Open Access Article
Open Access ArticleEvaluation of the freshness of rainbow trout (Oncorhynchus mykiss) fillets by the NIR, E-nose and SPME-GC-MS
Kunli Xu a,
Yuwen Yib,
Jing Dengb,
Yuanhui Wang
a,
Yuwen Yib,
Jing Dengb,
Yuanhui Wang a,
Bo Zhao
a,
Bo Zhao a,
Qianran Sun
a,
Qianran Sun c,
Chenhui Gong
c,
Chenhui Gong a,
Zepeng Yang
a,
Zepeng Yang a,
Hailun Wan
a,
Hailun Wan a,
Ruiyan Hea,
Xinyu Wu
a,
Ruiyan Hea,
Xinyu Wu a,
Bo Yao
a,
Bo Yao a,
Meichao Zhang
a,
Meichao Zhang a and
Yong Tang
a and
Yong Tang *a
*a
aSchool of Food and Biological Engineering, University of Xihua, Chengdu, Sichuan 610039, China. E-mail: jacktangy@gmail.com
bCuisine Science Key Laboratory of Sichuan Province, University of Sichuan Tourism, Chengdu, Sichuan 610100, China
cChengdu Esun Modern Technology Co., Ltd., Chengdu, Sichuan 610041, China
First published on 13th April 2022
Abstract
A comparison study on the freshness of rainbow trout (Oncorhynchus mykiss) fillets in the course of their sale was performed using near-infrared spectroscopy (NIRS), solid-phase microextraction combined with gas chromatography-mass spectrometry (SPME-GC-MS), and the electronic nose (E-nose) technique. Quantitative analysis of the volatile salt nitrogen (TVB-N) of rainbow trout fillets with different freshness using NIR combined with the partial least squares (PLS) method revealed that the predicted values of TVB-N of the samples were significantly correlated with the true values (P < 0.01). SPME-GC-MS combined with E-nose analysis demonstrated that there were significant differences in the volatile flavor components of rainbow trout fillets at different freshness, and E-nose combined with principal component analysis (PCA) and linear discriminant analysis (LDA) could achieve rapid and non-destructive freshness ranking of rainbow trout fillets based on volatile flavor characteristics. Consequently, the NIRS and E-nose non-destructive testing techniques are capable of acting as rapid screening tools for detecting the freshness of rainbow trout fillets during their sale.
1. Introduction
Rainbow trout (Oncorhynchus mykiss), which is widely promoted by the Food and Agriculture Organization of the United Nations (FAO), has become very popular among consumers worldwide. This is because they are not only rich in protein, polyunsaturated fatty acids, minerals, vitamins, and other nutrients, but also have high yields and high quality.1–3 However, the abundant endogenous enzymes and psychrotrophic bacteria in rainbow trout, its fragile tissue structure, and large contact area with air during the selling process easily lead to a decline in the quality of rainbow trout.4 The traditional method for the determination of freshness is usually based on sensory evaluation,5–7 physical and chemical analysis (such as texture,8,9 color variation,10,11 and volatile salt nitrogen (TVB-N) produced by the hydrolysis of specific amino acids by microorganisms and enzymes12,13), microbiological testing,14,15 etc. However, these methods are not only expensive, cumbersome, time-consuming, and destructive for the inspection of rainbow trout during their sale, but also difficult to meet the consumer requirements of fast and non-destructive testing of rainbow trout fillets. Definitely, these goals can be fulfilled using the near-infrared (NIR) and electronic nose (E-nose) non-destructive testing techniques, which have the advantages of high objectivity, fast detection, simple operation, good reproducibility and no requirement of complex sample pre-treatment.NIR spectroscopy in the near-infrared spectral region (wavelength range of 780–2500 nm) is based on the multiplicative and synergistic absorption of organic hydrogen-containing groups (C–H, O–H, and N–H) by molecules, jumping from the ground to upper energy due to the non-harmonic vibrations of molecules.16,17 NIR has been widely used in the evaluation of the quality and safety of meat and meat products in recent years.18,19 de Nadai Bonin et al.20 and Parastar et al.21 used NIR combined with chemometric methods to achieve the rapid detection of intramuscular fat in beef and authenticity of chicken, respectively, both resulting in effective inspection and prediction.
Due to its high sensitivity and superior separation ability, solid-phase microextraction coupled with the gas chromatography-mass spectrometry method (SPME-GC-MS) has been widely used in the analysis of volatile and semi-volatile flavor components of foods. The E-nose is capable of sensing different volatile odor substances, where each sensor in the sensor array has interactive sensitivity with a high capacity to analyze and identify the overall characteristics of volatile flavor substances in food.22,23 Zhang et al.24 used PCA with E-nose and electronic tongue combined with SDE-GC-MS to effectively discriminate the different drying methods of golden pomfret. Wang et al.25 established an E-nose combined with GC-MS method to identify lamb adulterated with inferior duck meat, which makes these technologies have certain practical application value in the identification and reduction of adulterated meat samples. Xu et al.26 reported that gas chromatography-time-of-flight mass spectrometry (GC-TOF-MS) and E-nose analysis revealed significant discrepancies in the content of volatile organic compounds (VOCs) in different parts of Chinese chicken, and subsequently combined with sensory evaluation showed that the flavor of chicken-like breast meat was superior to other parts. Adelina et al.27 demonstrated that PCA combined with HS-SPME/GC-MS and E-nose showed superior separation of two grafted pines under different roasting conditions.
Herein, the present study addresses the issue of the lack of a time-sensitive and non-destructive rapid detection method in the marketing process of rainbow trout fillets to determine their quality. Using NIRS, SPME-GC-MS combined with E-nose, we were able to analyze the quality and safety of rainbow trout fillets. To investigate the quality change pattern of rainbow trout fillets during their sale, a rapid and non-destructive freshness measurement method was established with a positive significance on their quality, ensuring, economic value and additional value, and providing a reference for the safety of rainbow trout consumption and quality evaluation.
