Open Access Article
Open Access ArticleComment on “Structural, electrical, and multiferroic characteristics of lead-free multiferroic: Bi(Co0.5Ti0.5)O3–BiFeO3 solid solution” by N. Kumar, A. Shukla, N. Kumar, R. Choudhary and A. Kumar, RSC Adv., 2018, 8, 36939”
Paweł E. Tomaszewski
Institute of Low Temperature and Structure Research, Polish Academy of Science, ul. Okólna 2, 50-422 Wrocław, Poland. E-mail: petomasz1@wp.pl
First published on 20th October 2022
Abstract
My comments concern the significant errors in the crystallographic part of the commented paper. It was found that the studied crystal is of the sillenite type and the correct formula should be Bi25FeO39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co,Ti instead of BiFeO3. Moreover, the type of unit cell is not correct. Due to the double doping the new type of unit cell, previously unknown, was proposed for such crystals. Furthermore, the authors did not find that the studied sample contains two slightly different phases, both of the sillenite type. The actual chemical composition of the studied crystals is not known.
Co,Ti instead of BiFeO3. Moreover, the type of unit cell is not correct. Due to the double doping the new type of unit cell, previously unknown, was proposed for such crystals. Furthermore, the authors did not find that the studied sample contains two slightly different phases, both of the sillenite type. The actual chemical composition of the studied crystals is not known.
Results
The commented paper1 presents the results of studies on the different physical properties of BiFeO3 ceramics modified by two different dopants, Co and Ti, in the nominal volume of 40%. However, it is clear that there are at least three major points which must be underlined and, if possible, corrected:(1) wrong attribution of the chemical formula and name of the crystal studied in the commented paper,
(2) wrong and illogical indexation of the presented diffraction patterns,
(3) the apparent splitting of the peaks is a sign of bi-phasic character of the studied sample. This is not discussed by the authors which leads to incorrect interpretation of all presented results.
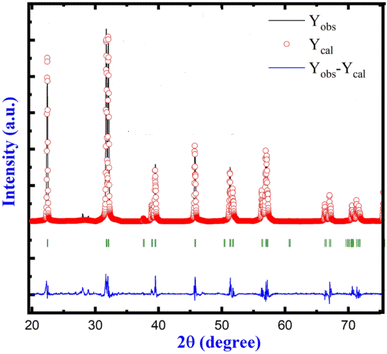
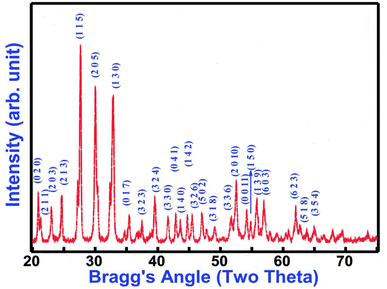
(Ad 1) The main error concerns the wrong chemical formula of studied crystals. The diffraction pattern published in the commented paper has a characteristic set of the diffraction peaks which can be compared with that of the sillenite-type crystal. It is sufficient to compare the X-ray powder diffraction patterns from BiFeO3 published by the same team of authors (N. Kumar, A. Shukla, N. Kumar & R. N. P. Choudhary – J. Mater. Sci.: Mater. Electron., 32, 5870–5885, (2021)) presented in upper figure, and the pattern from the commented paper (N. Kumar, A. Shukla, N. Kumar, R. N. P. Choudhary & A. Kumar – RSC Adv., 8, 36939, (2018)) shown in bottom drawing. The clear difference in both diffraction patterns (note that the scale is the same: 20–75°) indicate that the structures are different. Note, that the diffraction pattern is a fingerprint of the crystal structure.
Fig. 1 shows the diffraction pattern characteristic for BiFeO3 crystal in trigonal phase. However, the published pattern shown in Fig. 2 is not an image of other crystal phase of BiFeO3 crystal. This set of diffraction peaks is characteristic for the sillenite-type crystal, i.e. crystal of the other stoichiometry, Bi25FeO39. Thus, the correct chemical formula of studied sample is different than those supposed by authors. The studied samples are not a double-doted BiFeO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co,Ti, but the sillenite-type Bi25FeO39
Co,Ti, but the sillenite-type Bi25FeO39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co,Ti. The correct composition and structure of crystal from the commented paper will be possible only when the raw X-ray diffraction data can be accessible.
Co,Ti. The correct composition and structure of crystal from the commented paper will be possible only when the raw X-ray diffraction data can be accessible.
It is necessary to underline that the prepared mixture of ingredients can not give the crystal of a desired composition (and chemical formula). The Nature does not know what is the expected crystal composition, it has a well know his own chemical or crystallo-chemical rules of synthesis or crystal growth. It is well known that in some cases the crystals of desired chemical composition can be obtained only from a non-stoichiometrically mixture. Moreover, only the diffraction pattern is a unique tool for verification of crystal structure, i.e. crystal symmetry, unit cell parameters and atomic content of the unit cell.
One can suppose where is a source of failure in the growing of different crystal than those desired. This is due to the temperature of the sintering of the sample. They started with the Bi2O3 which is known to have the high temperature phase (γ-phase) of the sillenite structure. Thus, the synthesis at this region of temperature must give the final crystal of the same structure, i.e. Bi25FeO39. Note, that the nanocrystals often have the transition temperature lower that the bulk crystal; this is the case.
Moreover, it is well known that the sillenite-type phase can be observed as a parasite phase of small content. However, in the case of commented paper the sillenite phase is a MAJOR part of the sample, not minor as stated by Authors. The diffraction pattern (bottom figure) has no sign of the perovskite phase (the upper pattern) at all!
(Ad 2) The second error in the commented paper is the wrong set of lattice parameters. The published lattice parameters, a = 9.875(2), b = 8.485(2), c = 18.603(2) Å and V = 1558.8 Å3 (erroneously printed as 1585.8 Å3) are not related to those of pure BiFeO3 crystal. The indexation of the presented diffraction pattern is wrong. Moreover, it is not possible to obtain the published lattice parameters by DICVOL program. The presented set of indexes give the contradictory data on the lattice parameters calculated from different selected peaks. For example, the lattice parameter a is equal to 8.545 Å when use the 020 peak, 9.922 Å from 203 peak, 8.077 Å from 150 peak, etc. This clearly indicate that the indexations (hkl) presented by authors are wrong and MUST be corrected.
The crystal structure of sillenite Bi25FeO39 is described in cubic symmetry with space group I23 (No. 197) and lattice parameters a = b = c = 10.191 Å (ICSD #257493).2–5 Unfortunately, there was not possible to index the patterns in the cubic system. Fortunately, the parent structure of cubic sillenite may be also presented in the monoclinic symmetry reaching the unit cell with the lattice parameters of about a ≈ b ≈ 14.5 and c ≈ 10 Å, where a and b axes are oriented along the diagonal of the base of cubic unit cell. Thus, a and b are equal to about √2acubic. The monoclinic angle β could not significantly deviate from 90°. As I know, such unit cell was not observed until now in any of sillenite crystals.
It is obvious that the introducing of two different ions, e.g. Co and Ti, may result in the deformation of the basic crystal structure and a creation of a new unit cell – this of monoclinic symmetry.
(Ad 3) The third error is that the sample is bi-phasic and not a single-one. The apparent splitting of several peaks is, in reality, the effect of superposition of two similar diffraction patterns, both becoming from two different sillenite-type crystals, Bi25FeO39 instead of the assumed BiFeO3.2 This difference is a result of some kind of separation of dopants between two similar crystals. It is possible due to difference in the ionic radii of both dopants. Now, it is only possible to show that the lattice parameters of both phases are as follows: a = 14.790(12), b = 14.364(8), c = 10.088(6) Å, β = 90.88(6)° and V = 2142.9 Å3 for the phase I, and a = 14.706(4), b = 14.484(4), c = 10.464(8) Å, β = 90.42(5)° and V = 2228.6 Å3 for the phase II. Note, that such small deviation of the monoclinic angle from 90° can result in an apparent orthorhombic symmetry. The preliminary estimation of the possible space group for both phases gives two possible symmetries (for unique axis b and unit cell choice 1): C1c1 (no. 9) and C12/c1 (no. 15).
Thus, it is clear that the subsequent results of studies are also affected by the errors and should be corrected.
Conflicts of interest
The author does not receive support from any organization for the submitted work. The author has no conflicts of interest to declare that are relevant to the content of this article.References
- N. Kumar, A. Shukla, N. Kumar, R. N. P. Choudhary and A. Kumar, Structural, electrical, and multiferroic characteristics of lead-free multiferroic: Bi(Co0.5Ti0.5)O3-BiFeO3 solid solution –, RSC Adv., 2018, 8, 36939, 10.1039/c8ra02306a.
- S. S. F. Morales, J. A. L. Flores, J. L. P. Mazariego, V. M. Fabrega and R. W. G. Gonzalez, Synthesis of Bi25FeO39 by molten salts method and its Mössbauer spectrum, Physica B Condens., 2017, 504, 109, DOI:10.1016/j/physb.2016.10.019.
- M. Valant and D. Suvorov, A stoichiometric model for sillenites, Chem. Mater., 2002, 14, 3471–3476, DOI:10.1021/cm021173l.
- A. M. L. Lopes, J. P. Araújoa and S. Ferdov, Room temperature synthesis of Bi25FeO39 and hydrothermal kinetic relations between sillenite- and distorted perovskite-type bismuth ferrites, Dalton Trans., 2014, 43, 18010–18016, 10.1039/c4dt01825g.
- T. Elkhouni, M. Amami and A. Ben Salah, Structural, spectroscopic studies and magnetic properties of doped sillenites-type oxide Bi12[M]O20, M = Fe, Co, J. Supercond. Novel Magn., 2013, 26, 2997–3004, DOI:10.1007/s10948-013-2271-8.
| This journal is © The Royal Society of Chemistry 2022 |