Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Magnetic graphene oxide–lignin nanobiocomposite: a novel, eco-friendly and stable nanostructure suitable for hyperthermia in cancer therapy
Reza Eivazzadeh-Keihana,
Somayeh Asgharnasla,
Hooman Aghamirza Moghim Aliabadi bc,
Behnam Tahmasebid,
Fateme Radinekiyana,
Ali Maleki
bc,
Behnam Tahmasebid,
Fateme Radinekiyana,
Ali Maleki *a,
Hossein Bahreinizade,
Mohammad Mahdavi
*a,
Hossein Bahreinizade,
Mohammad Mahdavi f,
Mohammadhossein Shahsavari Alavijehg,
Reza Saberhi,
Senentxu Lanceros-Méndez
f,
Mohammadhossein Shahsavari Alavijehg,
Reza Saberhi,
Senentxu Lanceros-Méndez jl and
Ahmed Esmail Shalan
jl and
Ahmed Esmail Shalan jk
jk
aCatalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran 16846-13114, Iran. E-mail: maleki@iust.ac.ir; Fax: +98-21-73021584; Tel: +98-21-73228313
bProtein Chemistry Laboratory, Department of Medical Biotechnology, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
cAdvanced Chemistry Studies Lab, Department of Chemistry, K. N. Toosi University of Technology, Tehran, Iran
dSchool of Chemistry, College of Science, University of Tehran, Tehran, Iran
eMechanical Engineering Department, Sahand University of Technology, Tabriz, Iran
fEndocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
gDepartment of Mechanical Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran
hResearch Center for Science and Technology in Medicine, Tehran University of Medical Sciences, Tehran, Iran
iDepartment of Medical Nanotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran
jBCMaterials, Basque Center for Materials, Applications and Nanostructures, Martina Casiano, UPV/EHU Science Park, Barrio Sarriena s/n, Leioa 48940, Spain
kCentral Metallurgical Research and Development Institute (CMRDI), P. O. Box 87, Helwan, Cairo 11421, Egypt. E-mail: a.shalan133@gmail.com; ahmed.shalan@bcmaterials.net
lIKERBASQUE, Basque Foundation for Science, 48009 Bilbao, Spain
First published on 26th January 2022
Abstract
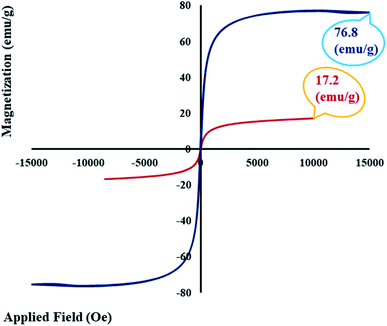
In this research, a novel magnetic nanobiocomposite was designed and synthesized in a mild condition, and its potential in an alternating magnetic field was evaluated for hyperthermia applications. For this purpose, in the first step, graphene oxide was functionalized with a natural lignin polymer using epichlorohydrin as the cross-linking agent. In the second step, the designed magnetic graphene oxide–lignin nanobiocomposite was fabricated by the in situ preparation of magnetic Fe3O4 nanoparticles in the presence of graphene oxide functionalized with lignin. The resultant magnetic nanobiocomposite possessed certain main properties, including stability and homogeneity in aqueous solutions, making it suitable for hyperthermia applications. The chemical and structural properties of the synthesized magnetic graphene oxide–lignin composite were characterized using FT-IR, EDX, FE-SEM, TEM, TG and VSM analyses. The saturation magnetization value of this magnetic nanocomposite was recorded as 17.2 emu g−1. Further, the maximum specific absorption rate was determined to be 121.22 W g−1. Given these results, this newly fabricated magnetic nanobiocomposite may achieve considerable performance under the alternating magnetic field in fluid hyperthermia therapy.