2. Materials and methods
2.1. Sample preparation
Freshly purchased rainbow trout was provided by Runzhao Fishery in 2020 (Tianquan, Yaan, Sichuan), with a mass of 3.5–4.5 kg per fish. After being executed in the laboratory, the head, tail, bone, and skin of all rainbow trout were removed and they were gutted, cleaned with distilled water, and cut into pieces of the same size (thickness 0.6 ± 0.1 cm and mass 10.0 ± 2.0 g). Subsequently, they were placed in trays and refrigerated at 4 °C, allowing the fillets to undergo natural decay in preparation for the subsequent determination and acquisition of their TVB-N values, NIR spectral information, GC-MS and E-nose fingerprinting information.2.2. Determination of TVB-N values
The TVB-N values of the rainbow trout fillet samples were measured using the automatic Kjeldahl method with reference to the Food Safety Chinese standard GB 5009.228-2016 “Determination of volatile salt nitrogen in food”,28 and three parallel experiments were performed for each group.2.3. Near-infrared spectral information acquisition
A SupNIR-2720 near-infrared analyzer (Polycom Technology Co. A, Ltd., Hangzhou, China) was used for spectral data acquisition in this experiment. The instrument was preheated for 30 min prior to the experiment, and then the instrument performance test was completed when it was stable, followed by calibration of the instrument with the reference white board. The rainbow trout fillets were removed from the 4 °C freezer, and then the surface water was absorbed using filter paper and the samples spread evenly in the test sample tray for rapid acquisition of NIR spectral information. The temperature of the spectral acquisition environment was 25 °C ± 2 °C, the humidity was 50% ± 5%, and the wavelength range of the instrument was 1000–1800 nm with a resolution of 12 nm. Each group consisted of 10 parallel samples, and to ensure a consistent light range, each parallel needed to reload samples of the same thickness 3 times, a total of 30 times.2.4. SPME-GC-MS analytical methods
The rainbow trout fillets were minced in a centrifuge tube and homogenized with saturated NaCl in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, where the sodium salt was added to increase the ionic strength of the water sample, thus reducing the solubility of the analytes in the aqueous phase and improving the extraction efficiency.29 Through optimization of the experimental conditions, the final solid-phase microextraction protocol was determined as follows: after the treated surimi supernatant was equilibrated by holding at 50 °C for 10 min, the samples were extracted by inserting a manual injection handle fitted with a polydimethylsiloxane/divinylbenzene (PDMS/DVB, 65 μm, 1 cm)-type extraction head at 50 °C for 40 min, and then resolved at 250 °C for 5 min at the GC inlet.
2, where the sodium salt was added to increase the ionic strength of the water sample, thus reducing the solubility of the analytes in the aqueous phase and improving the extraction efficiency.29 Through optimization of the experimental conditions, the final solid-phase microextraction protocol was determined as follows: after the treated surimi supernatant was equilibrated by holding at 50 °C for 10 min, the samples were extracted by inserting a manual injection handle fitted with a polydimethylsiloxane/divinylbenzene (PDMS/DVB, 65 μm, 1 cm)-type extraction head at 50 °C for 40 min, and then resolved at 250 °C for 5 min at the GC inlet.
The chromatographic column was an HP-5 quartz capillary column (30 m × 0.32 mm, 0.25 μm) with helium as the carrier gas. GC-MS method: the flow rate was 1.00 mL min−1; the inlet temperature was 250 °C, and the column was operated in non-split mode. The initial temperature of the column was held at 35 °C for 1 min, and the first stage was ramped up to 200 °C at 10 °C min−1 without holding and the second stage was ramped up to 280 °C at 20 °C min−1 and held for 5 min. MS method: the ionization mode was EI, the electron energy was 70 eV, the ion source temperature was 230 °C, the interface temperature was 260 °C, and the mass scan range was 35–500 m/z. For each sample, the composition of the volatiles was evaluated in terms of peak area percentage.30
2.5. E-nose detection
A PEN3 E-nose (Airsense Analytics, Germany) was used for the odor characterization of the rainbow trout fillets with 10 built-in selective metal oxide semiconductor sensors and Table 1 shows the types of sensitive substances corresponding to each sensor. The electronic nose detection was performed with slight modification according to Huang et al.31 After weighing 4 g of rainbow trout fillet samples in a 20 mL headspace vial and sealing it, the samples were placed in a constant temperature water bath at 60 °C for 30 min, and then taken out for E-nose fingerprinting data acquisition by headspace injection, with each group of samples detected 8 times in parallel. The specific measurement parameters of the E-nose were set as follows: pre-sampling time of 5 s, measurement time of 90 s, flush time of 90 s, zero-point trim time of 10 s, measuring interval time of 1 s, chamber flow of 300 mL min−1, and initial flow of 300 mL min−1.| Sensor number | Sensor name | Main applications (gas detector) |
|---|---|---|
| S1 | W1C | Aroma component |
| S2 | W5S | Oxynitride |
| S3 | W3C | Ammonia (aromatic component) |
| S4 | W6S | Hydrogen |
| S5 | W5C | Aromatic components of alkane |
| S6 | W1S | Methane |
| S7 | W1W | Sulfide |
| S8 | W2S | Alcohols, aldehydes and ketones |
| S9 | W2W | Aromatic and organic sulfide |
| S10 | W3S | Alkanes |
2.6. Data analysis
All statistical analysis was performed using SPSS 26.0 and plotted using Origin Pro 9.0. The analysis of the NIR spectral data was performed with the RIMP Client software, which came with the instrument. The analysis of volatile flavor substances was performed by controlled search in the NIST17 Spectral Library, where the substances with similarities greater than 80 were used as qualitative results, and the peak area normalization algorithm was used to calculate their relative percentage content for substance quantification. The E-nose data analysis was performed using its Winmuster software.3. Results and discussion
3.1. Analysis of TVB-N values of rainbow trout fillets
The results of the TVB-N values of the rainbow trout fillets stored in a refrigerated room at 4 °C for 0–5 days are shown in Fig. 1, where the TVB-N values of the fillets continued to increase with an increase in storage time. The increase in alkaline nitrogenous substances such as ammonia and amines produced by the decomposition of proteins under the action of enzymes and microorganisms was caused by the increase in amino acid destruction in food with the extension of time.32 According to the national food safety standard GB 2733-2015 “Fresh and Frozen Animal Fishery Products”,33 it is stipulated that the TVB-N value as the freshness index of animal food should not exceed 20 mg/100 g. As shown in Fig. 1, the TVB-N value of the rainbow trout fillets was 19.57 mg/100 g on the 2nd day, which reached the critical point of spoilage, while on the 5th day, the TVB-N value was as high as 29.05 mg/100 g. Accordingly, the freshness of rainbow trout fillets can be classified into three groups, as follows: fresh (TVB-N < 15 mg/100 g), sub-fresh (15 mg/100 g ≤ TVB-N ≤ 20 mg/100 g), and putrid (TVB-N > 20 mg/100 g).3.2. NIR analysis
