Open Access Article
Open Access ArticleSynthesis of bimetallic nickel cobalt selenide particles for high-performance hybrid supercapacitors†
Bei Jiangab,
Yang Liuab,
Jingchao Zhangb,
Yinhuan Wangb,
Xinyu Zhangb,
Renchun Zhangb,
Liang-Liang Huang *a and
Daojun Zhang
*a and
Daojun Zhang *b
*b
aSchool of Chemistry and Material Science, Liaoning Shihua University, Fushun 113001, Liaoning, P. R. China. E-mail: huangll@lnpu.edu.cn
bCollege of Chemistry and Chemical Engineering, Anyang Normal University, Anyang 455000, Henan, China. E-mail: zhangdj0410@sohu.com; Tel: +86 372 2900040
First published on 7th January 2022
Abstract
Supercapacitors are known as promising excellent electrochemical energy storage devices because of their attractive features, including quick charge and discharge, high power density, low cost and high security. In this work, a series of litchi-like Ni–Co selenide particles were synthesized via a simple solvothermal method, and the Ni–Co compositions were carefully optimized to tune the charge storage performance, charge storage kinetics, and conductivity for battery-like supercapacitors. Interestingly, the optimal sample Ni0.95Co2.05Se4 exhibits a high capacity of 1038.75 F g−1 at 1 A g−1 and superior rate performance (retains 97.8% of the original capacity at 4 A g−1). Moreover, an asymmetric supercapacitor device was assembled based on the Ni0.95Co2.05Se4 cathode and activated carbon anode. The device of Ni0.95Co2.05Se4//active carbon (AC) reveals a peak energy density of 37.22 W h kg−1, and the corresponding peak power density reaches 800.90 W kg−1. This work provides a facile and effective way to synthesize transition metal selenides as high-performance supercapacitor electrode materials.
1. Introduction
In recent years, with the depletion of fossil energy and the deterioration of the environment, the development of energy-efficient energy storage devices has become a hot research field.1–6 Due to the high power density and long life span for supercapacitors, more and more attention has been paid to them by researchers.7–12 Recently, transition metal selenide electrode materials have been extensively studied in the field of renewable energy for enhancing energy storage capabilities.13–16 The energy gap of selenides is smaller than that of oxides and sulfides of the same family, so selenides generally show superior electrochemical properties. Compared with Co or Ni single metal selenides,17,18 purposely designed binary metal selenide composites are an effective method to optimize electrode performance. For example, the (Ni,Co)Se2 bimetallic selenide-composite nanostructure was prepared by Wang et al., as a result, the (Ni,Co)Se2/NiCo LDH delivers a high capacity of 170 mA h g−1 (1224 F g−1) at a current density of 2 A g−1.19 The specific capacity of reported NiCo2Se4-rGO reaches 2038.55 F g−1 at a current density of 1 A g−1, and maintains 90% of the original capacity after 1000 cycles.20 Liu et al. designed and synthesized core–shell structure selenium-enriched bimetallic selenide balls as the battery–supercapacitor hybrid cathode electrode, the capacity of the electrode reaches 164.44 mA h g−1 at current density 1 A g−1, and it still maintains 85.72% of the initial capacity after 5000 cycles.21 Aside from considering the morphology effect on the electrochemical performance, control of the synergy between nickel and cobalt ions in the active material is another useful way to increase the capacitance.The pyrite-type NiCoSe4 is an ideal research model for supercapacitor, tuning of Ni/Co ratio in the structure may be an effect way to improve the sluggish kinetics.22 The NiCo2.1Se3.3NSs array electrode material with high conductivity and excellent specific capacitance of 742.4 F g−1 under 1 mA cm−2 was prepared by Wang et al., and still maintained 83.8% of the original capacity after 1000 cycles.23 In the all above reports, due to the good synergy between nickel and cobalt ions, the electrochemical performances of Co–Ni selenides have prominent advantages than single metal selenides, and according to current reports, researchers are often devoted to development of different structure materials to act as cathode materials for supercapacitor devices, however, rarely optimized Ni/Co compositions of Ni–Co–selenide carefully to tune charge storage performances for supercapacitor.
In this work, we present a simple one step solvothermal method to prepare spherical particles of Ni–Co–selenide with different ratios of nickel and cobalt ions, and the electrochemical properties of this series of products were studied. Surprisingly, the optimized Ni0.95Co2.05Se4 particles as supercapacitor electrode shows the highest capacity and distinguished cycling stability. Besides, the as-fabricated hybrid supercapacitor device (Ni0.95Co2.05Se4//AC) composed of selenide positive electrode and activated carbon negative electrode exhibits 45.86 mA h g−1 (104.68 F g−1) at 1 A g−1 and retains 95.21% of initial capacitance after 4000 cycles.
2. Experimental
2.1 Material preparation
Cobalt(II)2,4-pentanedionate, nickel(II)2,4-pentanedionate, cyclohexane, ethylene glycol, N,N-dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP), polyvinylidene fluoride (PVDF), polytetrafluoroethylene (PTFE), and graphite powder were purchased from Sinopharm Chemical Reagent Co, Ltd. All chemical reagents used in this work are analytically pure and are not further purification.2.2 Synthetic active material
Firstly, the total amount of 0.1 mmol mixture of nickel(II)2,4-pentanedionate and cobalt(II)2,4-pentanedionate and equal amounts of sodium selenide were added to the 20 mL Teflon autoclave. 2.5 mL of DMF, 6.0 mL of water, 0.5 mL of cyclohexane and 3.0 mL of ethylene glycol were then added in turn to stir on the magnetic agitator for an hour. The resulting uniform mixture solution was placed in an oven and kept for 12 h at 180 °C. Black solid products were washed three times with ethanol and deionized water and dried at 80 °C for 8 h. Under the same operating conditions, we also synthesized other samples of nickel–cobalt selenide with Ni/Co molar ratios of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,1
1,1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2, 1
2.2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.
5.