1. Introduction
With the discovery and identification of the wide useful properties of graphene, its two-dimensional nanosheet with a hexagonal lattice structure has been considered as one of the most important materials in the scientific world.1 Graphene results from very thin layers of graphite. This carbon-based material with a special structure and its derivatives and composites can be applied as supercapacitors, in biosensors, and different other applications.2,3 Oxidized derivatives of graphene, such as graphene oxide (GO), can be easily functionalized due to the oxygen-containing functional groups. Besides, the addition of these functional groups to the graphene surface make it an appropriate substrate for fabricating and designing new composites.4,5Nowadays, GO, which is generated from graphite, is one of the most interesting chemical materials among researchers. GO has the high purity of graphene but is much cheaper and much easier to prepare in bulk quantities. Moreover, the existence of oxygen functionalities in the GO structure facilitate other chemicals to grow on it, thereby making GO an important candidate for the fabrication of a wide range of composites and nanocomposites.6,7 The modification of GO with different substances has been shown to convert this carbon-based material to useful substrates for various applications.8,9 For instance, the functionalization of GO with magnetic nanoparticles changes it to a green and reusable catalyst for multicomponent reactions.10,11 On the other hand, GO functionalization is a multifunctional platform to developing adsorption agents for the removal of different kinds of pollutants from aqueous solutions.12 In the last decades, drug carriers have gathered great attention in cancer therapy and other branches of medicine towards enhancing the influence of drugs on target cells and reducing the side effect of drug resistance. GO presents high potential for designing versatile structures and drug binding. Therefore, it can be considered an efficient drug delivery system.13,14 Moreover, GO composites designed with natural polymers offer biocompatibility and biodegradability. Some of the most important and available polymers that enable efficient conversion on the base substrate are alginate,15 cellulose,16 chitosan,17 starch,18 and lignin.19 For example, the combination of GO with chitosan generated nanocomposites, which were employed as electrochemical DNA biosensors for the detection of typhoid.20 In another example, the combination of GO and natural cellulose lead to nanofiber composite films with strongly anisotropic thermal conductivity.21 Besides, supercapacitor composites are another achievement of the reaction between GO and polymers.22 Lignin is the main component of lignocellulosic biomass (LCBM); it is a biodegradable, amorphous, irregular three-dimensional, and highly branched phenolic and complex polymer.23 After cellulose, lignin, with a three-dimensional network of cross-linked phenylpropanoid subunits, is the most common polymeric structure in softwoods, hardwoods, and grasses.24 There are lots of lignin sources on the earth. But, unfortunately, most of them, such as the bark and wood of trees, are unusable. The main but small source of lignin, which is useable and exploitable, is the pulp and paper industry.25 Investigations on the chemical structure of lignin have demonstrated that it is made of three kinds of phenylpropanoid monomers; these carbon-based and aromatic monomers undergo radical coupling reactions to form the final complex structure of lignin. The existence of hydroxyl groups on the lignin structure is one of the reasons it is a suitable candidate for the reaction with GO. Despite the existence of the hydroxyl functional groups, lignin is insoluble in water and alcohol, and to induce this natural polymer to react with chemical substrates, it should be dissolved in alkaline solutions.26 In recent years, functionalizing natural polymers through green reactions and under mild conditions, such room temperature, green solvents, and short reaction times, as well as utilizing them in medicine as drug delivery agents, for targeted cancer therapy and in other branches has become common.27 Many studies have reported the potential of natural polymers is cancer therapy; starch and its nanoparticles are examples.28,29 Cancer treatment methods, such as surgery, radiation therapy, chemotherapy, targeted therapy, and hyperthermia, are one of the biggest issues in today's life. However, some of the cancer treatment methods like chemotherapy