| Spectral pre-processing method | Effects |
|---|---|
| Mean centering (MC) | Improves performance, while eliminating offset and avoiding numerical errors38 |
| Savitzky–Golay smoothing (SGS) | Reduces the random noise caused by the system itself and improves the spectrum signal-to-noise ratio39 |
| Savitzky–Golay derivative (SGD) | Removes baseline drift and improves the signal connected with organic compounds, highlighting characteristic spectra40 |
| Standard normal variate transformation (SNV) | Reduces the influence of inhomogeneous sample particles, linear baseline drift, scattering effects, noise, and other factors on the spectrum41 |
| Multiplication scatter correction (MSC) | Effectively reduces baseline compensation and multiplication effects and eliminates nonlinear baseline drift42 |
According to the sample spread shown in Table 3, the overall range of TVB-N values of the samples was wide, covering the variation of values for the different freshness of the rainbow trout fillets, which was representative. The collected near-infrared spectral sample sets of rainbow trout fillets were divided into calibration and validation sets in the ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to the KS classification method, which was used to establish a quantitative model of TVB-N values of freshness index and to verify the accuracy of the model. The standard deviation of the calibration set (SEC), the correlation coefficient of the calibration set (RC), the standard deviation of the validation set (SEP), the correlation coefficient of the validation set (RP), and standard deviation of the K-fold interaction test set (SECV) were used as model accuracy evaluation parameters to predict the accuracy of the mean deviation test model. Among them, with smaller values of SEC and SEP, the more accurate the prediction of the model, the closer RC and RP are to 1, the better the correlation between the predicted and true values obtained by the model; and the smaller the SECV, the better the regressive model.37
1 according to the KS classification method, which was used to establish a quantitative model of TVB-N values of freshness index and to verify the accuracy of the model. The standard deviation of the calibration set (SEC), the correlation coefficient of the calibration set (RC), the standard deviation of the validation set (SEP), the correlation coefficient of the validation set (RP), and standard deviation of the K-fold interaction test set (SECV) were used as model accuracy evaluation parameters to predict the accuracy of the mean deviation test model. Among them, with smaller values of SEC and SEP, the more accurate the prediction of the model, the closer RC and RP are to 1, the better the correlation between the predicted and true values obtained by the model; and the smaller the SECV, the better the regressive model.37
| Sample | Sample size | TVB-N/(mg/100 g) | |||
|---|---|---|---|---|---|
| Max | Min | Average value | Standard deviation | ||
| Total sample | 180 | 29.776 | 10.708 | 20.370 | 6.165 |
| Calibration | 135 | 29.776 | 10.296 | 20.406 | 6.313 |
| Validation | 45 | 29.336 | 10.708 | 20.273 | 5.812 |
As shown in Table 4, single and combined preprocessing methods were applied to build the quantitative PLS model with the raw spectral data as the blank control. The accuracy evaluation parameters of the model after SNV preprocessing were all good, with the smallest values of SEC and SECV, which indicated that the model had accurate prediction results and the best regression. The values of RC and RP were both greater than 0.95 and better than the model evaluation parameters without the pretreatment method, which indicated that there was a robust correlation between the predicted and true values. Fig. 3 shows the NIR spectra after SNV pre-processing. It can be seen that the SNV pre-processing used in the original NIR spectra of the rainbow trout fillets effectively weakened the influence of noise, scattering effects, and linear baseline drift on the spectra, while the spectral information of the characteristic bands was highlighted, which indicated that the model fitted the information in the spectrum and better reflected the TVB-N content of the rainbow trout fillets.43 Thereby, it can be concluded that pre-processing the raw NIR spectra of rainbow trout fillets with SNV alone can enhance the model performance and improve the accuracy of the model prediction.
| Pretreatment method | PLS components | Calibration set | Validation set | SECV | ||
|---|---|---|---|---|---|---|
| SEC | RC | SEP | RP | |||
| a The window parameter of SGS algorithm was 25 and the number of fits was 3.b The window parameter of SGD algorithm was 11, the number of fits was 2, and the order of derivation was 1. | ||||||
| No pre-processing | 14 | 0.4037 | 0.9981 | 1.5058 | 0.9604 | 1.3462 |
| MC | 14 | 0.3761 | 0.9985 | 1.4282 | 0.9694 | 1.2789 |
| SGS 25-3a | 14 | 0.9909 | 0.9887 | 1.3293 | 0.9739 | 1.4087 |
| SGD 11-2-1b | 9 | 0.8408 | 0.9915 | 1.3622 | 0.9724 | 1.3996 |
| SNV | 13 | 0.3209 | 0.9988 | 1.4314 | 0.9693 | 1.1496 |
| MSC | 11 | 0.6369 | 0.9952 | 1.4830 | 0.9671 | 1.3263 |
| MC + SGD 11-2-1 | 9 | 0.8238 | 0.9919 | 1.3885 | 0.9715 | 1.3930 |
| MC + SGS 25-3 | 14 | 0.9834 | 0.9891 | 1.5236 | 0.9651 | 1.3085 |
| MC + SNV | 9 | 1.8506 | 0.9540 | 3.5205 | 0.8803 | 2.3582 |
| MC + MSC | 4 | 5.9652 | 0.3542 | 5.3696 | 0.4048 | 9.1468 |
| SGS 25-3 + SGD 11-2-1 | 10 | 0.9181 | 0.9898 | 1.4000 | 0.9707 | 1.3788 |
| SGD 11-2-1 + SNV | 9 | 0.8830 | 0.9907 | 1.3825 | 0.9714 | 1.4644 |
| SGD 11-2-1 + MSC | 9 | 0.8895 | 0.9905 | 1.3864 | 0.9712 | 1.4722 |
| SGS 25-3 + SNV | 14 | 0.9250 | 0.9900 | 1.3672 | 0.9720 | 1.2886 |
| SGS 25-3 + MSC | 14 | 0.9391 | 0.9897 | 1.3785 | 0.9715 | 1.3054 |
| MC + SGD 11-2-1 + SGS 25-3 | 10 | 0.8919 | 0.9906 | 1.4266 | 0.9694 | 1.3276 |
| MC + SGD 11-2-1 + SNV | 6 | 1.7962 | 0.9595 | 1.6270 | 0.9605 | 2.0631 |
| MC + SGD 11-2-1 + MSC | 1 | 6.2676 | 0.1114 | 5.9062 | 0.2981 | 6.2378 |
| MC + SGS 25-3 + SNV | 11 | 1.8349 | 0.9567 | 3.6901 | 0.8611 | 2.1726 |
| MC + SGS 25-3 + MSC | 4 | 5.9708 | 0.3518 | 5.4062 | 0.3917 | 7.1418 |
| SGD 11-2-1 + SGS 25-3 + SNV | 10 | 0.9336 | 0.9899 | 1.4050 | 0.9704 | 1.3554 |
| SGD 11-2-1 + SGS 25-3 + MSC | 10 | 0.9382 | 0.9893 | 1.3097 | 0.9747 | 1.3790 |
| MC + SGS 25-3 + SGD 11-2-1 + SNV | 9 | 0.8959 | 0.9909 | 1.7215 | 0.9571 | 1.2067 |
| MC + SGS 25-3 + SGD 11-2-1 + MSC | 9 | 0.9777 | 0.9886 | 1.4028 | 0.9704 | 1.3830 |
Aiming to predict the TVB-N freshness index of the rainbow trout fillets, the measured true TVB-N values of the 10 sample sets that were not involved in the modeling were imported into the established best model, and the model predictions were compared with the true values and determined by correlation analysis, and the results are shown in Table 5. The deviation of the average TVB-N prediction in the model for the external validation samples was 0.16, and the correlation coefficient between the predicted and true values was 0.969, indicating that the predicted values obtained from the model were significantly correlated with the true values and the model had a good predictive ability for the TVB-N freshness index of rainbow trout fillets.
| External verification sample | TVB-N/(mg/100 g) | ||
|---|---|---|---|
| True value | Predicted value | Deviation | |
| 1 | 11.302 | 12.292 | 0.990 |
| 2 | 11.436 | 12.437 | 1.001 |
| 3 | 15.776 | 15.897 | 0.121 |
| 4 | 19.227 | 19.129 | −0.098 |
| 5 | 18.712 | 18.502 | −0.210 |
| 6 | 21.752 | 18.708 | −3.044 |
| 7 | 20.855 | 20.868 | 0.013 |
| 8 | 20.801 | 20.774 | −0.027 |
| 9 | 26.476 | 29.234 | 2.758 |
| 10 | 29.008 | 29.103 | 0.095 |
| Average value | 19.535 | 19.694 | 0.160 |
| Standard deviation | 5.720 | 5.837 | 1.440 |
| Correlation coefficient | 0.969** | ||
3.3. SPME-GC-MS analysis
| Keep time/min | Compounds | Relative content/% | ||
|---|---|---|---|---|
| Fresh | Sub-fresh | Putrid | ||
| a “—” means not detected. | ||||
| Hydrocarbons (47 types) | ||||
| 6.020 | (Z)-Tetradec-3-ene | 0.24 ± 0.03 | — | — |
| 8.500 | 7-Methyl-3-methyleneocta-1,6-diene | — | — | 4.47 ± 0.36 |
| 9.045 | 2,7-Dimethylocta-1,3,7-triene | — | — | 2.22 ± 0.15 |
| 9.115 | cis-1,1,3,5-Tetramethylcyclohexane | — | — | 1.29 ± 0.06 |
| 9.195 | 1,2,4,5-Tetramethylbenzene | — | 0.49 ± 0.02 | — |
| 9.260 | (+)-Limonene | 1.02 ± 0.04 | — | 10.32 ± 1.10 |
| 9.555 | (Z)-β-Ocimene | — | — | 0.65 ± 0.09 |
| 9.575 | alpha-Ocimene | — | 0.80 ± 0.05 | — |
| 9.640 | 2,3,5,8-Tetramethyldecane | 1.44 ± 0.13 | — | — |
| 9.805 | 1-Bromo-3,7-dimethylocta-2,6-diene | — | — | 5.81 ± 0.26 |
| 9.815 | 2,5-Dimethyl-2-hexene | — | 4.99 ± 0.27 | — |
| 10.025 | 1-Tetradecen-3-yne | 4.92 ± 0.33 | — | — |
| 10.170 | 2,5,5-Trimethyl-4-hydroxy-2,6-heptadien | — | — | 3.73 ± 0.41 |
| 10.430 | 2,3,5,8-Tetramethyldecane | 1.58 ± 0.29 | — | 2.01 ± 0.16 |
| 10.700 | 2,5-Dimethyl-6-methylidenespiro[2.4]heptane | 1.09 ± 0.12 | — | — |
| 12.110 | Dodecane | — | — | 1.75 ± 0.18 |
| 12.130 | (8Z,11Z,14Z)-Heptadeca-1,8,11,14-tetraene | 0.72 ± 0.09 | — | — |
| 12.580 | 5-Ethylidene-1-methylcycloheptene | — | — | 0.80 ± 0.04 |
| 13.050 | 5-Methyltetradecane | 0.07 ± 0.02 | — | — |
| 13.255 | 1-Iodotetradecane | 1.21 ± 0.14 | 2.14 ± 0.25 | — |
| 13.380 | 5-Methyl-5-propylnonane | 0.73 ± 0.08 | — | — |
| 13.400 | 5-Butylnonane | — | 0.69 ± 0.07 | — |
| 13.470 | Dodecane,4,6-dimethyl | 0.03 ± 0.00 | — | — |
| 13.580 | 5-(2-Methylpropyl)nonane | 0.18 ± 0.02 | — | — |
| 13.650 | Tridecane | 0.87 ± 0.11 | — | 0.81 ± 0.06 |
| 13.675 | 1-Chlorohexadecane | — | 1.20 ± 0.14 | — |
| 13.890 | 1-Iodo-decane | — | 0.62 ± 0.07 | — |
| 13.950 | Dodecane,4,6-dimethyl | 0.47 ± 0.03 | 0.70 ± 0.04 | 0.33 ± 0.08 |
| 14.020 | Isohexadecane | — | — | 0.44 ± 0.05 |
| 14.240 | 2,6,10-Trimethyltridecane | — | 0.90 ± 0.07 | — |
| 15.105 | Tetradecane | 0.62 ± 0.04 | 0.72 ± 0.03 | — |
| 15.120 | 1-Chlorooctadecane | — | — | 0.92 ± 0.11 |
| 15.390 | (1R,4Z,9S)-4,11,11-Trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | — | — | 0.39 ± 0.06 |
| 15.625 | β-Caryophyllene | — | 0.59 ± 0.09 | — |
| 16.215 | 4-Ethyl-3-nonen-5-yne | 0.26 ± 0.04 | — | — |
| 16.390 | 1-Tetradecene | 0.05 ± 0.01 | — | — |
| 16.400 | 1-Heptadecene | — | — | 0.21 ± 0.01 |
| 16.405 | 1-Pentadecene | — | 0.23 ± 0.04 | — |
| 16.475 | Pentadecane | 4.25 ± 0.26 | — | 6.60 ± 0.53 |
| 16.500 | N-Heptadecane | 0.27 ± 0.03 | 13.85 ± 1.19 | 5.02 ± 0.42 |
| 16.925 | Icosane | — | 0.36 ± 0.01 | — |
| 16.975 | (+/−)-trans-Calamanene | — | 0.29 ± 0.05 | 0.22 ± 0.03 |
| 17.325 | 1-Iodohexadecane | — | 0.12 ± 0.03 | — |
| 17.790 | Hexadecane | — | 0.57 ± 0.05 | 0.41 ± 0.07 |
| 18.360 | Pentacosane | — | 0.07 ± 0.01 | — |
| 18.770 | 1-Heptadecene | — | 0.35 ± 0.04 | — |
| 18.985 | N-Heneicosane | 4.55 ± 0.46 | — | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Alcohols (11types) | ||||