2.3 Electrode production
The as-prepared black solid samples, PVDF, and acetylene black in accordance with the ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the mortar of mixed grinding, then adding 120 μL of NMP to grind into an ink-like black solution. The ink was evenly coated on a 1 × 1 cm2 nickel foam and then pressed at 10 MPa. Finally, a cathode electrode was obtained after vacuum-dried at 110 °C for 12 h. The manufacturing method of other sample electrodes is the same as above. The difference is that when assembling asymmetric supercapacitors, anode electrode plate was prepared via mixed with activated carbon, PTFE, and acetylene black in 8
1 in the mortar of mixed grinding, then adding 120 μL of NMP to grind into an ink-like black solution. The ink was evenly coated on a 1 × 1 cm2 nickel foam and then pressed at 10 MPa. Finally, a cathode electrode was obtained after vacuum-dried at 110 °C for 12 h. The manufacturing method of other sample electrodes is the same as above. The difference is that when assembling asymmetric supercapacitors, anode electrode plate was prepared via mixed with activated carbon, PTFE, and acetylene black in 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and evenly coated on nickel foam.
1 ratio and evenly coated on nickel foam.
2.4 Electrochemical measurement
The electrochemical tests of these nickel–cobalt selenides modified electrodes were conducted on an electrochemical workstation (CHI 660E) at room temperature by three-electrode system in 2 M KOH solution. The Hg/Hg2Cl2 electrode was used as the reference electrode and the Pt foil was used as the counter electrode, respectively. The specific capacitance of electrodes can be calculated according to GCD curves under the three-electrode system by using the following equation.24
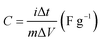 | (1) |
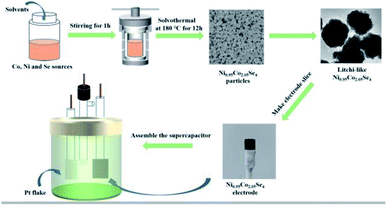 | ||
| Scheme 1 Schematic illustration for the synthesis of electrode materials and assemble the supercapacitor. | ||
2.5 Electrochemical performance of hybrid supercapacitor (HSC) device
In order to study the electrochemical properties of the optimized electrode, it is assembled into asymmetric supercapacitors with active carbon as negative. The useful area of the positive and negative electrodes is 1 × 1 cm2. The mass selection of the two electrodes is calculated according to the theory of charge balance (Q+ = Q−) (eqn (2)).
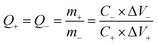 | (2) |
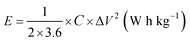 | (3) |
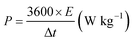 | (4) |
3. Results and discussion
In the one-step solvothermal synthesis of nickel–cobalt selenide particles, cobalt acetylacetonate and nickel acetylacetonate at the molar ratio of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2, 1
2.2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
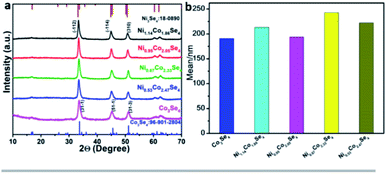
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to obtain a series of nickel cobalt selenides (NixCo3−xSe4). There was no significant difference in the morphology of the nickel–cobalt selenide particles, and their average diameters were around 191 to 243 nm, the detailed data is shown in Fig.S1, S2, and Table S1.† Fig. 1a shows the XRD patterns of the as-synthesized products at different proportions. The XRD peaks of pure Co3Se4 sample match well with the monoclinic phase Co3Se4 (ref. 28) with the PDF no. of 96-901-2804. With the increase of the content of nickel ions, the intensity of the diffraction peak remained unchanged, and the peak position shifted to left slightly. According to the Bragg equation 2d
5 to obtain a series of nickel cobalt selenides (NixCo3−xSe4). There was no significant difference in the morphology of the nickel–cobalt selenide particles, and their average diameters were around 191 to 243 nm, the detailed data is shown in Fig.S1, S2, and Table S1.† Fig. 1a shows the XRD patterns of the as-synthesized products at different proportions. The XRD peaks of pure Co3Se4 sample match well with the monoclinic phase Co3Se4 (ref. 28) with the PDF no. of 96-901-2804. With the increase of the content of nickel ions, the intensity of the diffraction peak remained unchanged, and the peak position shifted to left slightly. According to the Bragg equation 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sinθ = nλ, the radius of the metal nickel ions is larger than the radius of the cobalt ions, and this causing a slight negative shift of the diffraction peak in the Ni-doped products, the main diffraction peak of Ni1.14Co1.86Se4 at 33.2° matches well with the monoclinic Ni3Se4 (18-0890). Furthermore, the FWHM is inversely proportional to particle size, thus the pure Co3Se4 demonstrates the smallest particle size, the consequence is consistent with the statistical result shown in Fig. 1b. EDX analysis showed that the distribution of elements in each product was uniform and the proportion was close to the theoretical value (Fig. S3†). The calculated chemical formula of the synthesized samples is Co3Se4, Ni1.14Co1.86Se4, Ni0.95Co2.05Se4, Ni0.67Co2.33Se4, and Ni0.53Co2.47Se4, respectively.
sinθ = nλ, the radius of the metal nickel ions is larger than the radius of the cobalt ions, and this causing a slight negative shift of the diffraction peak in the Ni-doped products, the main diffraction peak of Ni1.14Co1.86Se4 at 33.2° matches well with the monoclinic Ni3Se4 (18-0890). Furthermore, the FWHM is inversely proportional to particle size, thus the pure Co3Se4 demonstrates the smallest particle size, the consequence is consistent with the statistical result shown in Fig. 1b. EDX analysis showed that the distribution of elements in each product was uniform and the proportion was close to the theoretical value (Fig. S3†). The calculated chemical formula of the synthesized samples is Co3Se4, Ni1.14Co1.86Se4, Ni0.95Co2.05Se4, Ni0.67Co2.33Se4, and Ni0.53Co2.47Se4, respectively.
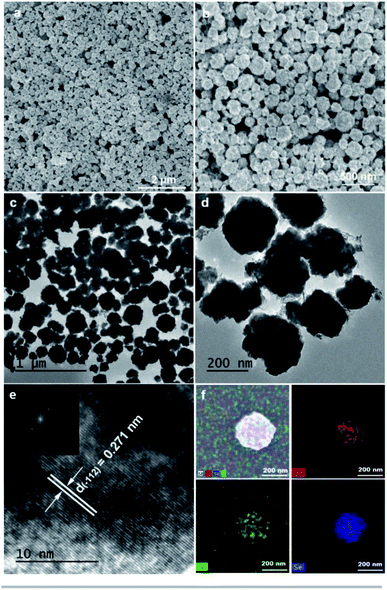
As shown in Fig. 2a and b, SEM images of Ni0.95Co2.05Se4 sample show that the particles were uniform distributed without impurities, which allows the active material to be evenly distributed on the electrode surface to improve electrochemical properties. The growth mechanism of Ni0.95Co2.05Se4 sample was furthermore studied at different conditions. The SEM images of different synthesis temperatures and times are shown in Fig. S4a–h and S4i–p,† it clearly seen that low temperature and short-term reaction time have a great influence on the morphology. It is difficult to form at a low temperature of 100 °C even the reaction time till to 12 h, while reaction temperature reaches 160 °C, the yield is low in reaction time of 1 h. In addition, we have further studied the influence of reagent dosage on the morphology as shown in Fig. S5a–f.† Sample morphology with the absence of cyclohexane (Fig. S5a and b†), ethylene glycol (Fig. S5c and d†) and both (Fig. S5e and f†) will cause adhesion and aggregation (Fig. S5g and h†), which shows that a small amount of cyclohexane and ethylene glycol can maintain a uniform morphology. When change Ni/Co ratio to 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the octahedron morphology can be obtained (Fig. S5g and h†). TEM images of Ni0.95Co2.05Se4 were further used to study the structure. The results are shown in Fig. 2c and d, which shows that the particles are about 194 nm solid architecture, and the lattice fringes of Ni0.95Co2.05Se4 sample can be clearly observed in high resolution TEM image (Fig. 2e). The lattice stripe spacing of 0.271 nm corresponds to the (−112) planes of Ni3Se4. Fig. 2f shows uniform distribution of Ni, Co, and Se elements in the sample of Ni0.95Co2.05Se4. The nitrogen sorption isotherms and the pore size distribution of five NixCo3−XSe4 samples with different ratios are shown in Fig. S6.† The BET specific surface area of Ni0.95Co2.05Se4 nanoparticles is 22.17 m2 g−1, where the presence of mesopores can be determined. However, the BET area of Co3Se4, Ni1.14Co1.86Se4, Ni0.67Co2.33Se4, and Ni0.53Co2.47Se4 is 15.92 m2 g−1, 12.64 m2 g−1, 9.0 m2 g−1, and 9.57 m2 g−1, respectively, which are all smaller than the Ni0.95Co2.05Se4 sample. It is clearly found that mean mesoporous size of Ni0.95Co2.05Se4 are smaller than others in the Fig. S6.† These data indicating that Ni0.95Co2.05Se4 particle has more active sites and a larger contact area with the electrolyte than other samples.
1, the octahedron morphology can be obtained (Fig. S5g and h†). TEM images of Ni0.95Co2.05Se4 were further used to study the structure. The results are shown in Fig. 2c and d, which shows that the particles are about 194 nm solid architecture, and the lattice fringes of Ni0.95Co2.05Se4 sample can be clearly observed in high resolution TEM image (Fig. 2e). The lattice stripe spacing of 0.271 nm corresponds to the (−112) planes of Ni3Se4. Fig. 2f shows uniform distribution of Ni, Co, and Se elements in the sample of Ni0.95Co2.05Se4. The nitrogen sorption isotherms and the pore size distribution of five NixCo3−XSe4 samples with different ratios are shown in Fig. S6.† The BET specific surface area of Ni0.95Co2.05Se4 nanoparticles is 22.17 m2 g−1, where the presence of mesopores can be determined. However, the BET area of Co3Se4, Ni1.14Co1.86Se4, Ni0.67Co2.33Se4, and Ni0.53Co2.47Se4 is 15.92 m2 g−1, 12.64 m2 g−1, 9.0 m2 g−1, and 9.57 m2 g−1, respectively, which are all smaller than the Ni0.95Co2.05Se4 sample. It is clearly found that mean mesoporous size of Ni0.95Co2.05Se4 are smaller than others in the Fig. S6.† These data indicating that Ni0.95Co2.05Se4 particle has more active sites and a larger contact area with the electrolyte than other samples.
X-Ray photoelectron spectroscopy (XPS) is used to analyze the surface element constitution and electronic state of the Ni0.95Co2.05Se4 sample. Fig. 3a shows the survey scan spectrum of Ni0.95Co2.05Se4 sample, which exhibits the chemical states of Ni 2p, Co 2p, and Se 2p.29 After Gaussian fitting, three sets of peaks can be obtained from the 2p map of Ni, corresponding to two spin orbital peaks and two shake-up satellites. The peaks at 868.44 and 851.18 eV represent 2p3/2 and 2p1/2 of Ni2+, respectively (Fig. 3b). Besides, the peaks at 854.13 and 871.88 eV are attributed to 2p3/2 and 2p1/2 of Ni3+ in Ni0.95Co2.05Se4 particles.30–32 The peaks with binding energies of 795.15 and 779.28 eV in Fig. 3c correspond to Co2+, while the peaks located at 791.22 and 776.40 eV correspond to Co3+. Moreover, there are two distinct satellite peaks located in the combined energy of 800.76 and 783.84 eV.33–35 For the Se 3d spectra (Fig. 3d), there is a pair of metal-selenium bonds, which indicates the presence selenium ions in Ni0.95Co2.05Se4 nanoparticles. The broad peak situated at 57.24 eV reflects surface Se species with a high oxidation state (SeOx),36,37 which is attributed to a small amount of oxidation on the surface.
 | ||
| Fig. 3 XPS spectra of Ni0.95Co2.05Se4 (a) wide-scan survey spectrum, (b) Ni 2p, (c) Co 2p, and (d) Se 3d spectra. | ||
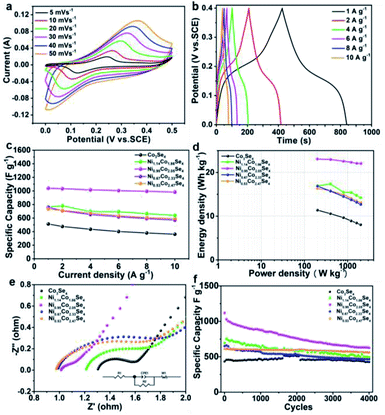
Using 2 M KOH aqueous solution as the electrolyte, the electrochemical properties of the working electrodes for supercapacitors were tested in a three-electrode system. Fig. S7† shows CV curves of Co3Se4, Ni1.14Co1.86Se4, Ni0.95Co2.05Se4, Ni0.67Co2.33Se4, and Ni0.53Co2.47Se4 samples at a scanning speed of 20 mV s−1. The curve of as-synthesized Ni0.95Co2.05Se4 exhibited the strongest redox peaks and largest closed area among the five samples. In Fig. S8,† obviously, the sample of Ni0.95Co2.05Se4 reveals the longest discharge time of the five electrodes, shows the best capacitance properties, which demonstrates the content of Ni/Co ratios has great influence on discharge capacity. Especially, the Ni0.95Co2.05Se4 delivers a capacity of 1038.75 F g−1 at a current density of 1 A g−1, while the corresponding capacity of Co3Se4, Ni1.14Co1.86Se4, Ni0.67Co2.33Se4, and Ni0.53Co2.47Se4 samples is 512.06, 762.74, 760.04, and 734.81 F g−1, respectively (Fig. S9†).
After comparing discharge curves of five different electrodes at 1 A g−1, it is concluded that the Ni0.95Co2.05Se4 electrode has the greatest energy storage capacity. Fig. 4a depicts the CV curves of Ni0.95Co2.05Se4 at different scan rates (5–50 mV s−1) under operating potential of 0–0.5 V. As the scan rate increased, the shape of these curves showed negligible changes but the intensity of these curves is increased. Therefore, the Ni0.95Co2.05Se4 electrode demonstrated fast electronic/ionic transport and high-rate capability. The GCD profiles of Ni0.95Co2.05Se4 in Fig. 4b exhibit quasi-symmetric shaped curves, indicating reversible redox reaction during the charging and discharging process. The specific capacities of the series of electrodes were calculated from the discharge time in the GCD curves and shown in Fig. 4c, remarkably, 94.58% of the initial capacitance could be retained for Ni0.95Co2.05Se4 electrode at a high current density of 10 A g−1, which also exhibits a higher specific capacity and rate performance compared with other samples. The energy density and power density curves of the samples were calculated according to the discharge curves, which shows in Fig. 4d. In general, the value of P increases but E decreases with the increase of current density. The Ni0.95Co2.05Se4 electrode can deliver a biggest energy density of 23.08 W h kg−1 at 1 A g−1 and the corresponding power density is 200.05 W kg−1. The energy density still reaches 22.04 W h kg−1 when the power density reaches 1609.54 W kg−1, and is higher than the energy density of the other samples.
The electrochemical impedance spectroscopy (EIS) of the nickel cobalt selenide series samples in the frequency range of 0.1 to 105 Hz are further studied. The Nyquist curves schematic of Co3Se4, Ni1.14Co1.86Se4, Ni0.95Co2.05Se4, Ni0.67Co2.33Se4, and Ni0.53Co2.47Se4 electrode materials are shown in Fig. 4e, the shapes of these five samples are similar, a semicircle in the high frequency region represents the charge transfer resistance, and the straight line in the low frequency region represents the diffusion resistance, this corresponds to the Bode plot diagram in Fig. S10.† After fitting, these electrodes have the same equivalent circuit. It is clear seen that the introducing Ni ions in Co3Se4 lattice can effectively decrease the solution resistance (Rs). Due to the intrinsic half-metallic behavior, Co3Se4 exhibits small charge transfer resistance, as increasing Ni content to 32% in Ni0.95Co2.05Se4, the Rct decrease dramatically, indicating that the charge transfer speed is much faster.38 Furthermore, according to the diffusion coefficient formula,39 the diffusion coefficient value of Ni0.95Co2.05Se4 is the largest among all the samples (Table S2†), this may be related to the effective diffusion of the OH− in Ni0.95Co2.05Se4 sample. Fig. 4f describes the cycling performance of the Co3Se4, Ni1.14Co1.86Se4, Ni0.95Co2.05Se4, Ni0.67Co2.33Se4 and Ni0.53Co2.47Se4 electrodes. The Ni0.95Co2.05Se4 electrode exhibits a specific capacitance of 625.27 F g−1 (61.55% capacitance retention) after 4000 cycles at a current density of 4 A g−1, whereas the others electrode exhibits a lower capacitance of 426.75, 493.47, 468.2, and 561.44 F g−1 after 4000 cycles at a current density of 4 A g−1, respectively. Although the retention rate is not higher than other sample electrodes, the capacity after cycling is still higher than other capacity values. The Ni0.95Co2.05Se4 electrode presents better electrochemical properties than some of the related supercapacitor electrode materials reported in the literature (Table S3†). Furthermore, the charge storage mechanism of the as-prepared series of electrode materials was investigated by fitting the plots of logarithmic anodic peak current versus logarithmic scan rate. According to the equation log(i) = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(v)+log(a), the plot slope b of 0.5 and 1.0 is the contribution of the electrochemical process dominated by ionic diffusion and surface capacitive control process.40 As shown in Fig. S11a,† the calculated b value of Co3Se4, Ni1.14Co1.86Se4, Ni0.95Co2.05Se4, Ni0.67Co2.33Se4, and Ni0.53Co2.47Se4 electrode is 0.795, 0.717, 0.707, 0.888, and 0.967, respectively, which indicated the diffusion contribution is dominant in Ni0.95Co2.05Se4 electrode. The result is consistent with the 58% diffusion contribution of the capacitance at low scan speed of 10 mV s−1, conversely, capacitive contribution enlarged at high rate for Ni0.95Co2.05Se4 electrode (Fig. S11b†).
log(v)+log(a), the plot slope b of 0.5 and 1.0 is the contribution of the electrochemical process dominated by ionic diffusion and surface capacitive control process.40 As shown in Fig. S11a,† the calculated b value of Co3Se4, Ni1.14Co1.86Se4, Ni0.95Co2.05Se4, Ni0.67Co2.33Se4, and Ni0.53Co2.47Se4 electrode is 0.795, 0.717, 0.707, 0.888, and 0.967, respectively, which indicated the diffusion contribution is dominant in Ni0.95Co2.05Se4 electrode. The result is consistent with the 58% diffusion contribution of the capacitance at low scan speed of 10 mV s−1, conversely, capacitive contribution enlarged at high rate for Ni0.95Co2.05Se4 electrode (Fig. S11b†).
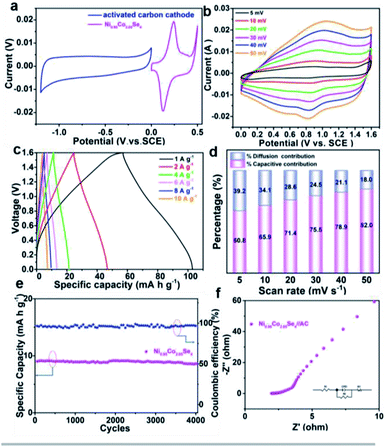
In order to further investigate the electrical properties of NixCo3−XSe4 in practical applications, the HSC devices are assembled by using activated carbon as the negative electrode and the series of NixCo3−XSe4 samples as the positive electrode. Fig. 5a displays the CV curves with a voltage window of −1.2–0 and 0–0.5 V for the activated carbon and Ni0.95Co2.05Se4 electrodes at 5 mV s−1 in a three-electrodes system, respectively. The charge and discharge of the carbon electrode forms an approximately rectangular CV curve, and anodic active material exhibits a pair of strong redox peaks. Fig. S12a† shows the CV curves of 10 mV s−1 under different test ranges from 1.2–2.0 V. When the voltage range from 1.2 V to 1.4 V is selected, the corresponding Faraday effect is not reflected well, and when the voltage is further increased to 1.8 V and 2.0 V, the electrode material is polarized and damaged for oxygen evolution reaction as shown in Fig. S11b,† therefore, a suitable voltage window of 0–1.6 V was selected for Ni0.95Co2.05Se4//AC device. Fig. 5b and Fig. S13† show the CV curves of the Ni0.95Co2.05Se4//AC and other HSCs at various scan rates, respectively. The current response gradually increases with the rise of the scan rates, and a little redox peak position was found to be stable at 0.85 and 1.1 V. The charges storage mechanism of Ni0.95Co2.05Se4//AC HSC was also characterized by CV technique at the scan speed of 5–50 mV s−1, the calculated value of b is 0.897 (Fig. S14†), it shows that the process is major dominated by surface capacitive control and involved in diffusion-controlled behavior. In order to compare the contribution ratios of the two behaviors at different scan rate, the calculated results were shown in Fig. 5d. The pseudocapacitive property increases from 60.8% to 82% with increase the scan rate from 5 to 50 mV s−1, indicating the capacitive contribution is participating in charge storage at high rate for Ni0.95Co2.05Se4//AC HSC. The charging–discharging voltage profiles of the Ni0.95Co2.05Se4//AC HSC and other HSCs at a series of current densities from 1 to 10 A g−1 are approximately symmetric (Fig. 5c and S15†), which manifests the special nature of the excellent coulombic efficiency and superior electrochemical reversibility. The specific capacitance of Ni0.95Co2.05Se4//AC HSC calculated from the discharge time is 45.9 mA h g−1 at 1 A g−1, and it still maintains 34.2 mA h g−1 at 10 A g−1. The impedance diagram of the HSC is shown in Fig. 5f, the fitting result of Rs and Rct is 2.961 Ω and 1.831 Ω, respectively. The cycling performance of Ni0.95Co2.05Se4//AC HSC was further investigated by repeated GCD tests at 4 A g−1 (Fig. 5e). The specific capacity Ni0.95Co2.05Se4//AC HSC retains 95.21% of the initial capacity and coulomb efficiency keeps 96.71% after 4000 cycles, respectively, suggesting that the constructed HSC possesses superior long-term electrochemical stability.
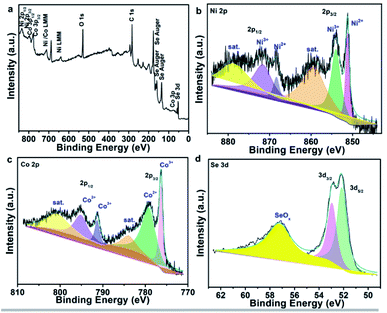
In practical applications, energy density and power density are two important factors that determine the quality of energy storage equipment. Fig. 6 shows the energy density and power density of the assembled Ni0.95Co2.05Se4//AC HSC and other related supercapacitor devices. By comparison, the specific energy/power densities of our constructed Ni0.95Co2.05Se4//AC HSC is no less than or even better than the previous related ASC devices. The corresponding devices are NiSe–Se@NF//AC@Ni (29.9 W h kg−1 at 594.46 W kg−1),41 NCS/NGF//AC (36.8 W h kg−1 at 375 W kg−1),42 NiSe@MoSe2//N-PMCN (32.6 W h kg−1 at 415 W kg−1),43 Ni0.85Se@MoSe2//GNS (25.5 W h kg−1 at 420 W kg−1),44 Ni3Se2-24//AC (22.3 W h Kg−1 at 160.4 W kg−1),32 NiSe2@CNT//AC (25.61 W h kg−1 at 810 W kg−1),45 NiCo2O4@Ni0.85Se//AC (29.3 W h kg−1 at 799 W kg−1),46 Ni3Se2 3D HMNN//AC (38.4 W h kg−1 at 794.5 W kg−1).37 These above results suggest that the Ni0.95Co2.05Se4//C device possesses remarkable energy storage capability and cycling stability, which demonstrate greatly practical applications in energy storage devices.
 | ||
| Fig. 6 Ragone plot the assembled Ni0.95Co2.05Se4//AC ASC supercapacitor and other related supercapacitors. | ||
4. Conclusion
In summary, a series of NixCo3−xSe4 particles with high electrochemical capacitance were synthesized with a simple and effective solvothermal method. The optimized Ni0.95Co2.05Se4 electrode specific capacitance can reach 1038.75 F g−1 at 1 A g−1, which is higher than the other four samples. The Ni0.95Co2.05Se4 electrode has excellent rate capability as well as outstanding cycling stability. The best electrochemical performance of Ni0.95Co2.05Se4 may be ascribed to its high surface area, low charge transfer resistance, and high diffusion coefficient in the series samples. Furthermore, an HSC device is assembled by using activated carbon as the negative electrode and Ni0.95Co2.05Se4 as the positive electrode, which present excellent stability and high coulomb efficiency during the 4000 cycles. Moreover, the Ni0.95Co2.05Se4//AC HSC arrives an energy density of 37.22 W h kg−1 and a power density of 800.90 W kg−1. The ASC device can keep 95.21% of original capacity after 4000 cycles. The electrochemical properties indicate the Ni0.95Co2.05Se4 is a promising electrode in future energy storage devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation of China (No. 21603004, U1604119), the Key Scientific Project of Higher Education of Henan Province (No. 22A150002), and the Program for Innovative Research Team of Science and Technology in the University of Henan Province (18IRTSTHN006).Notes and references
- X. Y. Yu and X. W. Lou, Mixed Metal Sulfides for Electrochemical Energy Storage and Conversion, Adv. Energy Mater., 2018, 8, 1701592 CrossRef.
- Y. Zhang, Q. Zhou, J. X. Zhu, Q. Y. Yan, S. X. Dou and W. P. Sun, Nanostructured Metal Chalcogenides for Energy Storage and Electrocatalysis, Adv. Funct. Mater., 2017, 27, 1702317 CrossRef.
- Q. B. Yun, Q. P. Lu, X. Zhang, C. L. Tan and H. Zhang, Three-Dimensional Architectures Constructed from Transition-Metal Dichalcogenide Nanomaterials for Electrochemical Energy Storage and Conversion, Angew. Chem., Int. Ed., 2018, 57, 626–646 CrossRef CAS PubMed.
- Y. H. Dou, L. Zhang, X. Xu, Z. Q. Sun, T. Liao and S. X. Dou, Atomically thin non-layered nanomaterials for energy storage and conversion, Chem. Soc. Rev., 2017, 46, 7338–7373 RSC.
- Y. Li, Y. X. Xu, W. P. Yang, W. X. Shen, H. G. Xue and H. Pang, MOF-Derived Metal Oxide Composites for Advanced Electrochemical Energy Storage, Small, 2018, 14, 1704 Search PubMed.
- Y. Gogotsi and R. M. Penner, Energy Storage in Nanomaterials-Capacitive, Pseudocapacitive, or Battery-like?, ACS Nano, 2018, 12, 2081–2083 CrossRef CAS PubMed.
- T. Brousse, D. Bélanger and J. W. Longd, To Be or Not To Be Pseudocapacitive?, J. Electrochem. Soc., 2015, 162, A5185–A5189 CrossRef CAS.
- J. L. Qi, Y. T. Yan, Y. F. Cai, J. Cao and J. C. Feng, Nanoarchitectured Design of Vertical-Standing Arrays for Supercapacitors: Progress, Challenges, and Perspectives, Adv. Funct. Mater., 2021, 31, 2006030 CrossRef CAS.
- Y. L. Shao, M. F. El-Kady, J. Y. Sun, Y. G. Li, Q. H. Zhang, M. F. Zhu, H. Z. Wang, B. Dunn and R. B. Kaner, Design and Mechanisms of Asymmetric Supercapacitors, Chem. Rev., 2018, 118, 9233–9280 CrossRef CAS PubMed.
- R. Lei, H. W. Ni, R. S. Chen, H. Z. Gu, H. Zhang and S. Dong, In situ growth of self-supported and defect-engineered carbon nanotube networks on 316L stainless steel as binder-free supercapacitors, J. Colloid Interface Sci., 2018, 532, 622–629 CrossRef CAS PubMed.
- R. Lei, H. Zhang, Q. Fang, H. W. Ni and H. Z. Gu, MnO2 nanowires electrodeposited on freestanding graphenated carbon nanotubes as binder-free electrodes with enhanced supercapacitor performance, Mater. Lett., 2019, 249, 140–142 CrossRef CAS.
- R. Lei, J. H. Gao, L. F. Qi, L. L. Ye, C. Wang, Y. Le, Y. Huang, X. G. Shi and H. W. Ni, Construction of MnO2 nanosheets@graphenated carbon nanotube networks core–shell heterostructure on 316L stainless steel as binderfree supercapacitor electrodes, Int. J. Hydrogen Energy, 2020, 45, 28930–28939 CrossRef CAS.
- H. J. Zhou, X. X. Li, Y. Li, M. B. Zheng and H. Pang, Applications of MxSey (M = Fe, Co, Ni) and Their Composites in Electrochemical Energy Storage and Conversion, Nano-Micro Lett., 2019, 11(40), 1–33 Search PubMed.
- T. T. Nguyen, J. Balamurugan, V. Aravindan, N. H. Kim and J. H. Lee, Boosting the Energy Density of Flexible Solid-State Supercapacitors via Both Ternary NiV2Se4 and NiFe2Se4 Nanosheet Arrays, Chem. Mater., 2019, 31, 4490–4504 CrossRef CAS.
- X. Song, C. Huang, Y. Qin, H. Li and H. C. Chen, Hierarchical hollow, sea-urchin-like and porous Ni0.5Co0.5Se2 as advanced battery material for hybrid supercapacitors, J. Mater. Chem. A, 2018, 6, 16205–16212 RSC.
- L. R. Hou, Y. Y. Shi, C. Wu, Y. R. Zhang, Y. Z. Ma, X. Sun, J. F. Sun, X. G. Zhang and C. Z. Yuan, Monodisperse Metallic NiCoSe2 Hollow Sub-Microspheres: Formation Process, Intrinsic Charge-Storage Mechanism, and Appealing Pseudocapacitance as Highly Conductive Electrode for Electrochemical Supercapacitors, Adv. Funct. Mater., 2018, 28, 1705921 CrossRef.
- H. Mei, L. Zhang, K. Zhang, J. Gao, H. Zhang, Z. Huang, B. Xu and D. Sun, Conversion of MOF into carbon-coated NiSe2 yolk–shell microspheres as advanced battery-type electrodes, Electrochim. Acta, 2020, 357, 136866 CrossRef CAS.
- S. Wang and S. Ma, Facile fabrication of Ni0.85Se nanowires by the composite alkali salt method as a novel cathode material for asymmetric supercapacitors, Dalton Trans., 2019, 48, 3906–3913 RSC.
- X. Li, H. Wu, C. Guan, A. M. Elshahawy, Y. Dong, S. J. Pennycook and J. Wang, (Ni,Co)Se2/NiCo-LDH core/shell structural electrode with the cactus-Like (Ni,Co)Se2 core for asymmetric supercapacitors, Small, 2019, 15, 1803895 Search PubMed.
- B. G. Amin, J. Masud and M. Nath, Facile one-pot synthesis of NiCo2Se4-rGO on Ni foam for high performance hybrid supercapacitors, RSC Adv., 2019, 9, 37939–37946 RSC.
- Y. L. Liu, C. Yan, G. G. Wang, F. Li, Q. Kang, H. Y. Zhang and J. C. Han, Selenium-rich nickel cobalt bimetallic selenides with core–shell architecture enable superior hybrid energy storage devices, Nanoscale, 2020, 12, 4040–4050 RSC.
- Z. Y. Xie, D. P. Qiu, J. N. Xia, J. Y. Wei, M. Li, F. Wang and R. Yang, Hollow Biphase Cobalt Nickel Perselenide Spheres Derived from Metal Glycerol Alkoxides for High-Performance Hybrid Supercapacitors, ACS Appl. Mater. Interfaces, 2021, 13, 12006–12015 CrossRef CAS PubMed.
- Y. Wang, W. Zhang, X. Guo, K. Jin, Z. Chen, Y. Liu, L. Yin, L. Li, K. Yin, L. Sun and Y. Zhao, Ni–Co selenide nanosheet/3D graphene/nickel foam binder-free electrode for high-performance supercapacitor, ACS Appl. Mater. Interfaces, 2019, 11, 7946–7953 CrossRef CAS PubMed.
- M. M. Vadiyar, S. B. Bandgar, S. S. Kolekar, J.-Y. Chang, Y.-C. Ling, Z. Ye and A. V. Ghule, Holey C@ZnFe2O4 Nanoflakes by Carbon Soot Layer Blasting Approach for High Performance Supercapacitors, ACS Appl. Energy Mater., 2019, 2, 6693–6704 CrossRef CAS.
- X. Zhou, J. Zhu, Y. Lu, Y. Zhang, Y. Hong, W. Wang, K. Karimov, I. Murtaza, Q. Wang and X. Dong, Three-Dimensional Co–S–P Nanoflowers as Highly Stable Electrode Materials for Asymmetric Supercapacitors, ACS Sustainable Chem. Eng., 2019, 7, 11448–11454 CrossRef CAS.
- A. Ray, A. Roy, P. Sadhukhan, S. R. Chowdhury, P. Maji, S. K. Bhattachrya and S. Das, Electrochemical properties of TiO2–V2O5 nanocomposites as a high performance supercapacitors electrode material, Appl. Surf. Sci., 2018, 443, 581–59122 CrossRef CAS.
- Y. Xiao, W. Wei, M. Zhang, S. Jiao, Y. Shi and S. Ding, Facile Surface Properties Engineering of High-Quality Graphene: Toward Advanced Ni-MOF Heterostructures for High-Performance Supercapacitor Electrode, ACS Appl. Energy Mater., 2019, 2, 2169–2177 CrossRef CAS.
- F. Javier García-García, A.-K. Larsson, L. Norèn and R. L. Withers, The crystal structures of Co3Se4 and Co7Se8, Solid State Sci., 2004, 6, 725–733 CrossRef.
- H. Chen, M. Fan, C. Li, G. Tian, C. Lv, D. Chen, K. Shu and J. Jiang, One-pot synthesis of hollow NiSe–CoSe nanoparticles with improved performance for hybrid supercapacitors, J. Power Sources, 2016, 329, 314–322 CrossRef CAS.
- Y. Wang, W. Zhang, X. Guo, K. Jin, Z. Chen, Y. Liu, L. Yin, L. Li, K. Yin, L. Sun and Y. Zhao, Ni–Co Selenide Nanosheet/3D Graphene/Nickel Foam Binder-Free Electrode for High-Performance Supercapacitor, ACS Appl. Mater. Interfaces, 2019, 11, 7946–7953 CrossRef CAS PubMed.
- J. Yang, Z. Sun, J. Wang, J. Zhang, Y. Qin, J. You and L. Xu, Hierarchical NiSe2 spheres composed of tiny nanoparticles for high performance asymmetric supercapacitors, CrystEngComm, 2019, 21, 994–1000 RSC.
- L. Zhao, P. Zhang, Y. Zhang, Z. Zhang, L. Yang and Z.-G. Chen, Facile synthesis of hierarchical Ni3Se2 nanodendrite arrays for supercapacitors, J. Mater. Sci. Technol., 2020, 54, 69–76 CrossRef.
- Y. Hu, C. Huang, S. Jiang, Y. Qin and H. C. Chen, Hierarchical nickel–cobalt selenide nanoparticles/nanosheets as advanced electroactive battery materials for hybrid supercapacitors, J. Colloid Interface Sci., 2020, 558, 291–300 CrossRef PubMed.
- S. Li, Y. Ruan and Q. Xie, Morphological modulation of NiCo2Se4 nanotubes through hydrothermal selenization for asymmetric supercapacitor, Electrochim. Acta, 2020, 356, 136837 CrossRef CAS.
- M. Sakthivel, S. Ramaraj, S.-M. Chen and K.-C. Ho, Bimetallic vanadium cobalt diselenide nanosheets with additional active sites for excellent asymmetric pseudocapacitive performance: comparing the electrochemical performances with M–CoSe2 (M = Zn, Mn, and Cu), J. Mater. Chem. A, 2019, 7, 12565–12581 RSC.
- L. Du, W. Du, H. Ren, N. Wang, Z. Yao, X. Shi, B. Zhang, J. Zai and X. Qian, Honeycomb-like metallic nickel selenide nanosheet arrays as binder-free electrodes for high-performance hybrid asymmetric supercapacitors, J. Mater. Chem. A, 2017, 5, 22527–22535 RSC.
- Y. Liu, Q. Xu, R. Wang, Y. Zheng, L. Zhu, Z. Wang and W. Zheng, Ionothermal synthesis of three-dimensional hierarchical Ni3Se2 mesoporous nanosheet networks with enhanced performance for asymmetric supercapacitors, J. Mater. Chem. A, 2020, 8, 797–809 RSC.
- L. Li, L. Jiang, Y. Qing, Y. Zeng, Z. Zhang, L. Xiao, X. Lu and Y. Wu, Manipulating nickel oxides in naturally derived cellulose nanofiber networks as robust cathodes for high-performance Ni–Zn batteries, J. Mater. Chem. A, 2020, 8, 565–572 RSC.
- S. Lalwani, S. Karade, J. H. Eum and H. Kim, Maximizing Redox Charge storage via Cation(V)–Anion(S) Dual-Doping on Nickel Diselenide Nanodiscs for Hybrid Supercapacitors, ACS Appl. Energy Mater., 2021, 4, 2430–2439 CrossRef CAS.
- A. Ray, A. Roy, S. Saha, M. Ghosh, S. R. Chowdhury, T. Maiyalagan, S. K. Bhattacharya and S. Das, Electrochemical energy storage properties of Ni–Mn-Oxide electrodes for advance asymmetric supercapacitor application, Langmuir, 2019, 35, 8257–8267 CAS.
- S. Subhadarshini, E. Pavitra, G. S. Rama Raju, N. R. Chodankar, D. K. Goswami, Y. K. Han, Y. S. Huh and N. C. Das, One-Dimensional NiSe–Se Hollow Nanotubular Architecture as a Binder-Free Cathode with Enhanced Redox Reactions for High-Performance Hybrid Supercapacitors, ACS Appl. Mater. Interfaces, 2020, 12, 29302–29315 CAS.
- Y. Chen, T. Liu, L. Zhang and J. Yu, NiCo2S4 Nanotubes Anchored 3D Nitrogen-Doped Graphene Framework as Electrode Material with Enhanced Performance for Asymmetric Supercapacitors, ACS Sustainable Chem. Eng., 2019, 7, 11157–11165 CrossRef CAS.
- H. Peng, J. Zhou, K. Sun, G. Ma, Z. Zhang, E. Feng and Z. Lei, High- Performance Asymmetric Supercapacitor Designed with a Novel NiSe@MoSe2 Nanosheet Array and Nitrogen-Doped Carbon Nanosheet, ACS Sustainable Chem. Eng., 2017, 5, 5951–5963 CrossRef CAS.
- H. Peng, C. D. Wei, K. Wang, T. Y. Meng, G. F. Ma, Z. Q. Lei and X. Gong, Ni0.85Se@ MoSe2 Nanosheet Arrays as the Electrode for High-Performance Supercapacitors, ACS Appl. Mater. Interfaces, 2017, 9, 17067–17075 CrossRef CAS PubMed.
- Y. Zheng, Y. Tian, S. Sarwar, J. Luo and X. Zhang, Carbon nanotubes decorated NiSe2 nanosheets for high-performance supercapacitors, J. Power Sources, 2020, 452, 227793 CrossRef CAS.
- Y. Sui, A. Ye, J. Qi, F. Wei, Y. He, Q. Meng, Y. Ren and Z. Sun, Construction of NiCo2O4@Ni0.85Se core–shell nanorod arrays on Ni foam as advanced materials for an asymmetric supercapacitor, J. Alloys Compd., 2019, 778, 234–238 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Size distribution of the of bimetallic nickel cobalt selenide particles, EDX spectrometography of these samples, SEM spectrometography of different samples. Co3Se4, Ni1.14Co1.86Se4, Ni0.67Co2.33Se4, Ni0.53Co2.47Se4. CV, GCD curves, and Nyquist plot of Co3Se4 and Ni0.53Co2.47Se4. See DOI: 10.1039/d1ra08678b |
| This journal is © The Royal Society of Chemistry 2022 |