are as harmful as they are effective due to the side effects. Recently, new treatments have been developed with less side effects.30 One of these new remedies is the use of magnetic nanoparticles for hyperthermia treatment.31–33 Due to the size-dependent tuneable magnetic feature, large surface area, super-paramagnetic behaviour and other physiochemical properties, applying these highly potent nano-agents as platform materials has been proven promising and ideal in catalysis,34–36 water treatment,37 and different biomedical fields.38–41 The Fe3O4 magnetic nanoparticles (Fe3O4 MNPs) present in the structure of magnetic-responsive materials play the main role in the hyperthermia process.42–44 In other words, Fe3O4 MNPs are introduced into the cancer tissue, and by applying an alternating magnetic field (AMF), the death of cancer cells is achieved. In addition to their easy synthesis process, biocompatibility, high penetration depth, and low toxicity, these nanoparticles must have reusability in the tumor region.45 After the insertion of MNPs in cancer cells and exerting AMF, the vibration of particles induces Brownian and Néel relaxation, increasing the temperature of the cells.46 This increase leads to the killing of the cancer cells. Heating the tumor has two general effects in cancer therapy. The first is, increasing the temperature to 42–46 °C by itself is a way of destroying the tumors. The second is, cell heating causes a better operation of chemotherapy.47 The two parameters of Fe3O4 MNPs essential for the hyperthermia process are concentration and the specific absorption rate (SAR). Moreover, different geometric shapes of nanoparticles effects in hysteresis loss and SAR reduction.48,49 In the hyperthermia process, a solution of Fe3O4 MNPs is prepared and injected into the target cells. Furthermore, the injection time depends on the injection rate. Above all, the prepared sample solution has to be homogenous during the injection process. The hydrophilic structure of the Fe3O4 MNPs used for inducing hyperthermia can aid in easily meeting this basic requirement.50 In this study, natural and non-toxic materials have been used to design a mild and eco-friendly reaction, which generates a hydrophilic and new magnetic nanobiocomposite. In the first synthetic step, GO was synthesized using a modified Hummers' method. Then, it was functionalized with natural lignin (GO–lignin). Following that, the in situ magnetization process of GO–lignin was implemented, and the new magnetic GO–lignin nanobiocomposite was prepared (Scheme 1). Given the homogeneity and constant stability in water, as well as its efficient magnetic property, the potency of the magnetic GO–lignin nanobiocomposite was evaluated using an alternating magnetic field (AMF).
2. Experimental section
2.1. Materials and methods
All the required chemical substances, such as solvents and reagents, were of high purity and bought from Merck, Aldrich and Fluka international companies. The FT-IR spectra were acquired on a Shimadzu IR-470 spectrometer (Japan) by the KBr pellet method. The morphology and structure of the synthesized magnetic nanobiocomposite were characterized by field-emission scanning electron microscopy (FE-SEM) (ZEISS-sigma VP model, Germany) and transmission electron microscopy (TEM) (Zeiss-EM10C-100KV, Germany). The vibrating sample magnetometry (VSM) analysis was performed by using a LBKFB model-magnetic Kashan Kavir (Iran) (5000 Oe). The energy-dispersive X-ray (EDX) analysis was accomplished using a Numerix DXP-X10P (France). The thermogravimetric (TG) analysis and thermal behavior of the magnetic biocomposite were analyzed by a Bahr-STA 504 instrument (Germany) under an argon atmosphere at the heating rate of 10°C min−1. Finally, the hyperthermia application of this composite was tested in varied frequencies by the device from NATSYCO, Iran.2.2. Synthesis of GO
A modified Hummers' method with sonication was used to synthesize GO.51,52 In this regard, at first 1.00 g of graphite and 0.5 g of NaNO3 were added to 23 mL of sulfuric acid (98%), The obtained liquid solution was stirred at 66 °C and then sonicated for 30 min in room temperature. In the next step, 3.00 g of KMnO4 was slowly added to the container, which was located in an ice bath, and the suspension was reacted for 1 h in the sonication bath. The temperature of the suspension was increased to 98 °C; then, 50 mL of distilled water was added, and a brown suspension was obtained. After that, 700 mL of warm distilled water was mixed with the brown suspension to dilute the mixture. In the end, 12 mL of H2O2 was added at once and a colour change from brown to yellow was observed. Finally, 2 mL of HCl and 98 mL of distilled water were added, and after one day, the product was repeatedly washed and dried at 60 °C.2.3. Functionalization of GO using lignin (GO–lignin)
According to a previously reported method,53 the coalition of GO and lignin was accomplished in a one-step procedure. In order to link GO to lignin, a solution was prepared according to previous work.54 In 100 mL of an aqueous solution containing 7.0 wt% NaOH/12.0 wt% urea/81.0 wt% distilled water, 0.3 g of GO was dispersed for 15 min. Then, 6 mL of epichlorohydrin (ECH), the cross-linking agent, was added to the suspension and stirred for 10 min. Finally, 3.00 g of lignin was added to the mixture, and the solution was kept under stirring for 48 h at room temperature. After the mentioned time, the product was centrifuged (4000 rpm, 5 min), washed with ethanol and dried in a freeze-drier overnight.2.4. Magnetization process of GO–lignin
For the first time, the GO–lignin composite was magnetized using Fe3O4 nanoparticles via an in situ procedure in this study. For this, 0.2 g of GO–lignin, 0.97 g FeCl3·6H2O and 0.43 g FeCl2·4H2O were added to 200 mL of distilled water in an N2 atmosphere, and the mixture was stirred for 30 min at 50 °C. Then, 10 mL of 25% aqueous ammonia was trickled into the solution in an N2 atmosphere. The resulting mixture was stirred for 3 h at 80 °C for completing the reaction. After the mentioned time, the obtained new magnetic nanocomposite was separated with an external magnet and washed with distilled water to reach natural pH (pH = 7).3. Results and discussion
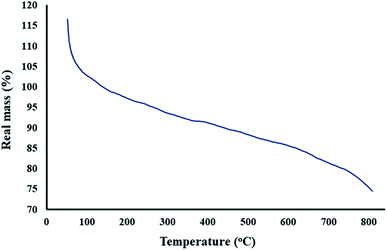
As we know, one of the main goals of organic and green chemistry is to design novel nanobiocomposites that are natural-product-based, eco-friendly, easy to fabricate and useful in biomedicine and medical applications. In this research, GO was functionalized with the natural complex polymer named lignin. Since the substance should be magnetic to be responsive to the alternating magnetic field (AMF) and applied in hyperthermia, the fabricated composite was made magnetic by the in situ construction of magnetic Fe3O4 nanoparticles (Scheme 1). The structure and properties of the designed magnetic GO–lignin nanobiocomposite were analysed by a wide range of spectroscopic and analytical techniques. The FT-IR spectra demonstrated the formation of new functional groups and occurrence of the reaction between the reagents; the FE-SEM images revealed the structure and morphology of the functionalized composite and the nanoparticles. The VSM analysis was used to show the magnetic property, and EDX analysis was carried out to determine the elemental combination and the inclusion of new elements in the structure. The TG curve showed the thermal resistance of the composite, and the TEM image illustrated the structure of magnetic GO–lignin.3.1. Characterization of the magnetic GO–lignin nanobiocomposite
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH and C–O) functional groups of GO,55 as well as the O–H deformation vibration mode.56 In Fig. 1B, the strong and wider absorption band at 1022–1198 cm−1 shows the formation of GO–lignin and demonstrates the presence of ether functional groups. The aromatic skeletal vibration mode of lignin can be observed at 1556 cm−1.57 Moreover, the small band at 1386 cm−1 could be attributed to the vibration mode of the syringyl and guaiacyl rings of lignin.58 In addition to this, the peak of the C–O functional groups of GO overlapped with the newly synthesized functional groups.59 Furthermore, the presence of a strong absorbance band at 557 cm−1 in Fig. 1C is because of the addition of the magnetic Fe3O4 nanoparticles to the composite.
O, C–OH and C–O) functional groups of GO,55 as well as the O–H deformation vibration mode.56 In Fig. 1B, the strong and wider absorption band at 1022–1198 cm−1 shows the formation of GO–lignin and demonstrates the presence of ether functional groups. The aromatic skeletal vibration mode of lignin can be observed at 1556 cm−1.57 Moreover, the small band at 1386 cm−1 could be attributed to the vibration mode of the syringyl and guaiacyl rings of lignin.58 In addition to this, the peak of the C–O functional groups of GO overlapped with the newly synthesized functional groups.59 Furthermore, the presence of a strong absorbance band at 557 cm−1 in Fig. 1C is because of the addition of the magnetic Fe3O4 nanoparticles to the composite.
3.2. Application of the magnetic GO–lignin nanobiocomposite in the hyperthermia procedure
 | (1) |
| Entry | MNPs | Nanocomposite | Optimum concentration of nanocomposite (mg mL−1) | SAR (W g−1) | Magnetic saturation (emu g−1) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Fe3O4 | Magnetic Fe3O4 | 15 | 18.5 | 58.8 | 45 |
| 2 | Fe3O4 | Poly (acrylic acid) coated Fe3O4 | 15 | 46.64 | 72.38 | 62 |
| 3 | Fe3O4 | Oleate coated Fe3O4 | 15 | 51.90 | 72.38 | 62 |
| 4 | Fe3O4 | Brick-like Ag@Fe3O4 | 1 | 100 | 37 | 63 |
| 5 | Fe3O4 | Fe3O4–chitosan | 2 | 118.85 | 49.96 | 64 |
| 6 | Fe3O4 | Graphene oxide–Fe3O4 | 1 | 98 | 78 | 65 |
| 7 | Fe3O4 | Magnetic GO–lignin | 0.5 | 121.22 | 17.2 | This work |
4. Conclusions
In this study, the three-dimensional complex polymer lignin was coupled with graphene oxide. Epichlorohydrin (ECH), a colourless liquid, was used as the cross-linking agent to bind GO and lignin and thereby design a new nanocomposite for use in the hyperthermia process. Lignin is the main compound that makes up plants and hence is natural, non-toxic and biodegradable and has the potential for use in medicine and drug-related applications. The fabricated composite of GO and lignin was magnetized, for the first time, in this study. Being magnetic, stable and homogenous in aqueous solutions would make it a suitable material for hyperthermia. Some analyses, including FT-IR, EDX, FE-SEM, TEM, TG, and VSM, demonstrated that the desired nanocomposite was formed. The nanocomposite performance and data from the alternating magnetic field (AMF) showed that the resultant substrate has the potential to be used in hyperthermia. Considering the high stability and good homogeneity of the magnetic GO–lignin nanobiocomposite in the aqueous phase, as well as the high specific absorption rate (121.22 W g−1) at the lowest concentration of the magnetic GO–lignin nanobiocomposite (0.5 mg mL−1) and the presence of the natural lignin polymer in the structure, the designed magnetic GO–lignin nanobiocomposite seems prospective. However, it must be evaluated more, and in vivo studies are needed to determine its applicability in cancer hyperthermia therapy. Furthermore, combining with therapeutic drugs, bioceramics, and other biomaterials may further develop the properties of our designed magnetic GO–lignin nanobiocomposite for application in drug delivery, tissue engineering, and other regenerative medicine strategies.Author contributions
R. E. K. and S. A. helped in preparing the material, characterization, and writing the manuscript. H. A. M. A., B. T., F. R. and H. B. helped in characterization of the obtained materials as well as the discussion of the obtained results. M. M., M. S. A. and R. S. participated in plotting the gained data in addition to interpreting these data. Furthermore, A. M., S. L.-M. and A. E. S. designed the research, contributed to supervising the work, discussed the results, and wrote the manuscript. All the authors participated in writing, editing, and revising the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
All authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology. Furthermore, AES thanks the National Research grants from MINECO, Spain, “Juan de la Cierva” [FJCI-2018-037717] and he is currently on leave from CMRDI.References
- M. Zhang, Y. Li, Z. Su and G. Wei, Polym. Chem., 2015, 6, 6107–6124 RSC.
- R. Eivazzadeh-Keihan, R. Taheri-Ledari, M. S. Mehrabad, S. Dalvand, H. Sohrabi, A. Maleki, S. M. Mousavi-Khoshdel and A. E. Shalan, Energy Fuels, 2021, 35, 10869–10877 CrossRef CAS.
- P. Pashazadeh-Panahi, M. Hasanzadeh and R. Eivazzadeh-Keihan, J. Mol. Recognit., 2020, 33, e2828 CAS.
- X. Huang, Z. Yin, S. Wu, X. Qi, Q. He, Q. Zhang, Q. Yan, F. Boey and H. J. Zhang, Small, 2011, 7, 1876–1902 CrossRef CAS PubMed.
- A. Sharma, M. Varshney, S. S. Nanda, H. J. Shin, N. Kim, D. K. Yi, K. H. Chae and S. O. Won, Chem. Phys. Lett., 2018, 698, 85–92 CrossRef CAS.
- A. F. Girão, S. Pinto, A. Bessa, G. Gonçalves, B. Henriques, E. Pereira and P. A. Marques, Advanced 2D Materials, Wiley-Scrivener Publishing LLC, 1st edn, 2016 Search PubMed.
- P. Tancredi, O. M. Londono, P. C. R. Rojas, M. Knobel and L. M. Socolovsky, Mater. Res. Bull., 2018, 107, 255–263 CrossRef CAS.
- R. Eivazzadeh-Keihan, A. Maleki, M. De La Guardia, M. S. Bani, K. K. Chenab, P. Pashazadeh-Panahi, B. Baradaran, A. Mokhtarzadeh and M. R. Hamblin, J. Adv. Res., 2019, 18, 185–201 CrossRef CAS PubMed.
- W. Yu, L. Sisi, Y. Haiyan and L. Jie, RSC Adv., 2020, 10, 15328–15345 RSC.
- R. Eyvazzadeh-Keihan, N. Bahrami, R. Taheri-Ledari and A. Maleki, Diamond Relat. Mater., 2020, 102, 107661 CrossRef CAS.
- R. Eivazzadeh-Keihan, R. Taheri-Ledari, N. Khosropour, S. Dalvand, A. Maleki, S. M. Mousavi-Khoshdel and H. Sohrabi, Colloids Surf., A, 2020, 587, 124335 CrossRef CAS.
- P. Shandilya, A. Sudhaik, P. Raizada, A. Hosseini-Bandegharaei, P. Singh, A. Rahmani-Sani, V. Thakur and A. K. Saini, Solid State Sci., 2020, 102, 106164 CrossRef CAS.
- E. Masoudipour, S. Kashanian and N. Maleki, Chem. Phys. Lett., 2017, 668, 56–63 CrossRef CAS.
- Y. Yun, H. Wu, J. Gao, W. Dai, L. Deng, O. Lv and Y. Kong, Mater. Sci. Eng., C, 2020, 108, 110380 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, F. Radinekiyan, H. Madanchi, H. A. M. Aliabadi and A. Maleki, Carbohydr. Polym., 2020, 248, 116802 CrossRef CAS PubMed.
- Z. Rao, H. Ge, L. Liu, C. Zhu, L. Min, M. Liu, L. Fan and D. Li, Int. J. Biol. Macromol., 2018, 107, 1184–1192 CrossRef CAS PubMed.
- F. A. Taher, F. H Kamal, N. A Badawy and A. E. Shrshr, Mater. Res. Bull., 2018, 97, 361–368 CrossRef CAS.
- C. Simi and T. E. Abraham, Bioprocess Biosyst. Eng., 2007, 30, 173–180 CrossRef CAS PubMed.
- M. Ghanbari, F. Davar and A. E. Shalan, Ceram. Int., 2021, 47, 9409–9417 CrossRef CAS.
- A. Singh, G. Sinsinbar, M. Choudhary, V. Kumar, R. Pasricha, H. Verma, S. P. Singh and K. Arora, Sens. Actuators, B, 2013, 185, 675–684 CrossRef CAS.
- W. Yang, Z. Zhao, K. Wu, R. Huang, T. Liu, H. Jiang, F. Chen and Q. Fu, J. Mater. Chem. C, 2017, 5, 3748–3756 RSC.
- W. Wu, Y. Li, L. Yang, Y. Ma, D. Pan and Y. Li, Electrochim. Acta, 2014, 139, 117–126 CrossRef CAS.
- Y. Lu, Y. C. Lu, H. Q. Hu, F. J. Xie, X. Y. Wei and X. Fan, J. Spectrosc., 2017, 2017, 8951658 Search PubMed.
- D. R. Ratnaweera, D. Saha, S. V. Pingali, N. Labbé, A. K. Naskar and M. Dadmun, RSC Adv., 2015, 5, 67258–67266 RSC.
- C. R. Poovaiah, M. Nageswara-Rao, J. R. Soneji, H. L. Baxter and C. N. Stewart Jr, Plant Biotechnol. J., 2014, 12, 1163–1173 CrossRef CAS PubMed.
- Z. Wu, W. Huang, X. Shan and Z. Li, Int. J. Biol. Macromol., 2020, 143, 325–333 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, F. Radinekiyan, A. Maleki, M. S. Bani, Z. Hajizadeh and S. Asgharnasl, Int. J. Biol. Macromol., 2019, 140, 407–414 CrossRef CAS PubMed.
- R. Sleightholm, B. Yang, F. Yu, Y. Xie and D. Oupický, Biomacromolecules, 2017, 18, 2247–2257 CrossRef CAS PubMed.
- Y. Jiang, T. Li, M. Lu, D. Li, F. Ren, H. Zhao and Y. Li, Int. J. Pharm., 2018, 547, 520–529 CrossRef CAS PubMed.
- W. A. Weber, J. Nucl. Med., 2005, 46, 983–995 CAS.
- R. Eivazzadeh-Keihan, S. Asgharnasl, M. S. Bani, F. Radinekiyan, A. Maleki, M. Mahdavi, P. Babaniamansour, H. Bahreinizad, A. E. Shalan and S. Lanceros-Méndez, Langmuir, 2021, 37, 8847–8854 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, M. Ghafori Gorab, H. Aghamirza Moghim Aliabadi, A. R. Akbarzadeh, A. Maleki and H. Ghafuri, Sci. Rep., 2021, 11, 1–13 CrossRef PubMed.
- H. Liu, A. Li, X. Ding, F. Yang and K. Sun, Solid State Sciences, 2019, 93, 101–108 CrossRef CAS.
- S. Asgharnasl, R. Eivazzadeh-Keihan, F. Radinekiyan and A. Maleki, Int. J. Biol. Macromol., 2020, 144, 29–46 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, E. B. Noruzi, F. Radinekiyan, M. S. Bani, A. Maleki, B. Shaabani and M. Haghpanahi, ChemistryOpen, 2020, 9, 735–742 CrossRef CAS PubMed.
- Z. Hajizadeh, A. Maleki, J. Rahimi and R. Eivazzadeh-Keihan, Silicon, 2019, 12, 1247–1256 CrossRef.
- R. Eivazzadeh-Keihan, F. Radinekiyan, S. Asgharnasl, A. Maleki and H. Bahreinizad, J. Mater. Res. Technol., 2020, 9, 12244–12259 CrossRef CAS.
- H. Bi and X. Han, Chem. Phys. Lett., 2018, 706, 455–460 CrossRef CAS.
- R. Eivazzadeh-Keihan, E. B. Noruzi, K. K. Chenab, A. Jafari, F. Radinekiyan, S. M. Hashemi, F. Ahmadpour, A. Behboudi, J. Mosafer and A. Mokhtarzadeh, J. Tissue Eng. Regener. Med., 2020, 14, 1687–1714 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, P. Pashazadeh-Panahi, T. Mahmoudi, K. K. Chenab, B. Baradaran, M. Hashemzaei, F. Radinekiyan, A. Mokhtarzadeh and A. Maleki, Microchim. Acta, 2019, 186, 329 CrossRef PubMed.
- A. Mokhtarzadeh, R. Eivazzadeh-Keihan, P. Pashazadeh, M. Hejazi, N. Gharaatifar, M. Hasanzadeh, B. Baradaran and M. de la Guardia, TrAC, Trends Anal. Chem., 2017, 97, 445–457 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan and A. Maleki, J. Nanostruct. Chem., 2021, 11, 571–767 Search PubMed.
- R. Eivazzadeh-Keihan, F. Radinekiyan, A. Maleki, M. S. Bani and M. Azizi, J. Mater. Sci., 2020, 55, 319–336 CrossRef CAS.
- Z. Hedayatnasab, A. Dabbagh, F. Abnisa and W. M. A. W. Daud, Mater. Res. Bull., 2020, 132, 110975 CrossRef CAS.
- S. M. Fotukian, A. Barati, M. Soleymani and A. M. Alizadeh, J. Alloys Compd., 2020, 816, 152548 CrossRef CAS.
- Y. Ling, X. Tang, F. Wang, X. Zhou, R. Wang, L. Deng, T. Shang, B. Liang, P. Li and H. Ran, RSC Adv., 2017, 7, 2913–2918 RSC.
- S. Kim, M. Ju Moon, S. Poilil Surendran and Y. Y. Jeong, Pharmaceutics, 2019, 11, 306 CrossRef CAS PubMed.
- X. Wang, F. Pan, Z. Xiang, W. Jia and W. Lu, Mater. Lett., 2020, 262, 127187 CrossRef CAS.
- M. S. Bani, S. Hatamie, M. Haghpanahi, H. Bahreinizad, M. H. S. Alavijeh, R. Eivazzadeh-Keihan and Z. H. Wei, Spin, 2019, 9, 1940003 CrossRef CAS.
- S. Dutz, M. Kettering, I. Hilger, R. Müller and M. Zeisberger, Nanotechnology, 2011, 22, 265102 CrossRef PubMed.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS PubMed.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- X. Chen, S. Zhou, L. Zhang, T. You and F. Xu, Materials, 2016, 9, 582 CrossRef PubMed.
- X. Chen, J. Chen, T. You, K. Wang and F. Xu, Carbohydr. Polym., 2015, 125, 85–91 CrossRef CAS PubMed.
- A. Emami, H. Ghafuri, M. K. Kenari and A. Maleki, ChemistrySelect, 2018, 3, 6349–6357 CrossRef CAS.
- D. He, Z. Peng, W. Gong, Y. Luo, P. Zhao and L. J. Kong, RSC Adv., 2015, 5, 11966–11972 RSC.
- S. Cheng, A. Huang, S. Wang and Q. Zhang, BioResources, 2016, 11, 4006–4016 CAS.
- C. G. Boeriu, D. Bravo, R. J. A. Gosselink and J. E. van Dam, Ind. Crops Prod., 2004, 20, 205–218 CrossRef CAS.
- M. Nabilah, M. Alwi, M. S. Su’ait, M. Imperiyka, S. A. Hanifah, A. Ahmad, N. H. Hassan and M. Y. A. Rahman, Russ. J. Electrochem., 2016, 52, 362–373 CrossRef CAS.
- Ł. Klapiszewski, K. Bula, M. Sobczak and T. Jesionowski, Int. J. Polym. Sci., 2016, 2016, 1–12 CrossRef.
- S. Wei, Q. Wang, J. Zhu, L. Sun, H. Lin and Z. Guo, Nanoscale, 2011, 3, 4474–4502 RSC.
- Y. V. Kolen'ko, M. Bañobre-López, C. Rodríguez-Abreu, E. Carbó-Argibay, A. Sailsman, Y. Piñeiro-Redondo, M. F. Cerqueira, D. Y. Petrovykh, K. Kovnir, O. I. Lebedev and J. Rivas, J. Phys. Chem. C, 2014, 118, 8691–8701 CrossRef.
- M. E. F. Brollo, J. M. Orozco-Henao, R. López-Ruiz, D. Muraca, C. S. B. Dias, K. R. Pirota and M. Knobel, J. Magn. Magn. Mater., 2016, 397, 20–27 CrossRef CAS.
- P. B. Shete, R. M. Patil, N. D. Thorat, A. Prasad, R. S. Ningthoujam, S. J. Ghosh and S. H. Pawar, Appl. Surf. Sci., 2014, 288, 149–157 CrossRef CAS.
- V. Narayanaswamy, I. M. Obaidat, A. S. Kamzin, S. Latiyan, S. Jain, H. Kumar, C. Srivastava, S. Alaabed and B. Issa, Int. J. Mol. Sci., 2019, 20, 3368 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |