunological factors (e.g. IL-1β, IL-6) that influenced inflammatory bowel disease. Furthermore, gut microbiota that were positively correlated with the levels of pro-inflammatory cytokines were belonged to the level of phylum Bacteroidetes and family Bacteroidaceae, unclassified_o__Bacteroidales, while positively correlated with anti-inflammatory cytokines levels or negatively correlated with pro-inflammatory cytokines levels were belonged to the levels of phylum Firmicutes and Actinobacteria and family Family_XI_o__Bacillales, Lactobacillaceae, Bifidobacteriaceae. The observations were in concordance with previous studies.10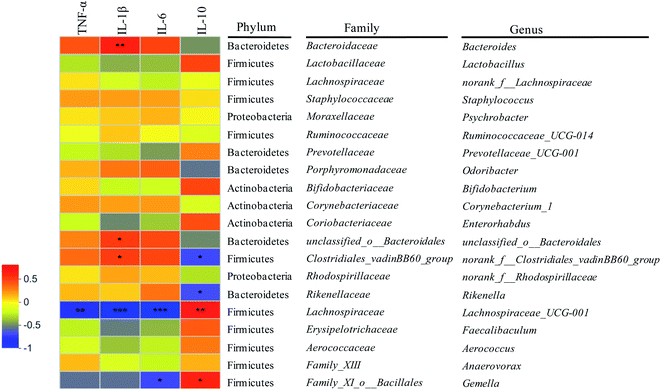 | ||
| Fig. 6 Correlations between gut microbiota and intestinal inflammatory cytokines. *: p < 0.05; **: p < 0.01; ***: p < 0.001. | ||
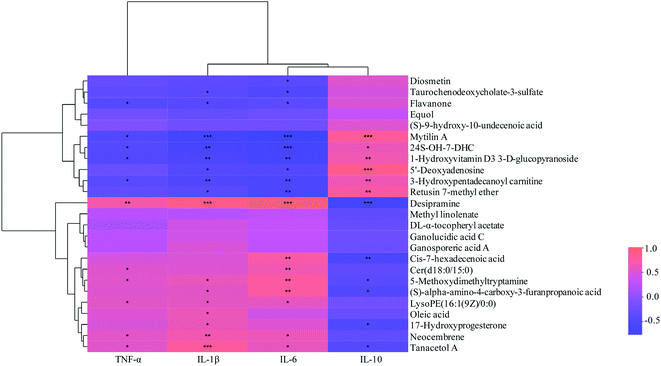 | ||
| Fig. 7 Correlations between fecal metabolites and intestinal inflammatory cytokines. *: p < 0.05; **: p < 0.01; ***: p < 0.001. | ||
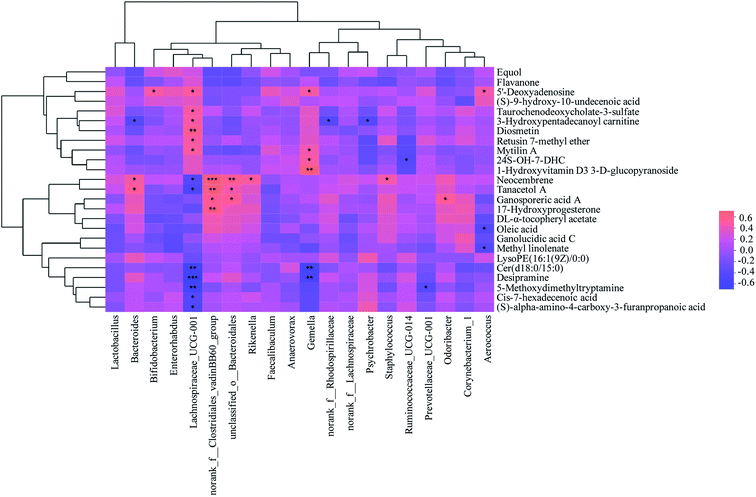 | ||
| Fig. 8 Correlations between metabolites and gut microbiota from mice faeces. *: p < 0.05; **: p < 0.01; ***: p < 0.001. | ||
The correlations between metabolites and immunological factors in colitis mice were shown in Fig. 7. Among the associated metabolites and inflammatory factors, 19 compounds were significantly correlated with more than one inflammatory factors. It was displayed that the concentrations of desipramine, cis-7-hexadecenoic acid, 5-methoxydimethyltryptamine, (S)-alpha-amino-4-carboxy-3-furanpropanoic acid, 17-hydroxyprogesterone and tanacetol were significantly positively correlated with the levels of pro-inflammatory factors (TNF-α, IL-1β and IL-6), while negatively correlated with that of anti-inflammatory factors (IL-10). The concentrations of mytilin A, 24S-OH-7-DHC, 1-hydroxyvitamin D3 3-D-glucopyranoside, 5′-deoxyadenosine, 3-hydroxypentadecanoyl carnitine and retusin 7-methyl ether were significantly positively correlated with the levels of anti-inflammatory factors, whereas negatively correlated with that of pro-inflammatory factors. Moreover, there were positive correlations between Cer(d18:0/15:0), LysoPE(16:1(9Z)/0:0), oleic acid, neocembrene and pro-inflammatory factors levels, while negative correlations between diosmetin, taurochenodeoxycholate-3-sulfate, flavanone 3 and anti-inflammatory factors levels (p < 0.05). Additionally, it was found that six compounds were significantly associated with PSWP-I, among which 5′-deoxyadenosine and flavanone were up-regulated and 17-hydroxyprogesterone, tanacetol A, oleic acid, neocembrene were down-regulated. These six compounds might be related to the anti-inflammatory properties of PSWP-I. 15 compounds were significantly associated with PSAP-I, among which mytilin A, 24S-OH-7-DHC, 1-hydroxyvitamin D3 3-D-glucopyranoside, 3-hydroxypentadecanoyl carnitine, retusin 7-methyl ether, diosmetin and taurochenodeoxycholate-3-sulfate were up-regulated, and desipramine, 17-hydroxyprogesterone, cis-7-hexadecenoic acid, (S)-alpha-amino-4-carboxy-3-furanpropanoic acid, 5-methoxydimethyltryptamine, Cer(d18:0/15:0), LysoPE(16:1(9Z)/0:0) and oleic acid were down-regulated. The 15 compounds might be related to the anti-inflammatory properties of PSAP-I. Furthermore, two common associated metabolites of oleic acid and 17-hydroxyprogesterone were found in PSWP-I and PSAP-I treatment group, and unique associated metabolites in that was four and thirteen, respectively. It was indicated that PSPPs (PSWP-I and PSAP-I) might modulate the levels of inflammatory factors by intestinal metabolites to alleviate the colonic inflammation. PSWP-I and PSAP-I had different regulating effects on intestinal compounds, which might be related to the monosaccharide composition and structure characteristics of branched chain in two polysaccharides.
The correlations between metabolites and gut microbiota in colitis mice were shown in Fig. 8. Among the associated metabolites and gut microbiota, 19 compounds were significantly correlated with more than one gut microbiota, while 15 gut microbiota at genus levels were significantly correlated with more than one compounds. Combined with Fig. 7, it was found that nine compounds of desipramine, cis-7-hexadecenoic acid, Cer(d18:0/15:0), 5-methoxydimethyltryptamine, (S)-alpha-amino-4-carboxy-3-furanpropanoic acid, oleic acid, 17-hydroxyprogesterone, neocembrene and tanacetol A were pro-inflammatory metabolites, while eight compounds of diosmetin, taurochenodeoxycholate-3-sulfate, mytilin A, 24S-OH-7-DHC, 1-hydroxyvitamin D3 3-D-glucopyranoside, 5′-deoxyadenosine, 3-hydroxypentadecanoyl carnitine and retusin 7-methyl ether were anti-inflammatory metabolites. The abundances of the gene norank_f__Clostridiales_vadinBB60_group, unclassified_o__Bacteroidales, Rikenella, Bacteroides, Staphylococcus, Odoribacter, norank_f__Rhodospirillaceae, Psychrobacter and Ruminococcaceae_UCG-014 showed significantly positive correlation with the concentrations of pro-inflammatory metabolites and negative correlation with that of anti-inflammatory metabolites. However, the abundances of the gene Aerococcus, Bifidobacterium, Lachnospiraceae_UCG-001, Gemella, Prevotellaceae_UCG-001 displayed significantly positive correlation with anti-inflammatory metabolites concentrations and negative correlation with pro-inflammatory metabolites concentrations (p < 0.05). Further analysis combined with Fig. 6 exhibited that 6 of the above 14 gut microbiota, including norank_f__Clostridiales_vadinBB60_group, unclassified_o__Bacteroidales, Rikenella, Bacteroides, Lachnospiraceae_UCG-001 and Gemella, were significantly correlated with inflammatory factors. And its correlation was completely consistent with the trend of the correlation between metabolites and inflammatory factors, suggesting intestinal metabolites had a close association with gut microbiota. Among the 6 gut microbiota, norank_f__Clostridiales_vadinBB60_group, unclassified_o__Bacteroidales, Bacteroides, Rikenella, Lachnospiraceae_UCG-001 were significantly correlated with PSWP-I, while norank_f__Clostridiales_vadinBB60_group, Bacteroides, unclassified_o__Bacteroidales, Rikenella, Lachnospiraceae_UCG-001 and Gemella were significantly correlated with PSAP-I. The results of above correlation analysis revealed a close relationship among intestinal metabolites, gut microbiota and colon immunological factors. PSPPs could alter the concentrations of intestinal metabolites by adjusting the abundances of gut microbiota, then regulate the levels of colon inflammatory factors, and finally ameliorate the intestinal inflammation in colitis mice. The effects of PSWP-I and PSAP-I on intestinal metabolites and gut microbiota in DSS-induced colitis mice were different, which might be related to intestinal flora composition modulated by polysaccharides and the decomposition and utilization characteristics of gut microbiota on polysaccharides.
The interaction between gut microbiota and the immune system runs through the entire life cycle of the host. Gut microbiota had a significant impact on the immune system. Abnormal changes of gut microbiota could induce the autoimmune diseases, such as IBD, rheumatoid arthritis and insulin-dependent diabetes.23 A recent study demonstrated that the interaction between gut microbiota and immune system was mainly mediated by the small-molecule metabolites. These intestinal metabolites played an important role in promoting host health and preventing diseases.24 Oleic acid, as a lipid inflammatory mediator, regulated the inflammatory response of adipocytes, macrophages and endothelial cells. Oleic acid concentration in intestinal tract was thus closely related to obesity, coronary heart disease, diabetes and neurological diseases.25 Gut microbiota metabolized stearic acid to produce oleic acid through the stearoyl CoA desaturase 1 (SCD1) pathway.26 SCD1 is a fat synthesis rate-limiting enzyme that plays an important effect in maintaining the balance of saturated fatty acids (SFAs) and mono-unsaturated fatty acids (MUFAs) in the body. SCD1 was related to fatty liver, diabetes, cardiovascular disease, cancer and many other diseases. Some researchers had documented that toll-like receptor 5 (TLR 5) deficiency in mice promoted the development of intestinal flora-dependent metabolic syndrome, and significantly enhanced SCD1 expression and oleic acid concentration.27 Oleic acid could adjust the phenotype of myeloid-derived suppressor cells (MDSCs) and enhance the expression of the pro-inflammatory cytokines, such as TNF-α, IL-6 and IL-1β.28 However, the intake of non-starch polysaccharides and resistant starch could maintain the intestinal health of host by regulating fatty acid metabolism pathways, especially that of related to oleic acid.29 Several intestinal probiotics had been reported to regulate the metabolism level of oleic acid.27,30 Administration of DSS elevated the concentrations of oleic acid in mice colon, which could promote the expression of IL-1β and inhibit the growth of Lactobacillus and other beneficial bacteria. The intake of PSPPs (PSWP-I, PSAP-I) significantly reduced the contents of free unsaturated fatty acids (such as oleic acid) in the intestine of mice with colitis, improved their metabolic pathways of cutin, suberine and wax biosynthesis, fatty acid biosynthesis, biosynthesis of unsaturated fatty acids. 17-Hydroxyprogesterone is a steroid hormone that is abnormally expressed in congenital adrenal hyperplasia due to the loss of 21-hydroxylase. In this study, the 17-hydroxyprogesterone level significantly increased in DSS-induced mice with colitis as compared to that of in normal control mice. This might be due to the disorder of lipid and amino acid metabolisms to inhibit 21-hydroxylase activity, and further affect the pathway of steroid hormone biosynthesis.29 An in vitro study showed that 17-hydroxyprogesterone had a pro-inflammatory effect and could enhance TNF-α level of the plasma in non-pregnant women treated with lipopolysaccharides (LPS).31 There was a high concentration of 17-hydroxyprogesterone in DSS-induced mice, which enhance the expression of IL-1β and block the expression of IL-10. Production of 17-hydroxyprogesterone might be related to the metabolism of gut microbiota, such as norank_f__Clostridiales_vadinBB60_group, Bacteroides. The oral administration of PSPPs (PSWP-I, PSAP-I) significantly lowered 17-hydroxyprogesterone concentration in mice colon and ameliorated the pathway of steroid hormone biosynthesis. In addition, PSPPs could decrease 5-methoxydimethyltryptamine level and increase 5′-deoxyadenosine level in mice with colitis. 5-Methoxydimethyltryptamine is an indoles compound that produces by tryptophan metabolism. Some microorganisms, such as Clostridia, Escherichia, might decompose and utilize tryptophan in host gut.32 Except the specific physiological activities, indoles compounds also regulated the host immune and inflammatory responses.33 Aryl hydrocarbon receptor (AhR), a cytoplasmic receptor and transcription factor, plays an important role in regulating adaptive immunity, barrier function, intestinal balance and intestinal inflammation. A study documented that the dysregulation of AhR signals exacerbated IBD conditions in mice.34 And indoles compounds modulated the immune response by activating AhR signals, caused the abnormal expression of inflammatory factors.35 Knarreborg et al.36 showed that increasing polysaccharides intake significantly reduced the activity of tryptophan metabolism and the concentration of indoles compounds in the intestine. In the present study, intake of DSS elevated 5-methoxydimethyltryptamine level of mice colon, which promoted the expression of IL-6, IL-1β, TNF-α, restrained the expression of IL-10, and inhibited the proliferation of Lachnospiraceae_UCG-001 and Prevotellaceae_UCG-001. Nevertheless, PSPPs significantly decreased 5-methoxydimethyltryptamine content in DSS-induced mice. 5′-Deoxyadenosine is an important intermediate product in the purine metabolism. A significantly negative correlation between 5′-deoxyadenosine concentration and inflammatory factors levels was revealed in genital inflammation mediated by human papilloma virus (HPV). 5′-Deoxyadenosine might possess an anti-inflammatory effect by modulating N-methyl-D-aspartate (NMDA) receptors to block the release of inflammatory factors.37,38 Our results indicated that DSS decreased the level of 5′-deoxyadenosine, while PSPPs increased that of 5′-deoxyadenosine in mice colon. 5′-Deoxyadenosine could inhibit the expression of IL-1β, IL-6, and enhance the expression of IL-10, which might be generated by Bifidobacterium, Lachnospiraceae_UCG-001, Aerococcus and Gemella. PSWP-I and PSAP-I alleviated the colonic inflammation of mice through improving the purine metabolism pathway and then hoisting the 5′-deoxyadenosine concentration.
Innate immune systems of human and animal used the pattern recognition receptors (PRRs) encoded by germ cells to monitor, regulate and respond the changes in gut microbiota and their metabolites. Transmembrane and cytoplasmic PRRs stimulated or modulated the immune responses by initiating the cascades of conservative signals, which were critical for host defense. Although the mechanism underlying the regulation of host immunity by gut microbiota was not fully understood, it was known that gut microbiota could induce innate immune responses based on the microorganism-associated molecular patterns (MAMPs).39 MAMPs were mainly the components of microbial cell wall, including lipopolysaccharides (LPS), peptidoglycans and formyl peptides. These molecules regulated immune responses via the PRR signaling pathways. Polysaccharide A (PSA) that produced by Bacteroides regulated intestinal immunity by activating toll-like receptor 2 (TLR2). PSA interacted with dendritic cells (DCs) in the lamina propria and then transmitted to CD4+ T cells, which activated transforming growth factor β (TGF β), and enhanced the function of FoxP3+CD25+ regulatory T cell with anti-inflammatory property and the expression of IL-10.40 Moreover, PSA also adjusted the balance between T helper 1 (TH1) and TH2. Formyl peptides that produced by microorganisms were mainly recognized and binded by formyl peptide receptors (FPRs), which always existed in immune cells such as neutrophils. It was reported that formyl peptide produced by Staphylococcus could induce neutrophils to accumulate at infection sites through FPR1, then caused toxic damage of host cells and malignant competition of microbial cells.41 Compared with MAMPs, some metabolites (such as fatty acids, vitamins) of intestinal flora were diet-dependent compounds. These metabolites mainly regulated host immunity through G protein-coupled receptors (GPCRs), AhRs, pregnane X receptors (PXRs) and others.42 Gut microbiota, such as Lachnospiraceae and Bifidobacterium, could ferment indigestible carbohydrates (for instance polysaccharides) to produce fatty acid especially short-chain fatty acids (SCFAs). Act as signaling molecules, the fatty acids modulated the mucosal immunity by activating GPCRs pathways. Short-chain fatty acids promoted the differentiation of FoxP3+CD4+ regulatory T cells to produce anti-inflammatory cytokine of IL-10 by activating G-protein-coupled receptor 43 (GPR43). And GPR43 could maintain intestinal homeostasis by directly acting on intestinal epithelial cells to activate inflammasomes for releasing IL-18. SCFAs also enhanced the function of DCs to express IL-10 through a GPR109A signaling pathway.43 Bifidobacterium, Lactobacillus and other intestinal flora could produce vitamins B, which induced IL-10 secretion by DCs and macrophages via a GPR109A-dependent mode to exert anti-inflammatory effects, thereby alleviating the intestinal inflammation.44 Gut microbiota metabolized tryptophan to produce indoles compounds (such as 5-methoxydimethyltryptamine), which had been confirmed as AhR ligands. As a ligand-inducible transcription factor, AhR directly targeted and activated the specific genes by binding to these tryptophan-derived compounds. In the process of inflammation, AhR was activated to regulate the expression of target genes, such as IL-6, IL-22 and prostaglandin G/H synthase 2 (PTGS2). And IL-22 also adjusted the release of antimicrobial peptides from intestinal epithelial cells and enhanced the expression of MAMPs.45
Overall, colonic metabolites had a close relationship with gut microbiota and host immunity. On one hand, gut microbiota affected the metabolites compositions and metabolic pathways. And on the other hand, metabolites played an important effect in regulating the levels of host immunological factors. PSPPs (PSWP-I, PSAP-I) enhanced host immunity through intestinal metabolites produced by gut microbiota, thus playing a role in modulating intestinal inflammation. This manuscript preliminary clarified the metabolomics mechanism of PSPPs in regulating ulcerative colitis based on the target of gut microbiota.
4. Conclusion
In short, this study exhibited the response of fecal metabolomic profiles in colitis mice to the administration of polysaccharides from purple sweet potato (Ipomoea batatas L.). Twenty-five significant metabolites and four strikingly metabolic pathways of cutin, suberine and wax biosynthesis, biosynthesis of unsaturated fatty acids, fatty acid biosynthesis, and steroid hormone biosynthesis were identified. Oleic acid and 17-hydroxyprogesterone were key biomarkers that responded to PSPPs. Furthermore, the change of fecal metabolomes in colitis mice impacted by PSPPs was correlated with the improvement of immune function and gut microbiota balance. This beneficial alteration might be partly due to the inflammation-regulating properties of PSPPs.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 31871803), China Agriculture Research System of MOF and MARA (CARS-10-B22), National Key R&D Program of China (2019YFD1001300 and 2019YFD1001302), Six Major Talent Summit Project of Jiangsu Province (SWYY-224).References
- A. Franke, Inflammatory bowel disease: A global disease that needs a broader ensemble of populations, Gastroenterology, 2017, 152, 14–16 CrossRef PubMed.
- D. Y. Jeong, S. Kim, M. J. Son, C. Y. Son, J. Y. Kim, A. Kronbichler, K. H. Lee and J. I. Shin, Induction and maintenance treatment of inflammatory bowel disease: A comprehensive review, Autoimmun. Rev., 2019, 18, 439–454 CrossRef.
- D. C. Baumgart and W. J. Sandborn, Inflammatory bowel disease: Clinical aspects and established and evolving therapies, Lancet, 2007, 369, 1641–1657 CrossRef CAS.
- R. Li, Y. Chen, M. Shi, X. Xu, Y. Zhao, X. Wu and Y. Zhang, Gegen Qinlian decoction alleviates experimental colitis via suppressing TLR4/NF-κB signaling and enhancing antioxidant effect, Phytomedicine, 2016, 23, 1012–1020 CrossRef PubMed.
- C. Tang, R. Ding, J. Sun, J. Liu, J. Kan and C. Jin, The impacts of natural polysaccharides on intestinal microbiota and immune responses-a review, Food Funct., 2019, 10, 2290–2312 RSC.
- S. Shao, D. Wang, W. Zheng, X. Li, H. Zhang, D. Zhao and M. Wang, A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors, Int. Immunopharmacol., 2019, 71, 411–422 CrossRef CAS PubMed.
- Y. Wang, N. Zhang, J. Kan, X. Zhang, X. Wu, R. Sun, S. Tang, J. Liu, C. Qian and C. Jin, Structural characterization of water-soluble polysaccharide from Arctium lappa and its effects on colitis mice, Carbohydr. Polym., 2019, 213, 89–99 CrossRef CAS.
- T. Yamada, D. Takahashi and K. Hase, The diet-microbiota-metabolite axis regulates the host physiology, J. Biochem., 2016, 160, 1–10 CrossRef CAS PubMed.
- M. Jin, H. Zhang, J. Wang, D. Shao, H. Yang, Q. Huang, J. Shi, C. Xu and K. Zhao, Response of intestinal metabolome to polysaccharides from mycelia of Ganoderma lucidum, Int. J. Biol. Macromol., 2019, 122, 723–731 CrossRef CAS PubMed.
- J. Sun, H. Chen, J. Kan, Y. Gou, J. Liu, X. Zhang, X. Wu, S. Tang, R. Sun, C. Qian, N. Zhang and C. Jin, Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice, Int. J. Biol. Macromol., 2020, 153, 708–722 CrossRef CAS PubMed.
- Y. Gou, J. Sun, J. Liu, H. Chen, J. Kan, C. Qian, N. Zhang and C. Jin, Structural characterization of a water-soluble purple sweet potato polysaccharide and its effect on intestinal inflammation in mice, J. Funct. Foods, 2019, 61, 03502 Search PubMed.
- H. Chen, J. Sun, J. Liu, Y. Gou, X. Zhang, X. Wu, R. Sun, S. Tang, J. Kan, C. Qian, N. Zhang and C. Jin, Structural characterization and anti-inflammatory activity of alkali-soluble polysaccharides from purple sweet potato, Int. J. Biol. Macromol., 2019, 131, 484–494 CrossRef CAS PubMed.
- J. Sun, B. Zhou, C. Tang, Y. Gou, H. Chen, Y. Wang, C. Jin, J. Liu, F. Niu, J. Kan, C. Qian and N. Zhang, Characterization, antioxidant activity and hepatoprotective effect of purple sweetpotato polysaccharides, Int. J. Biol. Macromol., 2018, 115, 69–76 CrossRef CAS PubMed.
- U. Daniluk, J. Daniluk, R. Kucharski, T. Kowalczyk, K. Pietrowska, P. Samczuk, A. Filimoniuk, A. Kretowski, D. Lebensztejn and M. Ciborowski, Untargeted metabolomics and inflammatory markers profiling in children with Crohn's disease and ulcerative colitis-a preliminary study, Inflammatory Bowel Dis., 2019, 25, 1120–1128 CrossRef PubMed.
- Y. M. Lee, B. Y. Kim and Y. Y. Park, Role of the PLA2-activated neutrophilic oxidative stress in oleic acid-induced acute lung injury, Tuberc. Respir. Dis., 2010, 68, 55–61 CrossRef.
- Y. Levy-Shraga and O. Pinhas-Hamiel, High 17-hydroxyprogesterone level in newborn screening test for congenital adrenal hyperplasia, BMJ Case Reports, 2016, 41, 1–4 Search PubMed.
- K. Setchell, N. M. Brown and L. O. Eva, The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones, J. Nutr., 2002, 12, 12 Search PubMed.
- C. R. Stevens, V. G. Oberholzer, J. A. Walker-Smith and A. D. Phillips, Lactosylceramide in inflammatory bowel disease: A biochemical study, Gut, 1988, 29, 580–587 CrossRef CAS PubMed.
- X. Liu, Purification and biological activity of carboxymethyl Poria cocos polysaccharide, S. China Univ. Technol., Guangzhou, 2018 Search PubMed.
- H. Wang, A. Liu, W. Zhao, L. Gong, E. Chen, N. Cui, X. Ji, S. Wang and H. Jiang, Metabolomics research reveals the mechanism of action of Astragalus polysaccharide in rats with digestive system disorders, Molecules, 2018, 23, 3333 CrossRef.
- M. Chen, B. Lu, Y. Li, Y. Wang, H. Zheng, D. Zhong, Z. Liao, M. Wang, F. Ma and Q. Liao, Metabolomics insights into the modulatory effects of long-term compound polysaccharide intake in high-fat diet-induced obese rats, Nutr. Metab., 2018, 15, 8 CrossRef PubMed.
- C. Li, Preparation of honey processed Astragalus polysaccharide and its effect on DSS-induced colitis mice, Guangdong Pharm. Univ., Guangzhou, 2019 Search PubMed.
- J. Gao, K. Xu, H. Liu, L. Gang, M. Bai, C. Peng, T. Li and Y. Yin, Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism, Front. Cell. Infect. Microbiol., 2018, 8, 13 CrossRef PubMed.
- R. W. P. Glowacki and E. C. Martens, In sickness and health: Effects of gut microbial metabolites on human physiology, PLoS Pathog., 2020, 16, e1008370 CrossRef CAS PubMed.
- D. Dai, Y. Tian, H. Guo, P. Zhang, Y. Huang, W. Zhang, F. Xu and Z. Zhang, A pharmacometabonomic approach using predose serum metabolite profiles reveals differences in lipid metabolism in survival and non-survival rats treated with lipopolysaccharide, Metabolomics, 2016, 12, 2 CrossRef.
- A. Kindt, G. Liebisch, T. Clavel, D. Haller and J. Ecker, The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice, Nat. Commun., 2018, 9, 3760 CrossRef PubMed.
- V. Singh, B. Chassaing, L. Zhang, B. S. Yeoh, X. Xiao, M. Kumar, M. T. Baker, J. Cai, R. Walker, K. Borkowski, K. J. Harvatine, N. Singh, G. C. Shearer, J. M. Ntambi, B. Joe, A. D. Patterson, A. T. Gewirtz and M. Vijay-Kumar, Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice, Cell Metab., 2015, 22, 983–996 CrossRef CAS PubMed.
- H. Wu, C. Weidinger, F. Schmidt, J. Keye, M. Friedrich, C. Yerinde, G. Willimsky, Z. Qin, B. Siegmund and R. Glaube, Oleate but not stearate induces the regulatory phenotype of myeloid suppressor cells, Sci. Rep., 2017, 7, 7498 CrossRef PubMed.
- Y. Sun, Y. Su and W. Zhu, Microbiome-metabolome responses in the cecum and colon of pig to a high resistant starch diet, Front. Microbiol., 2016, 7, 779 Search PubMed.
- W. Cheng, J. Lu, W. Lin, X. Wei and H. Li, Effects of a galacto-oligosaccharide-rich diet on fecal microbiota and metabolite profiles in mice, Food Funct., 2018, 9, 1612–1620 RSC.
- J. Amory, R. Lawler and L. Shields, Hydroxyprogesterone caproate and progesterone increase tumor necrosis factor-alpha production in lipopolysaccharide stimulated whole blood from non-pregnant women, J. Perinat. Med., 2005, 33, 506–509 CAS.
- X. Gu, Y. Song, Y. Chai, L. Feng, F. J. Gonzalez, G. Fan and Y. Qi, GC-MS metabolomics on PPARα-dependent exacerbation of colitis, Mol. BioSyst., 2019, 11, 1329–1337 Search PubMed.
- C. S. Kim, J. H. Li, B. Barco, H. B. Park, A. Gatsios, A. Damania, R. Wang, T. P. Wyche, G. Piizzi, N. K. Clay and G. M. Crawford, Cellular stress upregulates indole signaling metabolites in Escherichia coli, Cell Chem. Biol., 2020, 27, 1–10 CrossRef PubMed.
- R. Arsenescu, V. Arsenescu, J. Zhong, M. Nasser and W. J. D. Villiers, Role of the xenobiotic receptor in inflammatory bowel disease, Inflammatory Bowel Dis., 2011, 17, 1149–1162 CrossRef PubMed.
- R. Shinde, K. Hezaveh, M. J. Halaby, A. Kloetgen, A. Chakravarthy, T. D. S. Medina, R. Deol, K. P. Manion, Y. Baglaenko, M. Eldh, S. Lamorte, D. Wallace, S. B. Chodisetti, B. Ravishankar, H. Liu, K. Chaudhary, D. H. Munn, A. Tsirigos, M. Madaio, S. Gabrielsson, Z. Touma, J. Wither, D. D. D. Carvalho and T. L. Mcgaha, Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans, Nat. Immunol., 2018, 19, 571–582 CrossRef CAS PubMed.
- A. Knarreborg, J. Beck, M. T. Jensen, A. Laue, N. Agergaard and B. B. Jensen, Effect of non-starch polysaccharides on production and absorption of indolic compounds in entire male pigs, Anim. Sci., 2002, 74, 445–453 CrossRef CAS.
- Z. E. Ilhan, P. Łaniewski, N. Thomas, D. J. Roe, D. M. Chase and M. M. Herbst-Kralovetz, Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling, EBioMedicine, 2019, 44, 675–690 CrossRef PubMed.
- M. P. Kaster, D. G. Machado, A. R. S. Santos and A. L. S. Rodrigues, Involvement of NMDA receptors in the antidepressant-like action of adenosine, Pharmacol. Rep., 2012, 64, 706–713 CrossRef CAS.
- M. G. Rooks and W. S. Garrett, Gut microbiota, metabolites and host immunity, Nat. Rev. Immunol., 2016, 16, 341–352 CrossRef CAS PubMed.
- J. L. Round, S. M. Lee, J. Li, G. Tran, B. Jabri, T. A. Chatila and S. K. Mazmanian, The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota, Science, 2011, 332, 974–977 CrossRef CAS PubMed.
- D. A. Bloes, D. Kretschmer and A. Peschel, Enemy attraction: Bacterial agonists for leukocyte chemotaxis receptors, Nat. Rev. Microbiol., 2015, 13, 95–104 CrossRef CAS PubMed.
- W. J. Lee and K. Hase, Gut microbiota-generated metabolites in animal health and disease, Nat. Chem. Biol., 2014, 10, 416–424 CrossRef CAS PubMed.
- E. Blacher, M. Levy, E. Tatirovsky and E. Elinav, Microbiome-modulated metabolites at the interface of host immunity, J. Immunol., 2017, 198, 572–580 CrossRef CAS PubMed.
- N. Singh, A. Gurav, S. Sivaprakasam, E. Brady, R. Padia, H. Shi, M. Thangaraju, P. D. Prasad, S. Manicassamy, D. H. Munn, J. R. Lee, S. Offermanns and V. Ganapathy, Activation of GPR109A, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis, Immunity, 2014, 40, 128–139 CrossRef CAS PubMed.
- T. D. Hubbard, I. A. Murray and G. H. Perdew, Indole and tryptophan metabolism: Endogenous and dietary routes to ah receptor activation, Drug Metab. Dispos., 2015, 43, 1522–1535 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra00310d |
| This journal is © The Royal Society of Chemistry 2022 |