Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent trends and advances in polyindole-based nanocomposites as potential antimicrobial agents: a mini review
Hareesh Pradeepa,
Bindu M.b,
Shwetha Sureshb,
Anjitha Thadathila and
Pradeepan Periyat *b
*b
aDepartment of Chemistry, University of Calicut, Kerala, India-673635
bDepartment of Environmental Studies, Kannur University, Kerala, India. E-mail: pperiyat@uoc.ac.in; pperiyat@kannuruniv.ac.in
First published on 15th March 2022
Abstract
Infections caused by multi-drug resistant microbes are a big challenge to the medical field and it necessitates the need for new biomedical agents that can act as potential candidates against these pathogens. Several polyindole based nanocomposites were found to exhibit the ability to release reactive oxygen species (ROS) and hence they show excellent antimicrobial properties. The features of polyindole can be fine-tuned to make them potential alternatives to antibiotics and antifungal medicines. This review clearly portrays the antimicrobial properties of polyindole based nanocomposites, reported so far for biomedical applications. This review will give a clear insight into the scope and possibilities for further research on the biomedical applications of polyindole based nanocomposites.
1. Introduction
Microorganism induced resistance, based on the built-in abilities to nullify the activity of current antibiotics has been considered very crucial in regards to public health, especially in a scenario of the alarming increase in untreatable bacterial infections and scarcity in the production of new antibiotics.1–3 One alternative to address the impact of this issue is prevention, i.e., hampering the growth and development or simply preventing their adhesion.4–6 In this regard, the development of novel antibacterial materials to avoid the usage of antibiotics becomes an excellent approach. The replacement of conventional antibiotics by nanocomposites presents important advantages to deactivate new strategies of intrinsic resistance developed by multidrug-or even pan-drug-resistant microorganisms.7,8The exciting properties of nanotechnology have led to the development of antimicrobial nanomaterials in recent years. Nanomaterials can be used as an alternative to antibiotics, because of the ease in fine tuning of their properties such as particle size, crystal defect and morphology.9 Understanding the mechanism of antibacterial activity of nanomaterials is important in controlling the in vivo dosage.10 The ability of the material to produce reactive oxygen species (ROS) is of precise attention in regards to toxicity, owing to the oxidation of various cellular constituents by oxygen centered reactive species. ROS may comprise superoxide anions (O2˙−), hydroxyl radicals (OH˙), singlet oxygen and secondary oxygen centered species such as H2O2 (formed by the disproportionation of O2˙−) which then converted to OH˙ and singlet oxygen.11–13 Excellent literature reports are available based on the antibacterial activity of nano ZnO, TiO2, MgO, CuO, ZnO/TiO2 hybrids and Ag3PO4.14–18 It has been shown that, the ROS generated by nanomaterials can be used to treat cancer cells11,19–21 and ROS generation strongly depends upon the shape, size, surface area, charge and heterostructure of the nanomaterials.
Fig. 1(A) represents the influence of metallic and metallic oxide nanoparticles on the living systems and Fig. 1(B) represents the factors influencing the nanomaterials induced ROS generation. As the size of the materials becomes nano dimensions, there may be structural defects, owing to which alteration in the surface properties occurs. Electron donor or acceptor, then reacts with oxygen, leading to the formation of superoxide anions (O2˙−), which further undergoes Fenton type reactions22–24 to generate additional ROS. According to Fenton mechanism, the metal or metallic oxide nanoparticles react with H2O2 to form OH˙ and oxidized metal ion. There is one more mechanism, i.e. Haber Weiss mechanism, in which generation of OH˙ via the reaction between H2O2 and oxidized metal ions.25–28
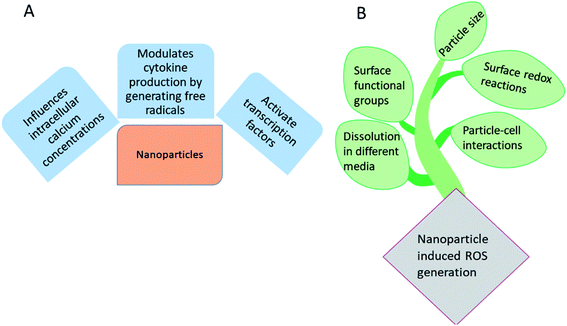 | ||
| Fig. 1 (A) Influence of metallic and metallic oxide nanoparticles on the living systems and (B) major factors involved in the nanomaterials induced ROS generation. | ||
Fig. 2 shows a schematic representation of the mechanism of nanoparticle induced ROS generation. Various steps involved are: (1) endocytosis (2) generation of endocytotic vesicles (3) release of nanoparticles from vesicles into the cell. The nanoparticle may then interact with mitochondria and NADPH oxidase, leading to the formation of ROS, owing to which DNA damage, cell cycle termination and alteration in apoptosis occurs.29
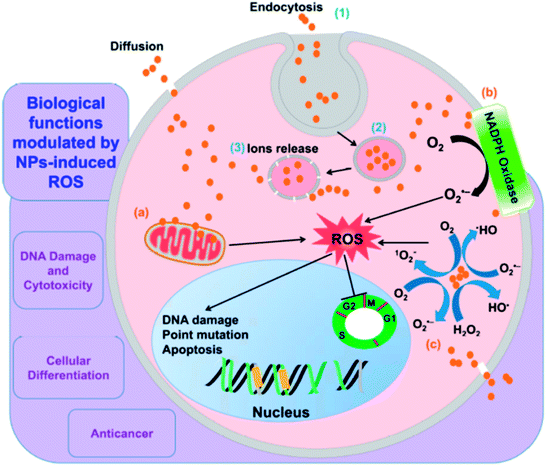 | ||
| Fig. 2 A schematic representation of mechanism of nanoparticle induced ROS generation. Reprinted with permission from ref. 29; copyright © MDPI. | ||
It has been reported that H2O2 can induce oxidative stress on living cells by forming ROS intracellularly.30,31 The intracellular ROS formation may happen either by some metabolic process (endogenous) or by other entities such as nanoparticles (exogenous).32–34 The ROS facilitated antibacterial activity has been found to be pro-inflammatory.35–37 Investigation of the antioxidant features of nanomaterials towards macrophages is of particular attention, owing to the fact that macrophage targeting may be employed to deliver anti-inflammatory drugs at the site of inflammation.
It has been reported that the inflammation (swelling at a particular area, pain and redness due to some injury or infection) is enhanced by some mechanism including ROS generation in macrophages.38,39 Studies reveal that the size of nanoparticles plays a crucial role in their uptake by macrophages.40 Materials which exhibit both antibacterial and anti-inflammatory properties simultaneously have potential for a variety of biomedical applications.41–45
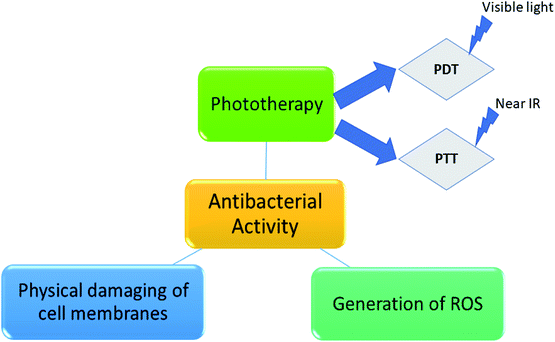
Photodynamic therapy (PDT) is a method of bacterial inactivation through oxidative stress by photosensitization. The photosensitizer absorbs specific wavelength of light, usually from laser sources, followed by visible light irradiation generates ROS.46,47 Uncontrolled formation of ROS leading to cell damage and cell death. The photosensitizer is either put into the blood stream via veins or applied directly on skin. After a certain period of time, drug is absorbed by the harmful cells. Upon irradiation to the area to be treated, the drug reacts and kills the cells. The time gap between the drug is given and light irradiation is known as drug to light interval.48
As a replacement of PDT by photothermal therapy (PTT), also requires photosensitizer which converts light energy into heat, owing to which cell impairment occurs. Generation of heat leads to aggregation and denaturation of the proteins, causes cell death. Here, the irradiation is done by near IR light.49,50 Scheme 1 shows different mechanistic pathway of antibacterial activity.
Nowadays, polymer nanocomposites have extensively been employed for antibacterial, tissue engineering, cancer therapy, medical imaging, drug delivery and dental applications. Polymer nanocomposites consist of a macromolecular matrix, in which nano fillers are embedded. Polymers are excellent hosting materials to fabricate composites, because of the easiness in tailoring their characteristics, to obtain a system, having good processability and durability. Addition of nanofillers to such matrices generates a material, with desired and fine-tuned properties than their counterparts.50–55 Polylactide, poly-glycolide and polycaprolactone are biodegradable and biocompatible polymers.56–58 Polymer nanocomposites, based on chitosan, poly(N-vinyl-2-pyrrolidone) (PVP), polyvinyl alcohol (PVA) and polyvinyl chloride (PVC) have been investigated for antimicrobial properties' evaluation and Table 1 lists a concise literature report of some commonly employed polymer nanocomposites as antimicrobial medicine.
| Polymer nanocomposites | Compositions | Species | CFU mL−1 | Antibacterial activity performances | Biocompatibility tests | Ref. |
|---|---|---|---|---|---|---|
| a BR: bactericidal rate, DIZ: diameter of inhibitory zone, MIC: minimum inhibitory concentration, CFU: colony forming units. | ||||||
| Chitosan/GO/iron oxide | 0.1 wt% GO/iron oxide | S. aureus (Gram positive) | 1 × 105 | DIZ ∼15 mm | Causing concentration dependent hemolysis of human red blood cells | 63 |
| E. coli (Gram negative) | 1 × 106 | DIZ ∼15 mm | ||||
| Chitosan/GO/ZnO | NG | S. aureus | 1 × 106 | MIC: 0.1 μg mL−1 | — | 64 |
| E. coli | 1 × 106 | MIC: 0.1 μg mL−1 | ||||
| Chitosan/GO/TiO2 | Chitosan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GO GO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 (1 TiO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
B. subtilis (Gram positive) | 1 × 108 | With 40 μg mL−1 of the material, the OD600 of B. subtilis drop from 0.79 to 0.33 for 12 h | Not causing cytotoxicity against mammalian somatic cells and plant cells | 65 |
| PVA/GO | 0.1 wt% GO | S. aureus | 2 × 105 | No obvious (24 h) | — | 66 |
| E. coli | 2 × 104 | |||||
| PVA/GO/AgNPs | 0.01 wt% GO, 10 wt% PVA, 3.9 mM AgNO3 | S. aureus | 1 × 106 | BR: 100% (3 h) | — | 67 |
| E. coli | 1 × 106 | BR: 100% (3 h) | ||||
| PLA/GO–ZnO | 0.2 wt% GO–ZnO | S. aureus | — | BR: 83% (24 h no light), 99% (24 h with light) | — | 68 |
| E. coli | — | BR: 52% (24 h no light), 98% (24 h with light) | ||||
| PAM/rGO/Ag | 1 wt% PAM/rGO | S. aureus | — | DIZ: 47 mm | — | 69 |
| Pseudomonas (Gram negative) | — | DIZ: 45 mm | ||||
2. Conducting polymer nanocomposites as biomedical agents
Conducting polymers (CPs) are a specific category of synthetic polymers with exceptional electrical and optical characteristics, which involve conjugated chains with alternating single and double bonds.59,60 Polyacetylene (PA), polythiophene (PT), poly[3,4-(ethylenedioxy)thiophene] (PEDOT), polypyrrole (PPy), polyindole (PIN), polyphenylene and polyaniline (PANi) are some examples of the most extensively used CPs in biomedical area.61 CPs have demonstrated promising candidates for numerous biomedical applications due to their biocompatibility, gifted response to electrical fields, high electrical conductivity, low-toxicity, good environmental stability, and nanostructured morphology.62 Recently, conducting polymers are widely used as antimicrobial and antifungal agents in various sectors such as bio-medical field, food industry, coating industry etc. The tendency for CPs to have low processability and not to be degradable, which can potentially be overcome by the synthesis of degradable CPs that are solution processable, and fabrication of CP blends and nanocomposites with various (bio) polymers and nanomaterials, respectively. Table 2 give a complete literature analysis of conducting polymer nanocomposite materials reported so far as antimicrobial medicine.| Conducting nanocomposites | Species | CFU mL−1 | Antibacterial activity performances | Biocompatibility tests | Ref. |
|---|---|---|---|---|---|
| a MBC: minimum bactericidal concentration. | |||||
| PPy–Pd | S. aureus (Gram positive) | — | MIC: 5.78 mg mL−1 | — | 70 |
| MBC: 23.12 mg mL−1 | |||||
| PPY–Zn@CuO | E. coli (Gram negative) | — | MIC: 0.078 mg mL−1 | PPY–Zn@CuO pertaining to minimal cytotoxicity | 71 |
| S. aureus | — | MIC: 0.156 mg mL−1 | |||
| PANI–Zn@CuO | E. coli | MIC: 0.144 mg mL−1 | PANI–Zn@CuO and PPY–Zn@CuO pertaining to minimal cytotoxicity | 71 | |
| S. aureus | MIC: 0.144 mg mL−1 | ||||
| PPy–NT Ag-NP | E. coli | — | MIC: 0.078 mg mL−1 | — | 72 |
| S. aureus | — | MIC: 0.15625 mg mL−1 | |||
| polyaniline/Pt–Pd | Staphylococcus sp (Gram positive) | MIC: 25 mg mL−1 | — | 73 | |
| MBC: 150 mg mL−1 | |||||
| AuNP–PTh | E. coli MTCC 433 | 1 × 106 | MBC: 112 μM | No harmful influence of AuNP–PTh treatment for various time periods (24 and 48 h) | 74 |
| L. monocytogenes Scott A (Gram positive) | 1 × 106 | MBC: 112 μM | |||
| Cu–PANI | E. coli | 1 × 106 | — | — | 75 |
| S. aureus | 1 × 106 | ||||
2.1. Polyindole (PIN)
Owing to the unique physical and electrochemical properties, polyindole (PIN) has gained marvellous consideration of the researchers, in the past couple of years. They belong to the fused ring compound family, which possesses a benzene and a pyrrole ring; so polyindole have the features of poly(para-phenylene) and polypyrrole76 as well. Several studies on polyindole revealed that they could be used as promising candidates for applications such as supercapacitors, batteries, electrochromic devices, sensors, electrocatalysis, catalysis, and anticorrosion.77–83 Graphene and silver nanoparticles loaded polyindole have been subjected to electro activity studies and found that such system can be used as an electrode material for various applications.84In the early 1976, initial studies have begun on the development of chemical polymerization methods to synthesize polyindole from indole.85 In 1982, Tourillon and Garnier synthesized conducting polyindole by employing electrochemical methods.86 Compared to polyaniline and polypyrrole, polyindole exhibit high thermal stability (crucial for sterilization, e.g.; in an autoclave), excellent oxidation–reduction activity (redox activity), chargeable electrical conductivity, slow rate of degradation and good blending properties.87 Because of its exceptional advantages in various domains, many scientists have done healthy research on polyindole and their derivatives in terms of their synthesis, properties, structure and applications. Two strategies have been employed for the synthesis PIN from indole monomers, chemical oxidative polymerization and electrochemical polymerization.88 We can precisely control the morphology of PIN formed such as nanowires, nanorods, nano- and micro-fibers, nano- and micro-spheres, and nanobelts.78 The chemical oxidative polymerization technique has been employed for the large scale production of PIN.89 The mechanism involves the formation of radical cations, by the oxidation of indole monomer and these indole radical cations couple together at 2 and 3 position. The deprotonation of the coupled species results in the formation of a dimer, which again undergoes oxidation, coupling and deprotonation, results in the formation of a trimer and the chain propagates to form the final product as polyindole. The mechanism has been depicted as Fig. 3.
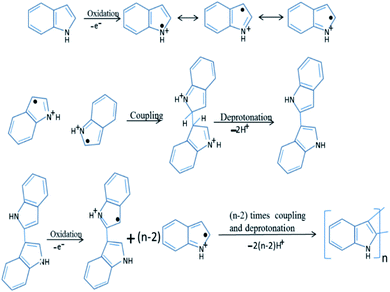 | ||
| Fig. 3 Mechanism of chemical polymerization of PIN. Reprinted with permission from ref. 88; copyright ©Elsevier. | ||
Unlike the chemical polymerization methods, electropolymerization produces PIN directly on a target electrode substrate in a three electrode system. A binder-free electrode has been achieved by using an organic or non-organic electrolyte and dopant material.89 Mechanism of the electrochemical polymerization of PIN has been shown as Fig. 4. The coupling position of indole moieties during the polymerisation strongly depends upon the nature of the solution and electrolyte used.90
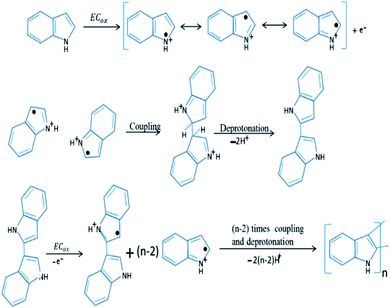 | ||
| Fig. 4 Electrochemical polymerization mechanism of PIN formation. Reprinted with permission from ref. 88; copyright © Elsevier. | ||
Polyindole based nanocomposites exhibit outstanding antimicrobial properties because of its promising capability to generate ROS, it can effectively inhibit microbial growth. Although many metal and metal oxide nanoparticles offer excellent antimicrobial activity, their cytotoxicity and safety concerns still exist as a challenge.91 Polyindole based nanocomposites have been found to exhibit enhanced antimicrobial activity than its partners due to the mutual synergistic enhancement of their properties.92 Also, they have less cytotoxic effects on human bodies. Hence, polyindole nanocomposites can be substituted as a potential alternative for antibiotics and can act as an effective biomedical agent.
2.2. Antimicrobial features of polyindole based nanocomposites
The oxidative polymerization of indole moieties produces positive charges at fixed intervals of monomers, along the polymeric chain of polyindole. This cationic nature is responsible for the antibacterial activity of the resulting PIN. The positive charge of polyindole chains electrostatically interacts with the negatively charged surfaces of bacterial cell wall, irreversibly interrupting the membrane structure of the bacteria, leading to penetration through the cells, and efficiently hindering the protein activity.87 Owing to the interaction with the charged surfaces and the diffusion of reactive species into the cell wall, cell death occurs by the leakage of vital components from the cells. A schematic representation of the electrostatic interactions and the step of cell death has been shown as Fig. 5.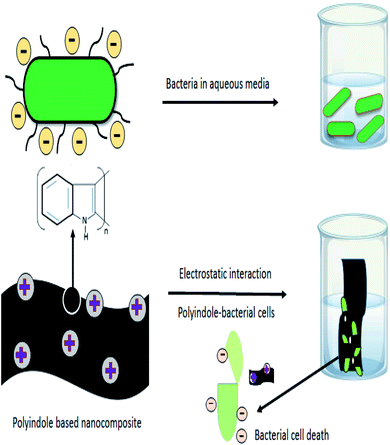 | ||
| Fig. 5 Schematic representation of electrostatic interactions involved in the mechanism of antibacterial activity of polyindole nanocomposites. | ||
The incorporation of nanomaterial into polyindole matrices enhance the performance against bacterial growth, proliferation, and the following cell death owing to the synergistic interaction of the components. With this goal, the polyindole has been combined with different fillers such as Ag, Ag–CuO, Ag–ZnO, Ag/CeO2, Ag/Co3O4, graphene, ZrO2, TiO2, and NiO/ZnO nanoparticles. In the following section, strategies for the optimization of polyindole-based nanocomposites as antibacterial agents have been discussed.
The antimicrobial activity of Ag and its ionic form is due to the binding of metallic ions to certain bio-macromolecular components. It has been reported that the cationic Ag targets binds to negatively charged components of the proteins and nucleic acid, leads to structural deformations in cell membrane and nucleic acids.98–100 Ag ions can also interact with electron rich functional groups such as thiols, hydroxyls, imidazoles, phosphates, indoles and amines.100 The binding of Ag ions to DNA, block transcription whereas those binds to cell surface inhibits bacterial respiration and ATP (adenosine triphosphate) synthesis and Ag ions have the potential to block the respiratory chain of microorganism in the cytochrome oxidase and NADH-succinate dehydrogenase region.10 Various mechanisms have been suggested to describe the antimicrobial activity of Ag nanoparticles. They are (1) slow release of Ag ions followed by suppression of ATP production and replication of DNA (2) cell membrane damaging directly (3) Production of ROS.101 Electron spin resonance (ESR) studies have been used to confirm the ROS generation.
Many polyindole derivatives have been prepared till now, in regards to the antimicrobial activity of indole monomer, some them shows fungicidal activity. In an interesting work, 1-allylindole-3 carbaldehyde (AIC) was used as the monomer and polymerization was carried out by atom transfer radical polymerization (ATRP) strategy, to form a polyindole derivative.92 The synthesis strategy has been depicted as Fig. 6.
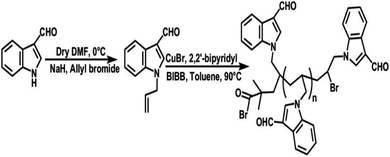 | ||
| Fig. 6 Synthesis strategy of poly(1-allylindole-3 carbaldehyde). Reprinted with permission from ref. 92; copyright © Wiley. | ||
Ag nanoparticles were prepared by using solutions of AgNO3 and NaBH4 as precursors. Addition of AgNO3 to NaBH4, causes the reduction of AgNO3 to Ag nanoparticles. A thin layer of borohydride anion got absorbed on the surface of nanoparticles, prevents their agglomeration. Ag nanoparticles synthesized were then introduced into the polymer matrix by ultrasonic-assisted method.93
Antibacterial studies were carried out against human pathogenic bacteria, by two different methods, disc diffusion method and broth dilution method.102 The results obtained from both the methods were similar, and observed that the monomer AIC doesn't exhibit any antibacterial properties at all, but the polymer PAIC [poly(1-allylindole-3 carbaldehyde) and the nanocomposite (PAICN) does. Table 3 shows the response of different systems towards pathogens.
| Sample | Gram positive bacteria | Gram negative bacteria | |||
|---|---|---|---|---|---|
| E. faecalis | S. aureus | E. coli | P. mirabilis | K. pneumoniae | |
| a —, no antibacterial activity; +, less than 7 mm; ++, 8–15 mm; +++, more than 15 mm. | |||||
| Disc diffusion data | |||||
| AIC | — | — | — | — | — |
| PAIC | — | + | — | ++ | +++ |
| PAICN | ++ | — | — | — | + |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Minimum inhibitory concentration (MIC, in μg mL−1) against pathogens, determined after one day of incubation | |||||
| AIC | — | — | — | — | — |
| PAIC | — | >50 | — | 50 | 40 |
| PAICN | 35 | — | — | — | >50 |
The association of aldehyde group of PAIC with unprotonated amines on the outer layer of bacterial cells is responsible for its antibacterial activity.103 An insignificant activity has been observed against S. aureus and E. faecalis. They exist as mucoid strains and their cells are being enclosed by a slime coating. The non-mucoid strains are affected more quickly compared to mucoid strains. PAICN shows activity against E. faecalis, with a minimum inhibitory concentration of 35 μgmL−1, owing to the slow release of Ag nanoparticles from the matrix. But, no activity was observed against S. aureus, P. mirabilis and K. pneumoniae, since the aldehyde group can't bind with amino acids on the cell surface, they are being participated in stabilizing Ag nanoparticles, owing to the affinity of oxygen atom towards metals.104
Antimicrobial features of ZnO nanoparticles are well known, which makes them suitable for agriculture and anticancer treatment.105–109 Oxidative stress mechanism involving ZnO nanoparticles against E. coli, have been well reported.102 For bulk ZnO, external generation of H2O2, is the reason for antibacterial activity. Being amphoteric, ZnO reacts with both acidic and alkaline medium, to generate Zn2+ ions. The free Zn2+ ions may then combine with proteins and carbohydrates, ceases the vital functions of bacteria.108
In view of the biological characteristics of ZnO nanoparticles, researchers are involved in the fabrication of hybrid materials, in combination with Ag nanoparticles, to achieve excellent antibacterial properties. Ag–ZnO nanocomposites can be used as an effective antimicrobial agent against a number of pathogenic bacteria. A recent study assessed the bactericidal effect of Ag–ZnO nanocomposites with S. aureus (Gram-positive) and GFP (green fluorescent protein, Gram-negative recombinant) expressing antibiotic resistant E. coli.109 By introducing these metal nanocomposites on a polymer matrix, its durability can be improved as well as the cytotoxic effects can be minimized.
In an interesting work, polyindole/Ag–ZnO nanocomposites were synthesized via chemical oxidation and co-precipitation methods and their antibacterial activities were explored.110 The antibacterial efficiency was assessed in terms of concentration of both AgNO3 and polyindole. Formation of the nanocomposites have been confirmed by using XRD, FTIR, SEM-EDAX and TEM. The selected bacterial strains for this study were E. coli, P. mirabilis, E. faecalis, B. subtilis, S. epidermidis, and S. aureus. The order of increasing bactericidal efficiency in terms of inhibition zone against the microbes follows the order, B. subtilis > E. coli > E. faecalis > S. aureus > P. mirabilis > S. epidermidis. The data of average zones of inhibition have been presented in Table 4.
| Microbes | Gram staining | ZA1 | ZA2 | ZA3 | ZA4 | ZA5 | ZnO | Ag | Pin |
|---|---|---|---|---|---|---|---|---|---|
| E. coli | Gram negative | 18 | 17 | 16 | 18 | 17 | 5 | 7 | 5 |
| P. mirabilis | 13 | 13 | 13 | 13 | 13 | 4 | 8 | — | |
| E. faecalis | Gram positive | 16 | 14 | 13 | 14 | 13 | 6 | 16 | 4 |
| B. subtilis | 23 | 20 | 20 | 20 | 20 | 4 | 11 | — | |
| S. epidermidis | 12 | 11 | 11 | 13 | 13 | 4 | 7 | 5 | |
| S. aureus | 15 | 13 | 12 | 12 | 13 | 3 | 12 | 4 |
It has been shown that the polyindole/Ag–ZnO nanocomposites possess good bactericidal efficiency than their constituents. However, the concentration of AgNO3 did not play any crucial role in enhancing the overall antibacterial effect. When the nanoparticles are incorporated into the polymer matrix, their exposure gets restricted, leading to a reduction in the cytotoxic effects towards healthy mammalian cells. Hence, the use of polyindole/Ag–ZnO nanocomposites promote a biocompatible, non-cytotoxic and thereby a safe approach in treating bacterial infections.
Elemental Cu and its compounds have been identified as antimicrobial agents by US environmental protection agency (EPA).111 Both Cu (+1) and Cu (+2) oxides in the nano-dimension exhibit excellent antimicrobial characteristics against many pathogens. The antimicrobial properties strongly depend upon their particle size, morphology and dissolution of copper ions in different media. The redox cycling between Cu+ and Cu2+ generates superoxide species, causes the degradation of biomolecules.112
Ag/CuO nanocomposites have been subjected to antimicrobial activity evaluation against Gram positive microbe Streptococcus pneumoniae.113 High surface-volume ratio of the nanoparticles makes their contact with the microbial cell surfaces, leading to cease the cellular functions.
Polyindole/Ag–CuO systems have been developed via a reflux strategy and their antimicrobial efficiency were assessed by well diffusion method.114 While preparing the nanocomposites, the concentration of both polyindole and AgNO3 has been varied and those of CuO kept constant. The structural characterisations of the prepared nanocomposites were done by FTIR, XRD and SEM analysis. The selected bacterial strains for this study were E. coli, P. mirabilis, E. faecalis, B. subtilis, S. epidermidis, and S. aureus. The antibacterial activity has been compared with the standard ciprofloxacin.73 Fig. 7 represent the antibacterial responses of polyindole/Ag–CuO systems against the pathogens.
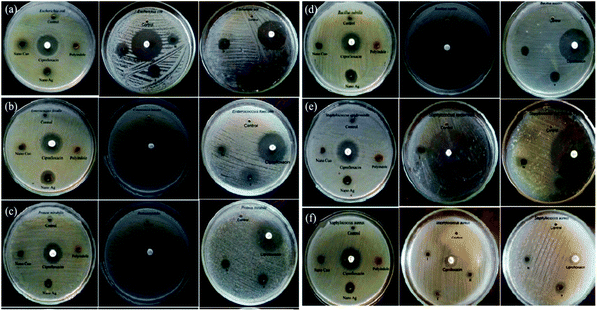 | ||
| Fig. 7 Antibacterial response of polyindole/Ag–CuO systems and its constituents against different bacterial strains. Reprinted with permission from ref. 114; copyright ©Taylor and Francis Ltd. | ||
It has been observed that the polyindole/Ag–CuO nanocomposites exhibit ∼50% activity in comparison with the reference antibiotic, against the pathogen. The antibacterial activity of the nanocomposites has been compared and shown as Fig. 8. The inhibition zone diameter found with nano CuO, Ag and polyindole were 6, 11 and 5 mm respectively. But for polyindole/Ag–CuO nanocomposites, an average zone diameter of 12 mm has been observed.
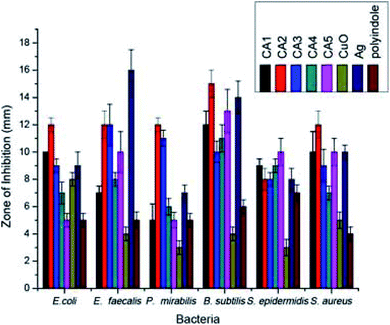 | ||
| Fig. 8 A Comparison of antibacterial activity of polyindole/Ag–CuO systems. Reprinted with permission from ref. 114; copyright © Taylor and Francis Ltd. | ||
Since the nanocomposites can easily interact with the bacterial cell wall, the released nanoparticles can effectively penetrate into the bacterial cell causing toxicity to the cells.99 Electrostatic interaction between the nanocomposites and cell wall of bacteria eventually leads to cell death.115 The results unveil the possibilities of exploring polyindole/Ag–CuO nanocomposites as an effective antimicrobial agent against pathogenic bacteria.
Cerium and cerium oxide-based nanomaterials have gained considerable attention as effective antibacterial agent against many pathogens, owing to the ROS induced by reversible conversion of oxidation state between Ce (+3) and Ce (+4).116 Literature reports are available based on the incorporation of Ce and CeO2 into many polymeric matrices for antibacterial applications.117,118
The antibacterial properties of Ag/CeO2 nanocomposites were comprehensively discussed in a recent article.119 The antibacterial activity of the nanocomposites has been assessed against S. aureus and P. aeruginosa, Gram-positive and Gram-negative bacteria respectively. For both the bacterial strains, the MIC upon treatment of Ag/CeO2 nanocomposites were observed to be 3.125 μg mL−1 and 6.25 μg mL−1 respectively.
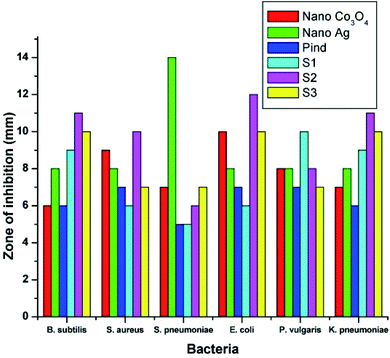
Polyindole based Ag doped CeO2 nanocomposites were explored for its antibacterial properties.120 Amorphous nature of the nanocomposites has been confirmed from the XRD results. The porous polyindole, spherical Ag and CeO2 nanoparticles were identified from the SEM and TEM investigations. Polyindole/Ag–CeO2 systems exhibited better antibacterial properties than their constituents. The average zone of inhibition against various bacterial strains have been presented as Table 5.
| S. no. | Bacteria | Gram staining | Nano CeO2 | Nano Ag | Polyindole | Nanocomposites | ||
|---|---|---|---|---|---|---|---|---|
| CM1 | CM2 | CM3 | ||||||
| 1 | B. subtilis | Gram positive | 8.1 | 7.2 | 6.3 | 10.1 | 12.2 | 14.3 |
| 2 | S. aureus | 9.2 | 6.3 | 8.2 | 10.3 | 8.2 | 9.1 | |
| 3 | S. pneumoniae | 4.2 | 6.2 | 2.3 | 7.2 | 8.3 | 10.2 | |
| 4 | E. coli | Gram negative | 2.3 | 7.3 | 8.3 | 14.2 | 12.1 | 11.3 |
| 5 | P. vulgaris | 8.2 | 6.3 | 4.2 | 7.3 | 8.2 | 10.2 | |
| 6 | K. pneumoniae | 12.2 | 6.2 | 10.3 | 10.2 | 9.3 | 10.1 | |
It has been shown that there is a direct relation between the AgNO3 concentration and antibacterial properties of the synthesized nanocomposites. As the Ag content increases, the antibacterial property also increases. Smaller size of the nanoparticles makes more impact of toxicity on the bacteria, due to the greater extend of adsorption at the surface.121
The antifungal activity evaluation of the systems was carried out by agar well diffusion method against the pathogenic fungal species such as Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Candida albicans, Aspergillus terreus, and Candida tropicalis. Fig. 9 shows the zone of inhibition against the selected pathogens.
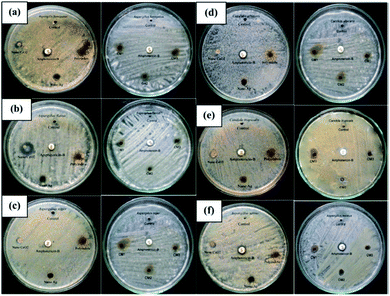 | ||
| Fig. 9 Antifungal response of polyindole/Ag–CeO2 systems and individual constituents against (a) A. fumigates, (b) A. flavus, (c) A. niger, (d) C. albicans, (e) A. terreus and (f) C. tropicalis. Reprinted with permission from ref. 120; copyright © Elsevier. | ||
It has been observed that, the polyindole does not exhibit any activity against the selected fungi. Also, the anti-fungal activity does not increase in the presence of Ag nanoparticles. The nanocomposites exhibit moderate antibacterial and minimum antifungal activities against the selected pathogens.
While comparing the antibacterial activity of the nanocomposites with its constituents, it has been found that the nanocomposites exhibited better antibacterial response against the pathogens. The same trend was observed for antifungal activity as well. This might be due to the synergistic enhancement in properties of the partners. From the above results, the polyindole/Ag–CeO2 nanocomposite proved to be an efficient antimicrobial agent.
Cobalt oxide nanoparticles are found to be well known antibacterial agent.122 In an interesting article, Ag nanoparticles, Co3O4 nanoparticles and Ag/Co3O4 nanocomposites of different weight ratio were synthesized via an environmental friendly, economical and green synthetic strategy.123 They were then subjected to antimicrobial activity evaluation against pathogenic microorganisms which include Gram-negative bacteria (Escherichia coli and Salmonella) as well as Gram-positive bacteria (Marsa, Listeria, Staphylococcus aureus, Bacillus subtilis) and also a pathogenic fungal species, Candidia. The results revealed that the systems displayed inhibition against the tested pathogens.
M. Elango et al. carried out a systematic study on the development, characterization and antimicrobial potency investigation of polyindole stabilized Ag–Co3O4 nanocomposites.124 The crystallinity was found to be increasing, with an increase in Ag content. XRD results show that the polyindole acts as a reducing as well as a stabilising agent for AgNO3 to develop polyindole/Ag–Co3O4 systems. Porous structure of the polyindole, as evident from TEM images (Fig. 10), makes effective incorporation of Ag and Co3O4 into it.
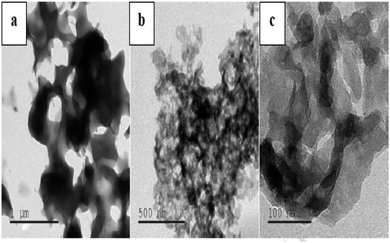 | ||
| Fig. 10 TEM images of polyindole at different magnifications. Reprinted with permission from ref. 124; copyright © Elsevier. | ||
The developed polyindole/Ag–Co3O4 nanocomposites were subjected to antibacterial and antifungal activity by disk diffusion method. Bacterial species selected for the studies were B. subtilis, S. aureus, S. pneumoniae, E. coli, P. vulgaris and K. pneumoniae. The fungal species used were A. fumigates, A. flavus, A. niger, C. albicans, A. terreus and C. tropicalis. Ciprofloxacin and amphotericin-B were used as the reference antibacterial and antifungal agents and a comparison of antimicrobial responses of the nanocomposites with these standards were made. It was a notable observation that the antimicrobial efficiency did not increase with an increase in Ag content.
Fig. 11 and 12 shows the comparative antibacterial and antifungal activity of the polyindole/Ag–Co3O4 systems, respectively. The polyindole/Ag–Co3O4 nanocomposites exhibited better antibacterial activity than its constituents. The antibacterial activity is due to the interaction between the nanocomposite surface and bacterial cell wall. As the size of the composite particles remains smaller, they can simply pierce the cell wall of bacteria, causing severe toxicity to the bacterial species.99 Conducting polymers have shown to cause cell death of bacteria, owing to their excellent antibacterial properties. The nanocomposites may have the sensitivity towards bacterial and fungal cell wall structures, which contribute to their antibacterial activities.125–127 All the developed systems exhibited good antibacterial activity against the selected pathogenic microorganisms. The results facilitate the need for further research in order to explore the applications of polyindole/Ag–Co3PO4 nanocomposites as efficient biomedical agents.
 | ||
| Fig. 11 Comparative antibacterial activity of the systems against the pathogens by well diffusion method. Reprinted with permission from ref. 124; copyright © Elsevier. | ||
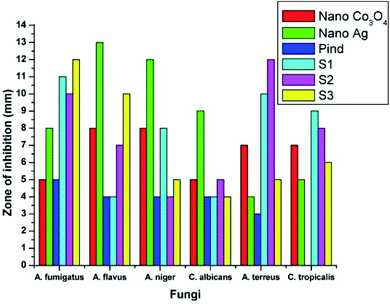 | ||
| Fig. 12 Comparative antifungal activity of the systems against the pathogens by well diffusion method. Reprinted with permission from ref. 124; copyright © Elsevier. | ||
Solubility product (Ksp) of Ag3PO4 is 1.4 × 10−16 and solubility is 0.02 g per litre at 25 °C, is partially soluble in water. Hence, it can slowly release Ag+ as an antibacterial agent.128 Simple solution-based precipitation methods may be employed for the controlled synthesis of Ag3PO4 nano-crystals, having good antibacterial properties.129 Their antibacterial activity depends upon the size and smaller crystals exhibit better antibacterial activity, owing to high specific surface areas. Upon irradiating with visible light, the antibacterial properties of Ag3PO4 could be greatly enhanced even more than commercial streptomycin.130,131
A latest study has been devoted for the evaluation of antibacterial, anti-cancerous and anti-inflammatory properties of bioactive Ag3PO4/polyindole nanocomposites. Ag3PO4 nanocrystals were grown in situ inside polyindole matrix to fabricate bioactive Ag3PO4/polyindole nanocomposites.132 XRD, SEM, TEM, EDX and Diffused Reflectance Spectroscopy techniques were used for the characterization of the synthesized samples. The antibacterial, anticancer and anti-inflammatory activity assays proved their outstanding ability to act as a potential biomedical agent. It is notable that even polyindole alone showed antibacterial properties for long time than Ag3PO4.
The intracellular ROS generation accounts for the long-standing antibacterial activity of the nanocomposites. When the composition of the polyindole was 50% of Ag3PO4, the intracellular ROS generation was greater, for a long time. Minimum inhibitory concentration (MIC) of Ag3PO4/polyindole has been observed to be equivalent to those of some metallic oxide nanoparticles.133,134 It was found that the nanocomposites show inhibition property against the bacterial stains, while virgin polyindole doesn't show any activity at all, this concentration range. This may be owing to the synergistic effect of the partners. Optical density of E. coli with virgin polyindole and Ag3PO4/polyindole nanocomposites was found to follow a similar pattern to that of the control, with lower optical density showing the suppression of bacterial growth and the nanocomposites considerably suppressed E. coli to larger level than the virgin polyindole (Fig. 13).
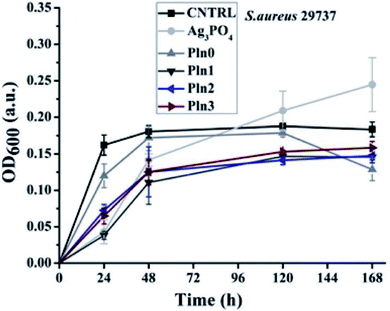 | ||
| Fig. 13 Optical density versus time plot of Ag3PO4/polyindole nanocomposites. Reprinted with permission from ref. 132; copyright © Royal Society of Chemistry. | ||
Furthermore, Ag3PO4 nanocomposites displayed anticancer activity with little toxicity towards other healthy cells. Therefore, more and more research programs have to be carried out to unveil their potential medical applications.
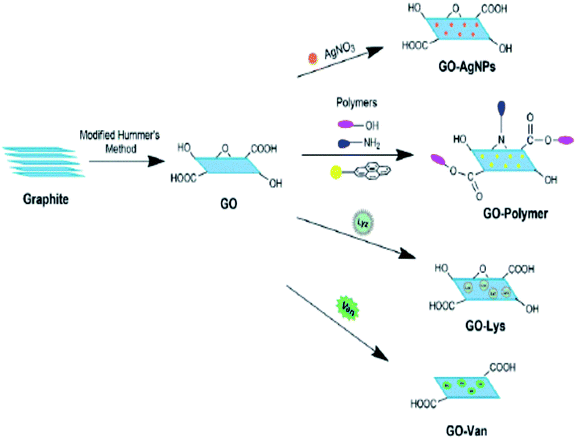 | ||
| Fig. 14 Schematic representation of possibility of functionalisation of graphene, for the development of antibacterial materials. Reprinted with permission from ref. 140; copyright © MDPI. | ||
A systematic investigation has been carried out for the in vitro and in vivo antimicrobial activity evaluation by using polyindole/graphene nanocomposites with methicillin resistant Staphylococcus aureus (SA) pathogen. The π–π interaction between the graphene and polyindole dramatically improved the dispersion of graphene in the polyindole matrix. The antibacterial potency of freshly prepared graphene@polyindole nanocomposites with resistant SA isolates have been evaluated.87 The standard antibiotic used was vancomycin. The interaction of graphene@polyindole nanocomposite with bacterial cell wall caused its disintegration, which was clearly understood from the electron microscopic studies. Significantly, the graphene@polyindole found to exhibit minimal toxicity to the mammalian cells and hence can effectively eradicate the MRSA strain with appreciable biocompatibility.
The evaluation of the mechanism of antibacterial property showed that firstly, the graphene@polyindole stick to the bacterial surface, and then it creates an irreversible interruption on the layer of the membrane of the bacteria. After that it eventually penetrated into the cells, and effectively hindered the activity of proteins, leads to bacterial apoptosis in vitro. Furthermore, the skin infection mediated by S. aureus in BALB/C mice was effectively treated with the synthesized graphene@polyindole nanocomposites. Fig. 15(A) shows the SEM micrographs of the bacteria treated with graphene@polyindole nanocomposites. In the case of untreated samples, the morphology was found to be spherical with smooth cell surfaces. The nanocomposite treated bacteria possesses wrinkled morphology with rough surfaces. Exposure of the bacteria to graphene@polyindole nanocomposites leads to cell lysis followed by the release of cellular components. Fig. 15(B) represent the TEM images indicating the interaction of the nanocomposites with bacterial strains.
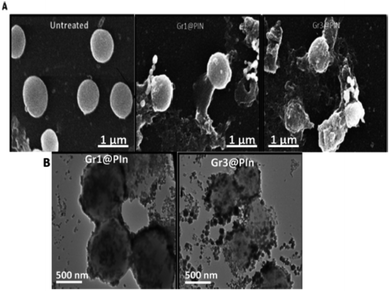 | ||
| Fig. 15 (A) SEM micrographs of the bacteria treated with graphene@polyindole nanocomposites. (B) TEM images indicating the interaction of the nanocomposites with bacterial strains. Reprinted with permission from ref. 87; copyright ©American Chemical Society. | ||
Polyindole-graphene synergy has been further confirmed from Raman spectroscopy as indicated by Fig. 16.
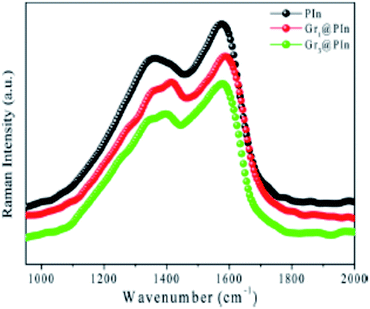 | ||
| Fig. 16 Raman spectrum of polyindole and graphene@polyindole nanocomposites. Reprinted with permission from ref. 87; copyright ©American Chemical Society. | ||
Presence of more sp3 carbon atoms, owing to the interaction between polyindole and graphene has been revealed from the intensity ratio of graphene and graphene polymer nanocomposites (Fig. 16). The D band to G band ratio varies from 1.26 to 1.36 for graphene and graphene@polyindole nanocomposites respectively.
The antibacterial potency of the systems, evaluated by agar diffusion assay has been shown as Fig. 17(A). Fluorescence microscopic techniques have been employed to evaluate the live-dead assay, against MRSA stains and represented as Fig. 17(B). Green fluorescence has been observed for control groups, while the samples treated for 3 hours appears to be red in colour, owing to the dye binding with bacterial DNA. The study showed that the nanocomposite is very effective for inhibition of the S. aureus-facilitated RBCs lysis. The work highlights the possibilities of future research for the development of a biocompatible and efficient biomedical agent against methicillin resistant Staphylococcus aureus (SA) pathogen.
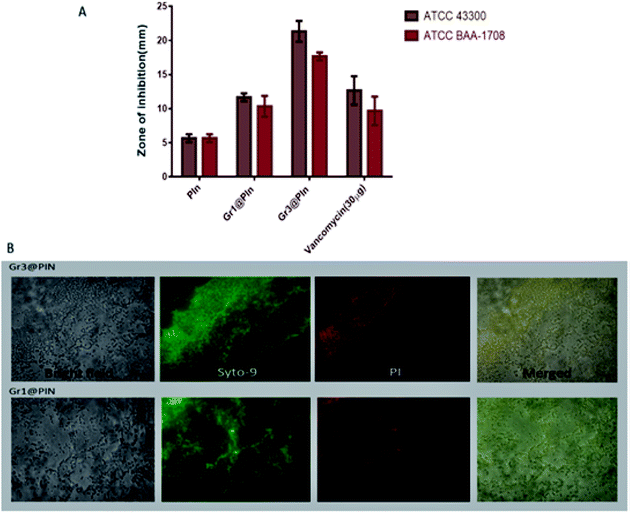 | ||
| Fig. 17 (A) Zone of inhibition against MRSA strains as a measure of anti-microbial activity. (B) The images of fluorescence of MRSA ATCC BAA-1708 were taken by a microscope after treating the cells using different graphene@polyindole formulations. Reprinted with permission from ref. 87; copyright ©American Chemical Society. | ||
Another group of researchers assessed nano zirconia for their antimicrobial activity via well disc diffusion method.142 B. subtilis and S. aureus (Gram positive) and E. coli and P. aeruginosa (Gram negative) were selected as reference bacterial strains. Since P. aeruginosa possess a negatively charged cell surface, nano zirconia shows an efficient inhibitory action at higher concentrations. The in vitro and in vivo experiments reveal the possibilities of exploiting the biomedical applications of ZrO2 nanoparticles.
S. Anandhi et al. synthesized polyindole/ZrO2 nanocomposites, by using mixing solution method.143 SEM analysis was used to understand the morphology of the synthesized polyindole, nano ZrO2 and polyindole/ZrO2 nanocomposites. FTIR, UV-Visible and NMR techniques have been employed for the structural confirmation of the synthesized nanocomposites. The degree of crystallinity and crystalline sizes were determined from XRD analysis. The thermal stability of the synthesized composites were analysed from TGA and DSC studies. EDAX technique was used to demonstrate elemental analysis and chemical characterization. The antibacterial activity of the synthesized nanocomposites were carried out on five microorganisms – Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Salmonella typhi and E. coli. Table 6 presents the antibacterial results of the polyindole/ZrO2 systems, and zone of inhibition have been represented as Fig. 18.
| Microbes | Gram staining | Inhibition zone (mm) | Antibiotic (1 mg mL−1) | ||
|---|---|---|---|---|---|
| Concentration of the samples (1 mg mL−1) | |||||
| Staphylococcus aureus | Gram positive | 20 | 13 | 10 | 35 |
| Bacillus subtilis | 10 | 8 | 8 | 20 | |
| E. coli | Gram negative | 15 | 10 | 9 | 26 |
| Salmonella typhi | 13 | 10 | 8 | 30 | |
| Pseudomonas aeruginosa | 15 | 10 | 7 | 30 | |
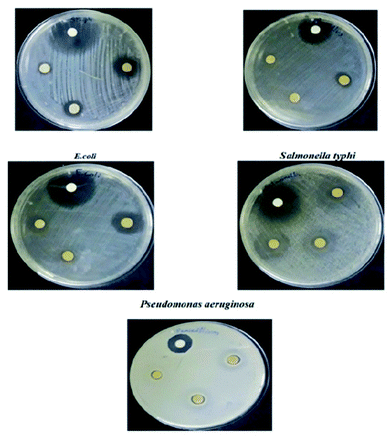 | ||
| Fig. 18 Antibacterial activity of polyindole/ZrO2 systems against selected bacterial strains as a measurement of zone of inhibition. Reprinted with permission from ref. 143; copyright © Elsevier. | ||
The polyindole/ZrO2 systems were also subjected to antifungal activity studies against pathogenic fungal strains – Candida albicans and Penicillium chrysogenum. The results were compared with the standard antibiotic – amphotericin-B. The synthesized polyindole/ZrO2 nanocomposites displayed excellent antifungal properties. Table 7 shows the antifungal data of the systems, and zone of inhibition have been represented as Fig. 19.
| Microbes | Zone of inhibition (mm) | Antibiotic (1 mg mL−1) | ||
|---|---|---|---|---|
| Sample concentration (1 mg mL−1) | ||||
| 1000 | 750 | 500 | ||
| Candida albicans | 16 | 15 | 14 | 17 |
| Penicillium chrysogenum | 11 | 10 | 8 | 18 |
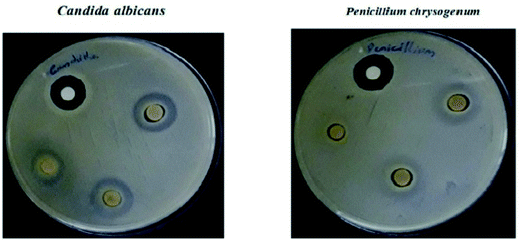 | ||
| Fig. 19 Antifungal activity of the composite material against selected bacterial strains as a measurement of zone of inhibition. Reprinted with permission from ref. 143; copyright © Elsevier. | ||
A systematic investigation was conducted on the development and antibacterial activity evaluation of polyindole/TiO2 nanocomposites.146 They employed ultrasound condition by aqueous in situ polymerization method. A representation of the synthesis strategy has been shown as Fig. 20. FTIR, UV-visible, XRD, TGA and SEM techniques were used for the structural characterization of the synthesized nanocomposite samples.
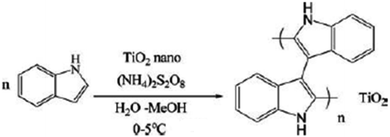 | ||
| Fig. 20 Synthesis strategy of polyindole/TiO2 nanocomposites. Reprinted with permission from ref. 146; copyright © Taylor and Francis Ltd. | ||
The XRD pattern indicated that TiO2 is in the anatase form. A significant antibacterial activity could be observed for the nanocomposite samples against B. subtilis and S. aureus (Gram-positive). However, a moderate activity was observed against E. coli (Gram-negative) whereas no activity was observed against S. typhi. The zone of inhibition has been indicated in Fig. 21.
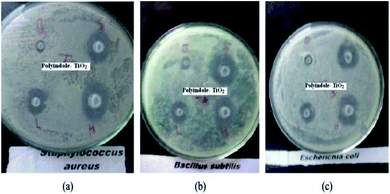 | ||
| Fig. 21 Antibacterial activity as a measure of zone of inhibition for polyindole/TiO2 nanocomposite against (a) Staphylococcus aureus, (b) Bacillus subtilis and (c) E. coli. Reprinted with permission from ref. 146; copyright © Taylor and Francis Ltd. | ||
It was proposed that the antibacterial activity of the polyindole/TiO2 nanocomposite materials is attributed to their ability to inhibit nucleic acids, thiol groups and essential enzymes present on the bacterial cell membranes. Hence, these materials act as a promising candidate and their use could be a novel approach to fight drug-resistant bacterial infections.
From this study, it is evident that when NiO/ZnO nanocomposite combines with polyindole matrix, there observed a higher antifungal activity than the single NiO/ZnO nanocomposites. This enhanced antimicrobial activity of the polymer nanocomposite has been due to the synergistic effect of both the polymer matrix and metal oxide nanoparticle counterparts in favour of appreciable ROS generation. From the above results, we can conclude that the synthesized polyindole/NiO–ZnO nanocomposite could act as a potential antifungal material to fight the fungal infection caused by Penicillium chrysogenum.
3. Future perspectives
Polyindole based nanocomposites were observed to be a potential biomaterial against the multi-drug resistant microbes. They can be used as an alternative to antibiotics and antifungal drugs. Clinical and in vivo applications are largely based on the size of the released nanoparticles from the polymer nanocomposites. Hence, it necessitates the need for effective toxicological studies and investigations of the polymer nanocomposites before initiating clinical trials. Another concern is at the development of an economical as well as an ecofriendly method for the synthesis of polyindole based nanocomposite is required. However, nowadays more and more researchers are coming up with some innovative green synthetic strategies, which may give progress to the field of polyindole based nanocomposites for biomedical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Dr Bindu M. is thankful to KSCSTE for her Back to Lab Post-Doctoral Fellowship, Grant No 161/2021/KSCSTE. Anjitha Thadathil is grateful to CSIR for her CSIR-JRF fellowship.References
- S. Alfei and A. M. Schito, Nanomaterials, 2020, 10, 2022 CrossRef CAS PubMed.
- J. Munguia and V. Nizet, Trends Pharmacol. Sci., 2017, 38, 473–488 CrossRef CAS PubMed.
- C. L. Ventola, Pharm. Therapeut., 2015, 40, 277 Search PubMed.
- N. F. Kamaruzzaman, L. P. Tan, R. H. Hamdan, S. S. Choong, W. K. Wong, A. J. Gibson, A. Chivu and M. d. F. Pina, Int. J. Mol. Sci., 2019, 20, 2747 CrossRef CAS PubMed.
- J. Hoque, S. Ghosh, K. Paramanandham and J. Haldar, ACS Appl. Mater. Interfaces, 2019, 11, 39150–39162 CrossRef CAS PubMed.
- D. Olmos and J. González-Benito, Polymers, 2021, 13, 613 CrossRef CAS PubMed.
- F. A. G. d. Silva, S. A. Vieira, S. d. A. Botton, M. M. d. Costa and H. P. d. Oliveira, Polimeros, 2021, 30, 1–9 Search PubMed.
- A. J. Huh and Y. J. Kwon, J. Controlled Release, 2011, 156, 128–145 CrossRef CAS PubMed.
- L. Wang, C. Hu and L. Shao, Int. J. Nanomed., 2017, 12, 1227 CrossRef CAS PubMed.
- L. C. Yun’an Qing, R. Li, G. Liu, Y. Zhang, X. Tang, J. Wang, H. Liu and Y. Qin, Int. J. Nanomed., 2018, 13, 3311 CrossRef PubMed.
- A. A. Dayem, M. K. Hossain, S. B. Lee, K. Kim, S. K. Saha, G.-M. Yang, H. Y. Choi and S.-G. Cho, Int. J. Mol. Sci., 2017, 18, 1–21 Search PubMed.
- F. Ciccarese, V. Raimondi, E. Sharova, M. Silic-Benussi and V. Ciminale, Antioxidants, 2020, 9, 211 CrossRef CAS PubMed.
- A. Manke, L. Wang and Y. Rojanasakul, BioMed Res. Int., 2013, 2013, 942916 Search PubMed.
- V. T. Nguyen, V. T. Vu, T. A. Nguyen, V. K. Tran and P. Nguyen-Tri, J. Compos. Sci., 2019, 3, 61 CrossRef CAS.
- A. Raghunath and E. Perumal, Int. J. Antimicrob. Agents, 2017, 49, 137–152 CrossRef CAS PubMed.
- S. V. Gudkov, D. E. Burmistrov, D. A. Serov, M. B. Rebezov, A. A. Semenova and A. B. Lisitsyn, Front. Phys., 2021, 9, 641481, DOI:10.3389/fphy.
- B. Abebe, E. A. Zereffa, A. Tadesse and H. A. Murthy, Nanoscale Res. Lett., 2020, 15, 1–19 CrossRef PubMed.
- L. M. Anaya-Esparza, E. Montalvo-González, N. González-Silva, M. D. Méndez-Robles, R. Romero-Toledo, E. M. Yahia and A. Pérez-Larios, Materials, 2019, 12, 698 CrossRef CAS PubMed.
- B. Yang, Y. Chen and J. Shi, Chem. Rev., 2019, 119, 4881–4985 CrossRef CAS PubMed.
- J. Singh, K. Vishwakarma, N. Ramawat, P. Rai, V. K. Singh, R. K. Mishra, V. Kumar, D. K. Tripathi and S. Sharma, 3 Biotech, 2019, 9, 1–14 CAS.
- N. Sanvicens and M. P. Marco, Trends Biotechnol., 2008, 26, 425–433 CrossRef CAS PubMed.
- A. M. Knaapen, P. J. Borm, C. Albrecht and R. P. Schins, Int. J. Cancer, 2004, 109, 799–809 CrossRef CAS PubMed.
- A. Nel, T. Xia, L. Mädler and N. Li, science, 2006, 311, 622–627 CrossRef CAS PubMed.
- B. Fubini and A. Hubbard, Free Radicals Biol. Med., 2003, 34, 1507–1516 CrossRef CAS PubMed.
- V. J. Thannickal and B. L. Fanburg, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2000, 279, L1005–L1028 CrossRef CAS PubMed.
- M. Valko, C. Rhodes, J. Moncol, M. Izakovic and M. Mazur, Chem.-Biol. Interact., 2006, 160, 1–40 CrossRef CAS PubMed.
- G. Oberdörster, A. Maynard, K. Donaldson, V. Castranova, J. Fitzpatrick, K. Ausman, J. Carter, B. Karn, W. Kreyling and D. Lai, Part. Fibre Toxicol., 2005, 2, 1–35 CrossRef PubMed.
- P. Riley, Int. J. Radiat. Biol., 1994, 65, 27–33 CrossRef CAS PubMed.
- A. Abdal Dayem, M. K. Hossain, S. B. Lee, K. Kim, S. K. Saha, G.-M. Yang, H. Y. Choi and S.-G. Cho, Int. J. Mol. Sci., 2017, 18, 120 CrossRef PubMed.
- X. Zhang, L. Wang, H. Lu, Z. Zong, Z. Chen, Y. Li, X. Luo and Y. Li, Sci. Rep., 2020, 10, 1–11 CrossRef CAS PubMed.
- C. Ransy, C. Vaz, A. Lombès and F. Bouillaud, Int. J. Mol. Sci., 2020, 21, 9149 CrossRef CAS PubMed.
- K. S. Kim, D. Lee, C. G. Song and P. M. Kang, Nanomedicine, 2015, 10, 2709–2723 CrossRef CAS PubMed.
- Y. Wang, H. Qi, Y. Liu, C. Duan, X. Liu, T. Xia, D. Chen, H.-l. Piao and H.-X. Liu, Theranostics, 2021, 11, 4839 CrossRef CAS PubMed.
- V. Aggarwal, H. S. Tuli, A. Varol, F. Thakral, M. B. Yerer, K. Sak, M. Varol, A. Jain, M. Khan and G. Sethi, Biomolecules, 2019, 9, 735 CrossRef CAS PubMed.
- R. Spooner and Ö. Yilmaz, Int. J. Mol. Sci., 2011, 12, 334–352 CrossRef CAS PubMed.
- F. Gao, T. Shao, Y. Yu, Y. Xiong and L. Yang, Nat. Commun., 2021, 12, 1–18 CrossRef PubMed.
- M. Herb and M. Schramm, Antioxidants, 2021, 10, 313 CrossRef CAS PubMed.
- M. Mittal, M. R. Siddiqui, K. Tran, S. P. Reddy and A. B. Malik, Antioxid. Redox Signaling, 2014, 20, 1126–1167 CrossRef CAS PubMed.
- X. Li, P. Fang, J. Mai, E. T. Choi, H. Wang and X.-f. Yang, J. Hematol. Oncol., 2013, 6, 1–19 CrossRef PubMed.
- H. H. Gustafson, D. Holt-Casper, D. W. Grainger and H. Ghandehari, Nano today, 2015, 10, 487–510 CrossRef CAS PubMed.
- R. A. Freitas Jr, Nanomedicine, 2005, 1, 2–9 CrossRef PubMed.
- L. Ge, Q. Li, M. Wang, J. Ouyang, X. Li and M. M. Xing, Int. J. Nanomed., 2014, 9, 2399 Search PubMed.
- Y. N. Slavin, J. Asnis, U. O. Häfeli and H. Bach, J. Nanobiotechnol., 2017, 15, 1–20 CrossRef PubMed.
- C. Bankier, R. Matharu, Y. Cheong, G. Ren, E. Cloutman-Green and L. Ciric, Sci. Rep., 2019, 9, 1–8 CAS.
- N. Dhull, V. Gupta and M. Tomar, Mater. Today: Proc., 2019, 17, 155–160 CAS.
- Z. Qiu, B. A. G. Hammer and K. Müllen, Prog. Polym. Sci., 2020, 100, 101179 CrossRef CAS.
- Q. Cui, H. Yuan, X. Bao, G. Ma, M. Wu and C. Xing, ACS Appl. Bio Mater., 2020, 3, 4436 CrossRef CAS PubMed.
- T. F. Abelha and A. R. L. Caires, Adv. NanoBiomed. Res., 2021, 1, 2100012 CrossRef.
- B. C. Wilson and R. A. Weersink, Photochem. Photobiol., 2020, 96, 219 CrossRef CAS PubMed.
- L. Liu, X. Wang, S. Zhu, C. Yao, D. Ban, R. Liu, L. Li and S. Wang, Chem. Mater., 2020, 32, 438 CrossRef CAS.
- S. Fu, Z. Sun, P. Huang, Y. Li and N. Hu, Nano Mater. Sci., 2019, 1, 2–30 CrossRef.
- M. Šupová, G. S. Martynková and K. Barabaszová, Sci. Adv. Mater., 2011, 3, 1–25 CrossRef.
- Y. Wang, G. J. Desroches and R. J. Macfarlane, Nanoscale, 2021, 13, 426–443 RSC.
- L. A. Savas and M. Hancer, Appl. Clay Sci., 2015, 108, 40–44 CrossRef CAS.
- Y. Dong, M. Argaiz, B. He, R. Tomovska, T. Sun and I. Martín-Fabiani, ACS Appl. Polym. Mater., 2020, 2, 626–635 CrossRef CAS.
- K. M. Nampoothiri, N. R. Nair and R. P. John, Bioresour. Technol., 2010, 101, 8493–8501 CrossRef PubMed.
- F. Asghari, M. Samiei, K. Adibkia, A. Akbarzadeh and S. Davaran, Artif. Cells, Nanomed., Biotechnol., 2017, 45, 185–192 CrossRef CAS PubMed.
- G. K. Arbade, J. Srivastava, V. Tripathi, N. Lenka and T. U. Patro, J. Biomater. Sci., Polym. Ed., 2020, 31, 1648–1670 CrossRef CAS PubMed.
- R. A. Green, S. Baek, L. A. Poole-Warren and P. J. Martens, Sci. Technol. Adv. Mater., 2010, 11, 014107 CrossRef PubMed.
- J. G. Ibanez, M. E. Rincón, S. Gutierrez-Granados, M. h. Chahma, O. A. Jaramillo-Quintero and B. A. Frontana-Uribe, Chem. Rev., 2018, 118, 4731–4816 CrossRef CAS PubMed.
- T. Nezakati, A. Seifalian, A. Tan and A. M. Seifalian, Chem. Rev., 2018, 118, 6766–6843 CrossRef CAS PubMed.
- K. Namsheer and C. S. Rout, RSC Adv., 2021, 11, 5659–5697 RSC.
- A. Konwar, S. Kalita, J. Kotoky and D. Chowdhury, ACS Appl. Mater. Interfaces, 2016, 8, 20625–20634 CrossRef CAS PubMed.
- A. Sanmugam, D. Vikraman, H. J. Park and H.-S. Kim, Nanomaterials, 2017, 7, 363 CrossRef PubMed.
- W. Xu, W. Xie, X. Huang, X. Chen, N. Huang, X. Wang and J. Liu, Food Chem., 2017, 221, 267–277 CrossRef CAS PubMed.
- Y.-C. Cao, W. Wei, J. Liu, Q. You, F. Liu, Q. Lan, C. Zhang, C. Liu and J. Zhao, Int. J. Polym. Sci., 2015, 2015, 1–7 Search PubMed.
- R. Surudžić, A. Janković, N. Bibić, M. Vukašinović-Sekulić, A. Perić-Grujić, V. Mišković-Stanković, S. J. Park and K. Y. Rhee, Composites, Part B, 2016, 85, 102–112 CrossRef.
- Y. Huang, T. Wang, X. Zhao, X. Wang, L. Zhou, Y. Yang, F. Liao and Y. Ju, J. Chem. Technol. Biotechnol., 2015, 90, 1677–1684 CrossRef CAS.
- H. Mahdavi, O. Rahmani and A. R. Shahverdi, J. Iran. Chem. Soc., 2017, 14, 37–46 CrossRef CAS.
- A. Salabat, F. Mirhoseini, M. Mahdieh and H. Saydi, New J. Chem., 2015, 39, 4109–4114 RSC.
- M. Maruthapandi, A. Saravanan, J. H. Luong and A. Gedanken, Polymers, 2020, 12, 1286 CrossRef CAS PubMed.
- J. Upadhyay, A. Kumar, B. Gogoi and A. Buragohain, Mater. Sci. Eng., C, 2015, 54, 8–13 CrossRef CAS PubMed.
- P. Boomi, H. G. Prabu and J. Mathiyarasu, Eur. J. Med. Chem., 2014, 72, 18–25 CrossRef CAS PubMed.
- M. D. Adhikari, S. Goswami, B. R. Panda, A. Chattopadhyay and A. Ramesh, Adv. Healthcare Mater., 2013, 2, 599–606 CrossRef CAS PubMed.
- U. Bogdanovic, V. Vodnik, M. Mitric, S. Dimitrijevic, S. D. Skapin, V. Zunic, M. Budimir and M. Stoiljkovic, ACS Appl. Mater. Interfaces, 2015, 7, 1955–1966 CrossRef CAS PubMed.
- R. Hassanien, M. Al-Hinai, S. A. Farha Al-Said, R. Little, L. Siller, N. G. Wright, A. Houlton and B. R. Horrocks, ACS Nano, 2010, 4, 2149–2159 CrossRef CAS PubMed.
- H. Mudila, P. Prasher, M. Kumar, A. Kumar, M. Zaidi and A. Kumar, Mater. Renew. Sustain. Energy, 2019, 8, 1–19 CrossRef.
- I. Marriam, W. Yuanhao and M. Tebyetekerwa, Energy Storage Mater., 2020, 336–359 CrossRef.
- J. Li, Q. Guo, Y. Lu and G. Nie, Eur. Polym. J., 2019, 113, 29–35 CrossRef CAS.
- K. Phasuksom, W. Prissanaroon-Ouajai and A. Sirivat, Sens. Actuators, B, 2018, 262, 1013–1023 CrossRef CAS.
- M.-T. Nguyen, B. Mecheri, A. Iannaci, A. D'Epifanio and S. Licoccia, Electrochim. Acta, 2016, 190, 388–395 CrossRef CAS.
- M. Mobin, F. Ansar, M. Shoeb, M. Parveen and J. Aslam, Nano Sel., 2021, 2, 293–302 CrossRef CAS.
- P. Chhattise, K. Handore, A. Horne, K. Mohite, A. Chaskar, S. Dallavalle and V. Chabukswar, J. Chem. Sci., 2016, 128, 467–475 CrossRef CAS.
- Q. Zhou, D. Zhu, X. Ma, J. Xu, W. Zhou and F. Zhao, RSC Adv., 2016, 6, 29840–29847 RSC.
- H. L. Youmans, J. B. Rush and V. H. Brown, J. Heterocycl. Chem., 1976, 13, 949–953 CrossRef CAS.
- G. Tourillon and F. Garnier, J. Electroanal. Chem. Interfacial Electrochem., 1982, 135, 173–178 CrossRef CAS.
- M. Shoeb, M. Mobin, M. A. Rauf, M. Owais and A. H. Naqvi, ACS Omega, 2018, 3, 9431–9440 CrossRef CAS PubMed.
- R. B. Choudhary, S. Ansari and B. Purty, J. Energy Storage, 2020, 29, 101302 CrossRef.
- W. Zhou and J. Xu, Polym. Rev., 2017, 57, 248–275 CrossRef CAS.
- P. Pandey and R. Prakash, J. Electrochem. Soc., 1998, 145, 999 CrossRef CAS.
- T. Puzyn, B. Rasulev, A. Gajewicz, X. Hu, T. P. Dasari, A. Michalkova, H.-M. Hwang, A. Toropov, D. Leszczynska and J. Leszczynski, Nat. Nanotechnol., 2011, 6, 175–178 CrossRef CAS PubMed.
- A. Srivastava, P. Singh, R. Kumar, S. K. Verma and R. N. Kharwar, Polym. Int., 2013, 62, 210–218 CrossRef CAS.
- P. Dallas, V. K. Sharma and R. Zboril, Adv. Colloid Interface Sci., 2011, 166, 119–135 CrossRef CAS PubMed.
- V. K. H. Bui, D. Park and Y.-C. Lee, Polymers, 2017, 9, 21 CrossRef PubMed.
- Z. Ding, M. Ding, C. Gao, C. Boyer and W. Zhang, Macromolecules, 2017, 50, 7593–7602 CrossRef CAS.
- H. Gong, K. Zhang, C. Dicko, L. Bülow and L. Ye, ACS Appl. Nano Mater., 2019, 2, 1655–1663 CrossRef CAS.
- E. Said-Galiev, A. Gamzazade, T. Grigor’ev, A. Khokhlov, N. Bakuleva, I. Lyutova, E. Shtykova, K. Dembo and V. Volkov, Nanotechnol. Russ., 2011, 6, 341–352 CrossRef.
- T. C. Dakal, A. Kumar, R. S. Majumdar and V. Yadav, Front. Microbiol., 2016, 7, 1831 Search PubMed.
- S. Prabhu and E. K. Poulose, Int. Nano Lett., 2012, 2, 1–10 CrossRef.
- S. Liao, Y. Zhang, X. Pan, F. Zhu, C. Jiang, Q. Liu, Z. Cheng, G. Dai, G. Wu and L. Wang, Int. J. Nanomed., 2019, 14, 1469 CrossRef CAS PubMed.
- A. B. Smetana, K. J. Klabunde, G. R. Marchin and C. M. Sorensen, Langmuir, 2008, 24, 7457–7464 CrossRef CAS PubMed.
- R. Kumar, S. K. Shrivastava and A. Chakraborti, Am. J. Biomed. Sci., 2010, 2, 202–208 CrossRef CAS.
- A. Ashnagar, N. G. Naseri and S. Alboghobesh, Biosci., Biotechnol. Res. Asia, 2007, 4, 65–70 CAS.
- K. Mallikarjuna, G. Narasimha, G. Dillip, B. Praveen, B. Shreedhar, C. S. Lakshmi, B. Reddy and B. D. P. Raju, Dig. J. Nanomater. Biostructures, 2011, 6, 181–186 Search PubMed.
- L. He, Y. Liu, A. Mustapha and M. Lin, Microbiol. Res., 2011, 166, 207–215 CrossRef CAS PubMed.
- J. W. Rasmussen, E. Martinez, P. Louka and D. G. Wingett, Expert Opin. Drug Delivery, 2010, 7, 1063–1077 CrossRef CAS PubMed.
- R. Singh, S. Cheng and S. Singh, 3 Biotech, 2020, 10, 1–13 CrossRef.
- K. S. Siddiqi, A. ur Rahman and A. Husen, Nanoscale Res. Lett., 2018, 13, 1–13 CrossRef PubMed.
- I. Matai, A. Sachdev, P. Dubey, S. U. Kumar, B. Bhushan and P. Gopinath, Colloids Surf., B, 2014, 115, 359–367 CrossRef CAS PubMed.
- M. Elango, M. Deepa, R. Subramanian and A. M. Musthafa, J. Alloys Compd., 2017, 696, 391–401 CrossRef CAS.
- G. Grass, C. Rensing and M. Solioz, Appl. Environ. Microbiol., 2011, 77, 1541–1547 CrossRef CAS PubMed.
- J. Ren, W. Wang, S. Sun, L. Zhang, L. Wang and J. Chang, Ind. Eng. Chem. Res., 2011, 50, 10366–10369 CrossRef CAS.
- N. Ghasemi, F. Jamali-Sheini and R. Zekavati, Mater. Lett., 2017, 196, 78–82 CrossRef CAS.
- M. Elango, M. Deepa, R. Subramanian and A. Mohamed Musthafa, Polym.-Plast. Technol. Eng., 2018, 57, 1440–1451 CrossRef CAS.
- M. Cabuk, Y. Alan and H. I. Unal, Carbohydr. Polym., 2017, 161, 71–81 CrossRef CAS PubMed.
- M. Qi, W. Li, X. Zheng, X. Li, Y. Sun, Y. Wang, C. Li and L. Wang, Front. Mater., 2020, 7, 213 CrossRef.
- A. B. Shcherbakov, V. V. Reukov, A. V. Yakimansky, E. L. Krasnopeeva, O. S. Ivanova, A. L. Popov and V. K. Ivanov, Polymers, 2021, 13, 924 CrossRef CAS PubMed.
- F. Mohammad, T. Arfin and H. A. Al-Lohedan, J. Ind. Eng. Chem., 2017, 45, 33–43 CrossRef CAS.
- K. Negi, A. Umar, M. Chauhan and M. S. Akhtar, Ceram. Int., 2019, 45, 20509–20517 CrossRef CAS.
- M. Elango, M. Deepa, R. Subramanian and G. Saraswathy, Mater. Today: Proc., 2020, 26, 3544–3551 CAS.
- K. Zheng, M. I. Setyawati, D. T. Leong and J. Xie, Coord. Chem. Rev., 2018, 357, 1–17 CrossRef CAS.
- M. Hafeez, R. Shaheen, B. Akram, S. Haq, S. Mahsud, S. Ali and R. T. Khan, Mater. Res. Express, 2020, 7, 025019 CrossRef CAS.
- H. A. Hassanin, A. Taha and E. Afkar, Ceram. Int., 2021, 47, 3099–3107 CrossRef CAS.
- M. Elango, M. Deepa, R. Subramanian and G. Saraswathy, Mater. Chem. Phys., 2018, 216, 305–315 CrossRef CAS.
- S. Jadoun, U. Riaz and V. Budhiraja, Med. Devices Sens., 2021, 4, e10141 CAS.
- S. S. Nair, S. K. Mishra and D. Kumar, Polym. Adv. Technol., 2019, 30, 2932–2953 CrossRef CAS.
- X. Chen and J. Li, Mater. Chem. Front., 2020, 4, 750–774 RSC.
- I. X. Yin, J. Zhang, I. S. Zhao, M. L. Mei, Q. Li and C. H. Chu, Int. J. Nanomed., 2020, 15, 2555 CrossRef CAS PubMed.
- A. Wu, C. Tian, W. Chang, Y. Hong, Q. Zhang, Y. Qu and H. Fu, Mater. Res. Bull., 2013, 48, 3043–3048 CrossRef CAS.
- Y. Seo, B.-E. Yeo, Y.-S. Cho, H. Park, C. Kwon and Y.-D. Huh, Mater. Lett., 2017, 197, 146–149 CrossRef CAS.
- U. Sulaeman, F. Febiyanto, S. Yin and T. Sato, Catal. Commun., 2016, 85, 22–25 CrossRef CAS.
- S. Podder, S. Paul, P. Basak, B. Xie, N. J. Fullwood, S. J. Baldock, Y. Yang, J. G. Hardy and C. K. Ghosh, RSC Adv., 2020, 10, 11060–11073 RSC.
- R. Y. Pelgrift and A. J. Friedman, Adv. Drug Delivery Rev., 2013, 65, 1803–1815 CrossRef CAS PubMed.
- A. Samanta, S. Podder, C. K. Ghosh, M. Bhattacharya, J. Ghosh, A. K. Mallik, A. Dey and A. K. Mukhopadhyay, J. Mech. Behav. Biomed. Mater., 2017, 72, 110–128 CrossRef CAS PubMed.
- L. Shi, J. Chen, L. Teng, L. Wang, G. Zhu, S. Liu, Z. Luo, X. Shi, Y. Wang and L. Ren, Small, 2016, 12, 4165–4184 CrossRef CAS PubMed.
- C. Liao, Y. Li and S. C. Tjong, Int. J. Mol. Sci., 2018, 19, 3564 CrossRef PubMed.
- S. Syama and P. Mohanan, Int. J. Biol. Macromol., 2016, 86, 546–555 CrossRef CAS PubMed.
- O. Erol, I. Uyan, M. Hatip, C. Yilmaz, A. B. Tekinay and M. O. Guler, Nanomedicine, 2018, 14, 2433–2454 CrossRef CAS PubMed.
- L. Ou, B. Song, H. Liang, J. Liu, X. Feng, B. Deng, T. Sun and L. Shao, Part. Fibre Toxicol., 2016, 13, 1–24 CrossRef PubMed.
- P. Kumar, P. Huo, R. Zhang and B. Liu, Nanomaterials, 2019, 9, 737 CrossRef CAS PubMed.
- M. Khan, M. R. Shaik, S. T. Khan, S. F. Adil, M. Kuniyil, M. Khan, A. A. Al-Warthan, M. R. H. Siddiqui and M. Nawaz Tahir, ACS Omega, 2020, 5, 1987–1996 CrossRef CAS PubMed.
- J. B. Fathima, A. Pugazhendhi and R. Venis, Microb. Pathog., 2017, 110, 245–251 CrossRef CAS PubMed.
- S. Anandhi, M. L. Edward and V. Jaisankar, Mater. Today: Proc., 2021, 40, S93–S101 CAS.
- L. Liu, R. Bhatia and T. J. Webster, Int. J. Nanomed., 2017, 12, 8711 CrossRef CAS PubMed.
- S. Jafari, B. Mahyad, H. Hashemzadeh, S. Janfaza, T. Gholikhani and L. Tayebi, Int. J. Nanomed., 2020, 15, 3447 CrossRef CAS PubMed.
- K. Handore, D. Walunj, P. Chhattise, A. Chabukswar, K. Mohite, S. Dallavalle, B. Bharat and V. Chabukswar, Polym.-Plast. Technol. Eng., 2017, 56, 1259–1266 CrossRef CAS.
- D. Devadathan and R. Raveendran, Int. J. Chem. Eng. Appl., 2014, 5, 240 CAS.
| This journal is © The Royal Society of Chemistry 2022 |