| 8.365 | Oct-1-en-3-ol | 6.79 ± 0.33 | 6.31 ± 0.59 | 4.71 ± 0.27 |
| 9.435 | 2,4-Dimethylcyclohexan-1-ol | 0.64 ± 0.08 | 1.81 ± 0.17 | — |
| 9.795 | 2-Octyn-1-ol | 2.14 ± 0.32 | — | — |
| 10.040 | cis-4-Thujanol | — | 13.55 ± 1.17 | — |
| 10.310 | 2-[(2R,5S)-5-Methyl-5-vinyltetrahydro-2-furanyl]-2-propanol | — | — | 4.82 ± 0.36 |
| 11.280 | cis-Sabinol | — | 1.47 ± 0.22 | — |
| 11.315 | (1alpha,2alpha,5alpha)-2-Methyl-5-(1-methyl-ethyl)bicyclo[3.1.0]hexan-2-ol | — | — | 1.02 ± 0.09 |
| 13.270 | Cyclooctanol | — | — | 1.43 ± 0.16 |
| 14.365 | Cerotin | 1.06 ± 0.07 | — | — |
| 18.250 | (+)-Cedrol | — | 0.05 ± 0.00 | — |
| 18.920 | 2-Hexyl-1-decanol | — | 0.71 ± 0.06 | 0.45 ± 0.04 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Aldehydes (14 types) | ||||
| 4.915 | Hexanal | 22.40 ± 1.41 | 4.65 ± 0.31 | 17.19 ± 1.06 |
| 6.005 | trans-2-Hexenal | — | 0.17 ± 0.01 | — |
| 6.860 | Heptanal | 2.46 ± 0.18 | — | — |
| 6.870 | Decanal | — | 1.77 ± 0.09 | 1.95 ± 0.06 |
| 7.950 | Heptenal | 0.84 ± 0.06 | — | 0.36 ± 0.02 |
| 8.715 | (E,E)-2,4-Heptadienal | 7.74 ± 0.57 | 7.62 ± 0.61 | 1.42 ± 0.13 |
| 8.765 | Octanal | 5.63 ± 0.29 | 4.60 ± 0.34 | 3.89 ± 0.15 |
| 10.570 | Nonanal | 5.15 ± 0.20 | — | — |
| 11.180 | alpha-Cyclocitral | — | — | 1.19 ± 0.03 |
| 13.695 | (2E,4E)-Deca-2,4-dienal | 3.40 ± 0.25 | 1.81 ± 0.17 | — |
| 13.840 | Undecanal | — | 0.51 ± 0.06 | 0.41 ± 0.04 |
| 14.110 | (E,E)-2,4-Dodecadienal | — | 1.92 ± 0.11 | — |
| 15.310 | Tridecanal | 0.25 ± 0.03 | — | 0.35 ± 0.04 |
| 15.320 | Lauryl aldehyde | — | 0.43 ± 0.08 | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Acids (1 type) | ||||
| 7.005 | 2-Amino-5-methylbenzoic acid | 5.73 ± 0.26 | 5.83 ± 0.15 | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Ketones (4 types) | ||||
| 8.405 | 2,3-Octanedione | 10.33 ± 0.53 | 10.70 ± 0.68 | 5.04 ± 0.42 |
| 11.160 | 1-(Furan-2-yl)butan-2-one | 0.19 ± 0.06 | — | — |
| 11.580 | 1-Propan-2-ylbicyclo[3.1.0]hexan-4-one | — | 0.12 ± 0.03 | 0.68 ± 0.08 |
| 13.135 | 3-Methyl-6-(1-methylethyl)-2-cyclohexen-1-one | — | — | 0.67 ± 0.10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Esters (11 types) | ||||
| 7.315 | Methyl hexanoate | — | — | 1.48 ± 0.12 |
| 8.670 | Ethyl hexanoate | — | — | 2.23 ± 0.17 |
| 9.280 | (Z)-3,7-Dimethyl-2,7-octadien-1-yl propanoate | — | 2.59 ± 0.15 | — |
| 10.175 | Methyl 5-oxooxolane-2-carboxylate | — | 3.34 ± 0.21 | — |
| 11.275 | (1S,3R,5S)-4-Methylidene-1-(propan-2-yl)bicyclo[3.1.0]hex-3-yl acetate | — | — | 1.55 ± 0.18 |
| 12.360 | n-Propyl methacrylate | — | — | 0.46 ± 0.07 |
| 14.825 | (3-Hydroxy-2,2,4-trimethylpentyl) 2-methylpropanoate | 0.26 ± 0.01 | — | — |
| 15.020 | Nonyl-2,2,2-trichloroacetat | — | 0.38 ± 0.04 | — |
| 17.695 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | — | — | 0.10 ± 0.00 |
| 18.895 | Nonyl-2-methylpropanoate | 0.33 ± 0.03 | — | — |
| 20.625 | Diisobutyl phthalate | 0.10 ± 0.00 | — | 0.22 ± 0.01 |
 | ||
| Fig. 6 Heatmap of main volatile flavor components of rainbow trout fillets with different freshness. | ||
The composition and content of the volatile flavor substances of the rainbow trout fillets of different freshness are shown in Table 7 and Fig. 7. In the rainbow trout fillets of different freshness, 38, 40, and 42 volatile flavor substance components were identified, respectively, where hydrocarbon compounds were the highest number of volatile profiles classified in all the rainbow trout fillet samples, followed by aldehydes. Furthermore, the changes in hydrocarbons, alcohols, and aldehydes were more pronounced in the rainbow trout fillets from freshness to putridness than that in the other three compounds.
| Ingredient category | Classification number/species (relative content/%) | Average relative content/% | ||
|---|---|---|---|---|
| Fresh | Sub-fresh | Putrid | ||
| Hydrocarbons | 20 (24.55) | 19 (29.67) | 20 (48.38) | 34.20 |
| Alcohols | 4 (10.64) | 6 (23.89) | 5 (26.77) | 20.43 |
| Aldehydes | 8 (47.87) | 9 (23.47) | 8 (12.47) | 27.94 |
| Acids | 1 (5.73) | 1 (5.83) | 0 (0.00) | 3.85 |
| Ketones | 2 (10.51) | 2 (10.82) | 3 (6.39) | 9.24 |
| Esters | 3 (0.7) | 3 (6.32) | 6 (6.03) | 13.05 |
| Total | 38 | 40 | 42 | |
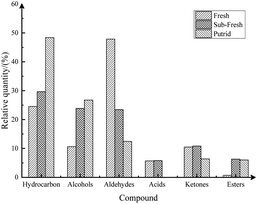 | ||
| Fig. 7 Comparison of the content of various volatile substances in rainbow trout fillets with different freshness. | ||
The production of hydrocarbons is mainly attributed to the homogeneous cleavage of fatty acid alkoxy radicals. In the detection of volatile flavor substances in the rainbow trout fillets of different freshness, hydrocarbons were the most diverse, including alkanes, olefins, and alkynes, and their average relative content was 34.20%, the highest among the substance types. It has been found that various alkanes (C6 to C19) are present in the volatile composition of fish and have the effect of enhancing the overall flavor of fish.44 In this experiment, a total of 20 hydrocarbons were detected in the fresh rainbow trout fillets. Among them, 14 alkanes were detected, being the most volatile category, and mainly concentrated between C7 and C21, where the relative contents of pentadecane and n-heneicosane were higher, in accordance with the results by Josephson et al.46
Alcohols are mainly produced by the oxidative decomposition of lipids and some come from the reduction of carbonyl compounds (such as aldehydes and ketones). The accumulation of some alcoholic volatile components was caused by the metabolism of putrid microorganisms, mainly by the oxidation of polyunsaturated fatty acids.47 The average relative content of alcohols in the detection of volatile flavor substances among the rainbow trout fillets of different freshness was 20.43%, and the relative content showed a stable increasing trend from freshness to putridness. This indicated that alcohols explained the flavor of the putrid rainbow trout fillets to some extent. A small amount of cis-sabinol was detected in the sub-fresh rainbow trout fillets, but not in the putrid rainbow trout fillets, which may be due to the fact that the metabolites produced during the onset of putridness can be further metabolized by microorganisms.48
Aldehydes were one of the main volatile species in the rainbow trout fillets, and they had a lower flavor threshold than alcohols. The test results of the volatiles indicated that 14 types of aldehydes were detected, more than alcohols, but their average relative content was 27.94%, lower than that of alcohols. Similar results were found for French rainbow trout samples.49 These samples also showed a significant decreasing trend as the freshness of the rainbow trout fillets decreased, which indicated that aldehydes contributed more to the aroma and characterized the flavor of fresh rainbow trout fillets.50 Among them, hexanal was one of the most abundant carbonyl compounds in the fresh fish, and it produced a green plant-like aroma within seconds after death.51 In addition, heptanal, n-octanal, and nonanal have also been reported to be present in fresh fish.52 However, (E,E)-2,4-heptadienal detected in aldehydes has been considered as an important correlate of the fishy taste and its formation has been associated with the oxidation of polyunsaturated fatty acids in fish.53
The detection of volatile flavor substances in rainbow trout fillets of different freshness was low for acids, ketones, and esters, with 1, 4, and 11 compounds, respectively. The production of ketones may be due to the thermal oxidation or degradation of unsaturated fatty acids,54 which had a much higher threshold than aldehydes, and therefore contributed relatively little to the fish odor. Among them, the detected 2,3-octanedione showed an “inverted V” trend, which may be representative of the transition from freshness to putrid in fish, in agreement with the results by Duflos et al.55 In contrast, esters were less in type and relative content in the raw fish fillets, probably because esters need to accumulate under high-temperature conditions.
3.4. Rapid nondestructive testing of rainbow trout fillets by E-nose for freshness grading
As shown in Fig. 8, the response trend of the E-nose to the rainbow trout fillet samples with different freshness was consistent, but the freshness of three rainbow trout fillet samples has obvious pattern differences. The response of the sensor to rainbow trout fillets with different freshness was strong to weak in order from putrid > sub-fresh > fresh, which was because the putrid rainbow trout fillets had a more intense and abundant volatile odor than the sub-fresh and fresh fillets. Fig. 9 presents a histogram containing a comparative analysis of the E-nose sensor response values for rainbow trout fillets with different freshness based on the radar plot. The response of W5S, W1S, W1W, and W2S to the rainbow trout fillet samples was stronger than that of the other six sensors, and there were significant differences among the different freshness groups. This result indicates that the volatile odor substances of the rainbow trout fillets were mainly nitrogen oxides, alkanes, sulfides, alcohols, and other aromatic components.
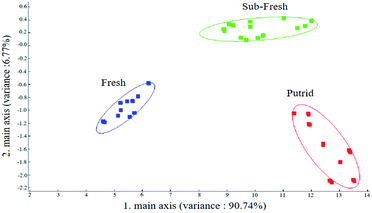
Linear discriminant analysis (LDA) is the projection of high-dimensional sample signal data into a low-dimensional space with good categorical differentiability to maximize the ratio of between-group differences to within-group differences.62,63 Fig. 12 shows the LDA of the rainbow trout fillets with different freshness, which had a better differentiation effect than PCA because the linear discriminant minimized the intra-group variation. As shown in Fig. 12, the contributions of LDA-1 and LDA-2 were 66.40% and 23.97%, respectively, with a cumulative contribution of 90.37%, which can represent the main information of the rainbow trout fillets. The E-nose combined with LDA was effective in distinguishing rainbow trout fillets of different freshness, where the samples of the fresh rainbow trout fillets were farther away from that of the sub-fresh and putrid rainbow trout fillets, which indicates that the volatile odor of the fresh rainbow trout fillets was very different than that of the other two freshness.
According to the research results of the E-nose combined with radar plot analysis, load analysis, PCA and LDA analysis of the rainbow trout fillets with different freshness, it can be concluded that the variations in nitrogen oxides, alkanes, sulfides, alcohols, and other aromatic compounds play a major role in distinguishing the volatile odors of the rainbow trout fillets, and further validate the results of the SPME-GC-MS analysis of volatile flavor substances of the rainbow trout fillets with different freshness. Meanwhile, the results indicate that the E-nose could realize the non-destructive rapid inspection of freshness grading based on the volatile flavor characteristics of rainbow trout fillets, which provides another new idea for non-destructive rapid inspection technology of freshness.
4. Conclusions
The results of the linear regression fitting analysis based on NIR combined with PLS showed that there was a good correlation between the true and predicted values of TVB-N for the different freshness of rainbow trout fillets during the pending sale process. The composition and content of volatile flavor substances in the rainbow trout fillets of three different freshness were found to be very different based on SPME-GC-MS, and the detected main compounds were highly correlated with the response of the E-nose sensors. It was also demonstrated that the E-nose combined with PCA and LDA could achieve rapid non-destructive freshness grading of fresh, sub-fresh, and putrid rainbow trout fillets based on their volatile flavor characteristics, which provides a new thought for freshness non-destructive rapid inspection technology.Author contributions
Conceptualization, Bo Zhao, Yuanhui Wang and Kunli Xu; data curation, Bo Zhao and Kunli Xu; formal analysis, Yuanhui Wang; funding acquisition, Yong Tang; investigation, Hailun Wan, Qianran Sun, Chenhui Gong, Ruiyan He, Xinyu Wu and Bo Yao; methodology, Kunli Xu; project administration, Chenhui Gong and Meichao Zhang; resources, Yuwen YI, Jing Deng, and Yong Tang; software, Zepeng Yang; supervision, Qianran Sun; visualization, Zepeng Yang and Meichao Zhang; writing – original draft, Kunli Xu and Bo Zhao; writing – review & editing, Kunli Xu and Yong Tang.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Chunhui Project of the Ministry of Education (Grant No. Z2016142); the Key R&D projects of the Department of Science and Technology of Sichuan Province (Grant No. 2020YFN0022); the Key R&D Projects of the Department of Science and Technology of Zhejiang Province (Grant No. 2019C02075); Project of Science and Technology Department of Sichuan Province (Grant No. 2020YFH0130); the Innovation and Entrepreneurship Program for College Students (Grant No. S201910622061).References
- L. Xiao, Effect of temperature fluctuation during circulation on quality change and microbial diversity of bigorder tuna, Shanghai Ocean University, Shanghai, 2018 Search PubMed.
- Q. He, Z. Li, Z. Yang, Y. Zhang and J. Liu, Aquaculture, 2020, 514, 734506 CrossRef CAS.
- M. M. Reis, E. Martínez, E. Saitua, R. Rodríguez, I. Pérez and I. Olabarrieta, Lebensm.-Wiss. Technol., 2017, 78, 129–137 CrossRef CAS.
- L. Wu, H. Pu and D. W. Sun, Trends Food Sci. Technol., 2019, 83, 259–273 CrossRef CAS.
- S. O. Knowles, N. D. Grace, J. R. Rounce and C. E. Realini, Food Res. Int., 2020, 137, 109655 CrossRef CAS PubMed.
- J. Zhang, B. Bowker, Y. Yang, B. Pang and H. Zhuang, Lebensm.-Wiss. Technol., 2020, 120, 108939 CrossRef CAS.
- D. P. Green, Handb. Seafood Qual., Saf. Health Appl., 2010, 29–38 Search PubMed.
- A. Aussanasuwannakul, S. D. Slider, M. Salem, J. Yao and P. Brett Kenney, J. Food Sci., 2012, 77, S335–S341 CrossRef CAS PubMed.
- J. H. Cheng, D. W. Sun, Z. Han and X. A. Zeng, Compr. Rev. Food Sci. Food Saf., 2014, 13, 52–61 CrossRef.
- Y. H. Kim, J. T. Keeton, H. S. Yang, S. B. Smith, J. E. Sawyer and J. W. Savell, Meat Sci., 2009, 82, 234–240 CrossRef CAS PubMed.
- D. J. Livingston and W. D. Brown, Food Technol., 1981, 35, 238–252 Search PubMed.
- Y. Zhang, Q. Luo, K. Ding, S. G. Liu and X. Shi, Sens. Actuators, B, 2021, 335, 129708 CrossRef CAS.
- Y. Li, X. Tang, Z. Shen and J. Dong, Food Chem., 2019, 287, 126–132 CrossRef CAS.
- A. Naila, S. Flint, G. C. Fletcher, P. J. Bremer, G. Meerdink and R. H. Morton, Food Chem., 2012, 135, 2650–2660 CrossRef CAS PubMed.
- L. Huang, J. Zhao, Q. Chen and Y. Zhang, Food Res. Int., 2013, 54, 821–828 CrossRef CAS.
- M. Zareef, Q. Chen, M. Hassan, M. Arslan, M. M. Hashim, W. Ahmad, F. Kutsanedzie and A. A. Agyekum, Food Eng. Rev., 2020, 12, 173–190 CrossRef CAS.
- K. B. Beć, J. Grabska and C. W. Huck, Chem.–Eur. J., 2021, 27, 1514–1532 CrossRef PubMed.
- S. Savoia, A. Albera, A. Brugiapaglia, L. Di Stasio, A. Ferragina, A. Cecchinato and G. Bittante, Meat Sci., 2020, 161, 108017 CrossRef CAS PubMed.
- M. Á. Fernández-Barroso, S. Parrini, M. Muñoz, P. Palma-Granados, G. Matos, L. Ramírez, A. Crovetti, J. M. García-Casco and R. Bozzi, J. Food Compos. Anal., 2021, 102, 104018 CrossRef.
- M. de Nadai Bonin, S. da Luz e Silva, L. Bünger, D. Ross, G. L. Dias Feijó, R. da Costa Gomes, F. Palma Rennó, M. H. de Almeida Santana, F. Marcondes de Rezende, L. C. Vinhas Ítavo, F. J. de Novais, L. M. A. Surita, M. de Nadai Bonin, M. W. Filgueira Pereira and J. B. S. Ferraz, Meat Sci., 2020, 163, 108077 CrossRef PubMed.
- H. Parastar, G. van Kollenburg, Y. Weesepoel, A. van den Doel, L. Buydens and J. Jansen, Food Control, 2020, 112, 107149 CrossRef CAS.
- H. Zeng, Q. Li and Y. Gu, Chin. Phys. B, 2016, 25, 024201 CrossRef.
- M. Ghasemi-varnamkhasti and M. Aghbashlo, Trends Food Sci. Technol., 2014, 38, 158–166 CrossRef CAS.
- J. Zhang, J. Cao, Z. Pei, P. Wei, D. Xiang, X. Cao, X. Shen and C. Li, Food Res. Int., 2019, 123, 217–225 CrossRef CAS PubMed.
- Q. Wang, L. Li, W. Ding, D. Zhang, J. Wang, K. Reed and B. Zhang, Food Control, 2019, 98, 431–438 CrossRef CAS.
- Y. Xu, Y. P. Chen, S. Deng, C. Li, X. Xu, G. Zhou and Y. Liu, Food Res. Int., 2020, 137, 109669 CrossRef CAS PubMed.
- N. M. Adelina, H. Wang, L. Zhang and Y. Zhao, Food Res. Int., 2021, 140, 110026 CrossRef CAS PubMed.
- GB 2009.228-2016, Determination of Volatile base Nitrogen in Food, Standards Press of China, Beijing, 2016 Search PubMed.
- J. Pawliszyn, Z. Zhang and T. Gorecki, Theory and Practice of Solid Phase Microextraction, Federation of Analytical Chemistry, United States, 1995 Search PubMed.
- E. Kafkas, T. Cabaroglu, S. Selli, A. Bozdoğan, M. Kürkçüoğlu, S. Paydaş and K. H. C. Başer, Flavour Fragrance J., 2006, 21, 68–71 CrossRef CAS.
- X. H. Huang, L. B. Qi, B. S. Fu, Z. H. Chen, Y. Y. Zhang, M. Du, X. P. Dong, B. W. Zhu and L. Qin, Food Chem., 2019, 286, 241–249 CrossRef CAS PubMed.
- A. E. D. A. Bekhit, B. W. B. Holman, S. G. Giteru and D. L. Hopkins, Sci. Technol., 2021, 109, 280–302 CAS.
- GB 2733-2015, Fresh and Frozen Animal Aquatic Products, Standards Press of China, Beijing, 2015 Search PubMed.
- M. Qassem and P. A. Kyriacou, J. Biomed. Opt., 2014, 19, 87007 CrossRef PubMed.
- N. Prieto, R. Roehe, P. Lavín, G. Batten and S. Andrés, Meat Sci., 2009, 83, 175–186 CrossRef CAS PubMed.
- X. Yu, J. Wang, S. Wen, J. Yang and F. Zhang, Biosyst. Eng., 2019, 178, 244–255 CrossRef.
- Seema, A. K. Ghosh, B. S. Das and N. Reddy, Geoderma Regional, 2020, 23, e00349 CrossRef.
- M. Juybar, M. Khanmohammadi Khorrami, A. Bagheri Garmarudi and S. Zandbaaf, Spectrochim. Acta, Part A, 2020, 228, 117539 CrossRef CAS.
- S. Weng, B. Guo, P. Tang, X. Yin, F. Pan, J. Zhao, L. Huang and D. Zhang, Spectrochim. Acta, Part A, 2020, 230, 118005 CrossRef CAS.
- H. Jiang, Y. Ru, Q. Chen, J. Wang and L. Xu, Spectrochim. Acta, Part A, 2021, 249, 119307 CrossRef CAS PubMed.
- H. Jiang, X. Jiang, Y. Ru, J. Wang, L. Xu and H. Zhou, Infrared Phys. Technol., 2020, 110, 103467 CrossRef CAS.
- T. Tiecher, J. M. Moura-Bueno, L. Caner, J. P. G. Minella, O. Evrard, R. Ramon, G. Naibo, C. A. P. Barros, Y. J. A. B. Silva, F. F. Amorim and D. S. Rheinheimer, Geoderma, 2021, 384, 114815 CrossRef CAS.
- A. A. Agyekum, F. Y. H. Kutsanedzie, V. Annavaram, B. K. Mintah, E. K. Asare and B. Wang, Vib. Spectrosc., 2020, 108, 103044 CrossRef CAS.
- J. Iglesias and I. Medina, J. Chromatogr. A, 2008, 1192, 9–16 CrossRef CAS PubMed.
- D. Yu, Y. Xu, J. M. Regenstein, W. Xia, F. Yang, Q. Jiang and B. Wang, Food Chem., 2018, 242, 412–420 CrossRef CAS PubMed.
- D. B. Josephson, R. C. Lindsay and D. A. Stuiber, J. Food Sci., 1987, 52, 596–600 CrossRef CAS.
- F. F. Parlapani, A. Mallouchos, S. A. Haroutounian and I. S. Boziaris, Int. J. Food Microbiol., 2014, 189, 153–163 CrossRef CAS PubMed.
- F. Leduc, P. Tournayre, N. Kondjoyan, F. Mercier, P. Malle, O. Kol, J. L. Berdagué and G. Duflos, Food Chem., 2012, 131, 1304–1311 CrossRef CAS.
- S. Selli, C. Prost and T. Serot, Food Chem., 2009, 114, 317–322 CrossRef CAS.
- C. Alasalvar, K. D. A. Taylor and F. Shahidi, J. Agric. Food Chem., 2005, 53, 2616–2622 CrossRef CAS.
- D. Josephson and R. Lindsay, Biogeneration of Aromas, T. H. Parliament and R. Croteau, 1155 Sixteenth Street N.W.Washington, DC, United States of America, 1986, pp. 201–219 Search PubMed.
- T. Kawai and M. Sakaguchi, Crit. Rev. Food Sci. Nutr., 1996, 36, 257–298 CrossRef CAS PubMed.
- C. F. Ross and D. M. Smith, Compr. Rev. Food Sci. Food Saf., 2006, 5, 18–25 CrossRef CAS PubMed.
- Y. J. Cha, K. R. Cadwallader and H. H. Baek, J. Food Sci., 1993, 58, 525–530 CrossRef CAS.
- G. Duflos, F. Leduc, A. N'Guessan, F. Krzewinski, O. Kol and P. Malle, J. Sci. Food Agric., 2010, 90, 2568–2575 CrossRef CAS PubMed.
- J. Tan and J. Xu, Artif. Intell. Med., 2020, 4, 104–115 Search PubMed.
- S. Trirongjitmoah, Z. Juengmunkong, K. Srikulnath and P. Somboon, Comput. Electron. Agric., 2015, 113, 148–153 CrossRef.
- H. Zhang, J. Wang, X. Tian, H. Yu and Y. Yu, J. Food Eng., 2007, 82, 403–408 CrossRef.
- A. H. Gómez, J. Wang, G. Hu and A. G. Pereira, Sens. Actuators, B, 2006, 113, 347–353 CrossRef.
- H. Yu, Y. Wang and J. Wang, Sensors, 2009, 9, 8073–8082 CrossRef.
- Z. Wei, J. Wang and W. Zhang, Food Chem., 2015, 177, 89–96 CrossRef CAS PubMed.
- M. Ghasemi-Varnamkhasti, M. Tohidi, P. Mishra and Z. Izadi, Postharvest Biol. Technol., 2018, 138, 134–139 CrossRef.
- Radi, S. Ciptohadijoyo, W. S. Litananda, M. Rivai and M. H. Purnomo, Comput. Electron. Agric., 2016, 121, 429–435 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |