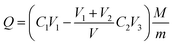Open Access Article
Open Access ArticlePreparation and characterization of three-dimensional hierarchical porous carbon from low-rank coal by hydrothermal carbonization for efficient iodine removal
Yufeng Yina,
Dingcheng Liang *a,
Deqian Liua and
Qianjun Liub
*a,
Deqian Liua and
Qianjun Liub
aSchool of Chemical and Environmental Engineering, China University of Mining & Technology (Beijing), Beijing, 100083, China. E-mail: liangdc@cumtb.edu.cn
bDepartment of Petroleum and Geosystems Engineering, The University of Texas at Austin, Austin, TX 78712, USA
First published on 24th January 2022
Abstract
Low-rank coal, such as Shengli lignite (SL) and Datong bitumite (DT), has abundant reserves and is low in cost. Due to its high moisture content, abundant oxygen-containing groups, high ash content and low calorific value, low-rank coal is mainly used in a low-cost method of direct combustion. For better value-added utilization of SL and DT, a novel strategy has been developed for the preparation of oxygen-rich hierarchical porous carbons (HPCs) by hydrothermal carbonization (HTC), followed by steam activation. In this paper, firstly, the physical and chemical properties of SL and DT were improved by HTC pretreatment, bringing them closer to high rank coal. Then, the effects of HTC pretreatment and activation temperature on the properties of the HPCs were investigated in detail. The results show that the HPCs have mainly microporous structures (the microporosity of 200-SLHPC-900 is 79.58%) based on the N2 adsorption–desorption isotherm analysis and exhibit a higher specific surface area (SSA) and larger pore volume (25.02% and 2.69% improvement for 200-SLHPC-900; 4.93% and 14.25% increase for 200-DTHPC-900, respectively) after HTC pretreatment. The two types of HPCs also present good adsorption performance. The iodine adsorption value of lignite-based HPC presents an increase of 13.72% from 503 mg g−1 to 572 mg g−1, while the value of bitumite-based HPC increases up to 924 mg g−1. A preliminary additional HTC step is therefore an effective method by which to promote the performance of low-rank coal based porous carbon. The process of hydrothermal carbonization and steam activation is a cost-effective and environmentally-friendly preparation method, which omits the use of a chemical activator and reduces the step of alkaline waste liquid discharge compared with the route of carbonization and chemical activation.
1. Introduction
Iodine-containing wastewater produced in the pharmaceutical industries, nuclear power plants and disinfection process of iodophor can result in a series of serious problems, such as contaminating underground water sources and threatening the life of creatures due to its toxicity.1–4 Therefore, iodine removal from wastewater is an urgent problem to be solved globally and it is of great importance to improve civil health and boost the sustainable development of the environment. Multiple methods, such as membrane separation, chemical reaction binding and adsorption, have been proposed and applied for the removal of iodine.5,6 Of these methods, adsorption has become one of the most attractive wastewater treatment technologies because of its high efficiency, simple operation and low cost. Specifically, porous carbons (PCs) have higher chemical and structural stabilities compared with metal–organic frameworks and nanoporous organic polymer networks,7,8 and have been primarily prepared from biomass wastes,9 coal,10 pitch,11 petroleum and their derived products11 via template-based, chemical activation or physical activation methods.12,13 Of a variety of raw materials, coal is still the main material used to produce PCs due to its easy availability, low cost and high carbon content.Coal is the primary energy resource in China,14 and the country has abundant reserves, especially of low-rank coal, of around 200 billion tons, such as Shengli lignite from Inner Mongolia and Datong low metamorphism bitumite from Shanxi, China. Unfortunately, the energy density of low-rank coal is lower than that of anthracite coal due to its higher inherent moisture, oxygen content, ash content and lower carbon content, which limits its application in combustion and gasification.15 Nonetheless, the high reactivity and oxygen-containing functional groups content of low-rank coal have attracted increased attention towards its use as a precursor for porous carbon.16,17
In the past 10 years, hydrothermal carbonization (HTC) has attracted increasing interest in the field of materials science engineering, as it can be used to synthesize functional carbon materials.18 HTC products feature abundant oxygen-containing functional groups, thus making them suitable for many applications, such as adsorption, drug delivery, catalyst supports and the synthesis of porous carbon.19,20 Moreover, the presence of oxygen-containing species in the precursor increases the number of activable sites, thus leading to an increase in the porosity of PCs.21 Simultaneously, the obtained hydrochar can be easily modified, allowing the introduction of heteroatoms such as N, S, P, and Fe, besides O, which is conducive to different functionalized products being obtainable.22 The process of producing synthetic coal from biomass at low temperatures has been known for more than 100 years and has recently regained greater attention in the synthesis of functional carbon materials because of its crucial applications in adsorption, energy storage, separation science, catalysis, and biomedicine, among others.23,24 Furthermore, hydrothermal pretreatment is a mild, green and environmentally-friendly carbonization technology, as the reactions are carried out in enclosed system conditions at relatively low carbonization temperatures. Recently, several researchers synthesized PCs, carbon spheres, carbon nanotubes, carbon quantum dots and graphene via the hydrothermal treatment of biomass.25,26 For instance, Hao et al.27 used waste sugar solution to prepare porous carbon spheres with a high specific surface area of up to 2293 m2 g−1 and good electrochemical performance for supercapacitors. Falco et al.23 prepared activated carbon successfully from lignocellulosic biomass via HTC and its chemical activated form shows high performance in CO2 capture and high-pressure CH4 storage. It is well-known that low-rank coals are not suitable as coking raw materials and are frequently carbonized and upgraded via HTC treatment. However, PCs derived from low-rank coal via HTC pretreatment and applied in the field of adsorption are rarely reported.
In this paper, lignite and bitumite were selected as raw materials to prepare three-dimensional HPCs via hydrothermal carbonization and steam activation. Firstly, the two low-rank coals were pretreated by HTC in a Teflon-lined autoclave and the as-obtained hydrochar was activated by steam. Besides this, the effects of HTC pretreatment and activation temperature on the pore structure properties, the surface chemistry and adsorption performance of the coal samples were investigated. The results achieved in this study not only have certain industrial reference significance for HPCs for the removal of iodine from wastewater, but also help to improve the high value-added utilization of two types of low-rank coal.
2. Experimental
2.1. Raw materials
Shengli lignite (SL) from Inner Mongolia and Datong bitumite (DT), which was collected from Shanxi, China, were used as the raw materials in this study. The proximate and ultimate analyses of SL and DT are listed in Table 1. Reagent grade chemicals were used in all of the experiments. Iodine and potassium iodide were provided by Xilong Chemical Co. Ltd. Sodium thiosulfate, hydrochloric acid and starch were purchased from Tianjin Chemical Reagent Research Institute Co. Ltd.| Sample | Proximate analysis (wt%) | Ultimate analysis (wt%) | AO/C | AH/C | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | FCdafb | Cdaf | Hdaf | Odafb | Ndaf | Sdaf | |||
| a Note, ad: air dry basis; d: dry basis; daf: dry ash-free.b By difference. | |||||||||||
| SL | 13.41 | 14.48 | 45.59 | 54.41 | 66.15 | 4.30 | 25.88 | 1.21 | 2.46 | 0.391 | 0.065 |
| 200-SLhydrochar | 8.43 | 12.44 | 43.48 | 56.52 | 70.20 | 3.62 | 23.60 | 1.12 | 1.46 | 0.336 | 0.052 |
| DT | 6.97 | 4.34 | 33.36 | 66.64 | 76.65 | 7.10 | 14.11 | 1.13 | 1.01 | 0.184 | 0.093 |
| 200-DThydrochar | 5.83 | 4.01 | 32.31 | 67.69 | 78.04 | 6.45 | 13.75 | 1.05 | 0.71 | 0.176 | 0.083 |
2.2. Preparation of the HPCs
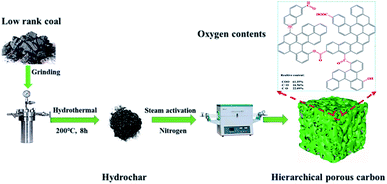
The HPCs were prepared via a two-stage process involving HTC and steam activation. Step 1, 15.0 g of low-rank coal (SL and DT) and 100 mL of deionized water were added to 200 mL Teflon-lined autoclaves, which were then heated at a preset temperature of 200 °C for 8 h. After the autoclave was allowed to cool to room temperature, the resultant product was filtered and washed with deionized water more than three times, followed by desiccation at 110 °C for 24 h. The obtained hydrothermal products were named 200-SLhydrochar and 200-DThydrochar, where 200 represents the HTC temperature. For comparison, SLchar-600 was obtained via direct pyrolysis at 600 °C for 30 min under a N2 atmosphere.Step 2, the hydrochar was filled into a home-made mold and extruded into a cake block with a diameter of 25 mm and a thickness of 8 mm under a pressure of 200 MPa. After natural air drying, the block was broken into granular material with a particle size of 3–10 mm. The hydrochar was then activated by steam (0.8 mL ghydrochar−1 h−1) produced from a steam generator in a horizontal tube furnace at a heating rate of 10 °C min−1 up to the final temperatures (900, 950 and 1000 °C) and held for 3 h under a N2 atmosphere. After cooling, the activated samples were dried at 150 °C under vacuum for 2 h. The obtained HPCs were denoted as 200-DTHPC-900 (200 represents the HTC temperature and 900 is the activation temperature) and 200-SLHPC-y (200 represents the HTC temperature and y is 900, 950, and 1000, representing the activation temperature). For comparison, SL and DT were also individually activated by the same steam flux at 900 °C for 3 h without hydrothermal carbonization pretreatment and were designated as SLPC-900 and DTPC-900. Besides this, the graphical schematic for the preparation of HPCs is illustrated in Fig. 1.
2.3. Sample characterization
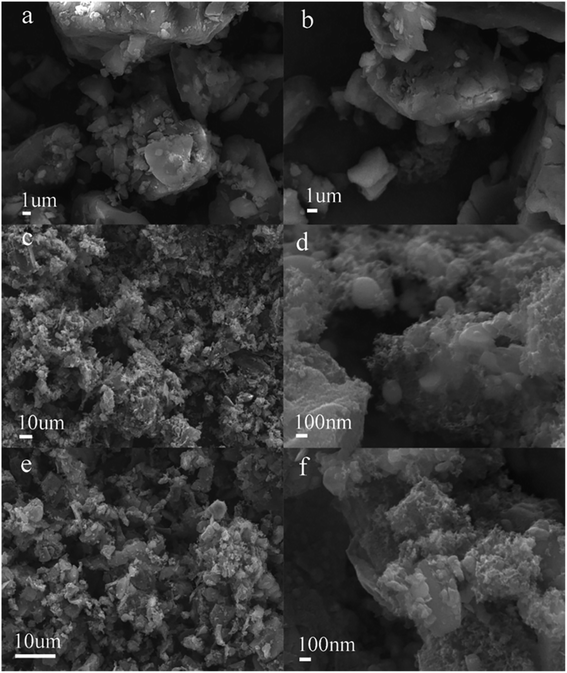
All samples to be characterized were pulverized through 200 mesh and dried at 110 °C for 24 h. X-ray diffraction (XRD) measurements were performed on a Bruker D8 Advance diffractometer using Cu Kα radiation operated at 40 mA and 40 kV (10° min−1 from 10 to 90°). Raman analysis of the hydrochar and HPC samples was carried out on a Bruker Senterra Raman spectrometer by 532 nm laser excitation using a microscope. The Raman spectra were obtained in the range of 1000–2000 cm−1, and all scans were performed three times. Field-emission scanning electron microscopy (FESEM) images were measured using a Merlin Zeiss microscope operated at 5.0 kV to observe the morphology of the samples. The surface chemical properties of the samples were characterized qualitatively using a Nicolet Magna IR560 Fourier-transform infrared (FTIR) spectrometer. X-ray photoelectron spectroscopy (XPS) data were collected using a Thermo ESCALAB 250Xi spectrometer to analyze the surface oxygen-containing functional groups. N2 adsorption–desorption isotherms were measured at −196 °C using a Quantachrome Autosorb-iQ instrument. The SSA was obtained using the Brunauer–Emmett–Teller (BET) method. Micropore surface area and micropore volume were calculated using the t-plot method. The total pore volume was determined at a relative pressure, P/P0, of 0.99. The pore size distribution was calculated from the N2 adsorption data using density functional theory (DFT).2.4. Adsorption experiments
HPCs was selected as adsorbents to evaluate the adsorption performance of iodine (I2). The I2 adsorption tests were carried out following the ASTM D4607-86 method. Dried HPCs were weighed and then added to 250 mL flasks containing 10 mL of 5 wt% HCl solution. The flasks were then gently swirled until the HPCs were completely wetted; 100 mL of 0.1 N I2 solution was added to the flasks and then they were strongly shaken for 30 s before the products were filtered. A standardized 0.1 N sodium thiosulfate solution was used to titrate the filtered solutions using starch indicator. The I2 adsorption value Q (mg g−1) on the HPCs was calculated based on the following equation:where C1 and C2 (mol L−1) are the concentrations of I2 and sodium thiosulfate solutions, respectively, and V1, V2 and V3 (mL) are the consumed volumes of I2, hydrochloric acid and sodium thiosulfate solution, respectively. V (mL), M (126.9 mg g−1) and m (g) represent the volumes of filtrate, half the molecular weight of I2 and the weight of porous carbon, respectively.
3. Results and discussion
3.1. Structural characterization of hydrochar and porous carbon samples
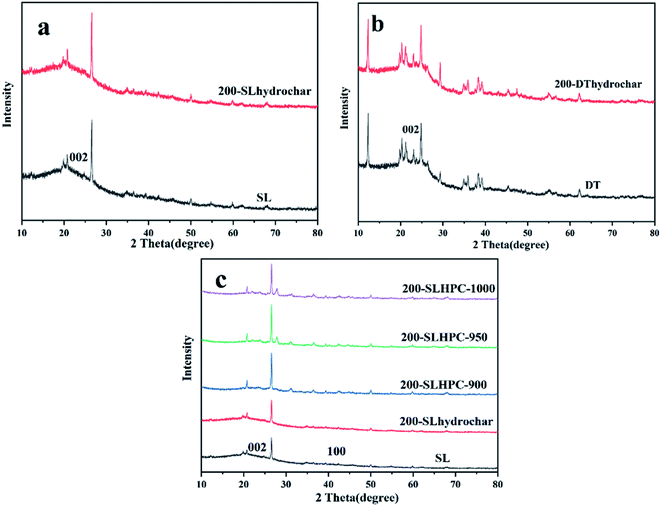
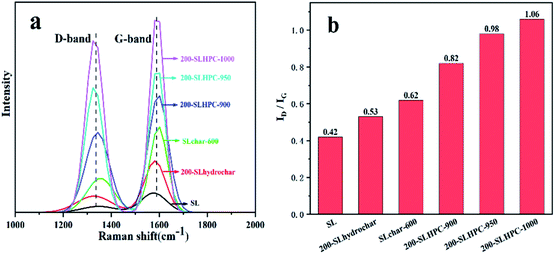
Ultimate analysis of SL and DT shows that carbon content increased, whereas the oxygen content decreased significantly after the HTC process. Decreases in the atomic ratios of oxygen to carbon (AO/C) and hydrogen to carbon (AH/C), which are related to coal rank, indicate partial removal of oxygen-containing functional groups and an improvement in properties in terms of coal rank. Furthermore, the losses in oxygen are related to the dehydration, decarboxylation and reduction reactions.28
In summary, it is worth noting that low-rank coals after HTC modification might be considered to be somewhat similar to higher-rank coals, and that HTC can be regarded as somewhat analogous to the coalification process, which can be greatly accelerated by the application of temperature and pressure.
3.2. Surface chemistry characterization of the hydrochar and porous carbon samples
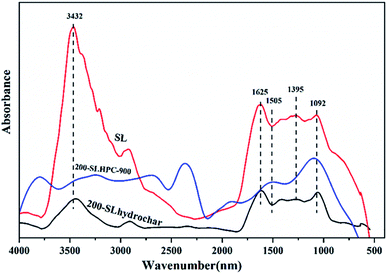
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carbonyl, ester, or carboxyl groups and 1395 cm−1 can be attributed to C–H asymmetrical bending, respectively.34 The strong bands at around 1350–1000 cm−1 represent the stretching vibrations of C
O in carbonyl, ester, or carboxyl groups and 1395 cm−1 can be attributed to C–H asymmetrical bending, respectively.34 The strong bands at around 1350–1000 cm−1 represent the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in hydroxyl, ester, and ether groups and the bending vibration of –OH. The peak at around 783 cm−1 may be assigned to the bending of hydrogen in various locations in the aromatic rings and the peak at 990 cm−1 can be assigned to C–O.35 After HTC pretreatment, some oxygen-containing functional groups of 200-SLhydrochar showed significant changes. For instance, the intensities of the –COOH (1620 cm−1) and –OH (3600–3200 cm−1) peaks become weaker, while the intensity of the C
O in hydroxyl, ester, and ether groups and the bending vibration of –OH. The peak at around 783 cm−1 may be assigned to the bending of hydrogen in various locations in the aromatic rings and the peak at 990 cm−1 can be assigned to C–O.35 After HTC pretreatment, some oxygen-containing functional groups of 200-SLhydrochar showed significant changes. For instance, the intensities of the –COOH (1620 cm−1) and –OH (3600–3200 cm−1) peaks become weaker, while the intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1690–1640 cm−1) peaks increase, which can be assigned to the stretching of the conjugated aromatic carbonyl moiety. These phenomena might be caused by intramolecular and intermolecular decarboxylation, dehydration and aromatization during the HTC process.36 It is also worth noting that some peaks can be observed to appear and disappear after steam activation, and the newly increased peak intensities of the functional groups are those of oxygen-containing species, such as the C–O (1300–1100 cm−1) of an ether. This might be due to activation by steam resulting in structural damage to the organic macromolecules of 200-SLhydrochar, thus generating new functional groups. Some scholars have reported that steam works not only as a dehydrating agent but also as an oxidizing agent during the activation process.37 Furthermore, these oxygen-containing species improve the adsorptive performance of the HPCs via improved wettability and the formation of chemisorption active sites.
O (1690–1640 cm−1) peaks increase, which can be assigned to the stretching of the conjugated aromatic carbonyl moiety. These phenomena might be caused by intramolecular and intermolecular decarboxylation, dehydration and aromatization during the HTC process.36 It is also worth noting that some peaks can be observed to appear and disappear after steam activation, and the newly increased peak intensities of the functional groups are those of oxygen-containing species, such as the C–O (1300–1100 cm−1) of an ether. This might be due to activation by steam resulting in structural damage to the organic macromolecules of 200-SLhydrochar, thus generating new functional groups. Some scholars have reported that steam works not only as a dehydrating agent but also as an oxidizing agent during the activation process.37 Furthermore, these oxygen-containing species improve the adsorptive performance of the HPCs via improved wettability and the formation of chemisorption active sites.
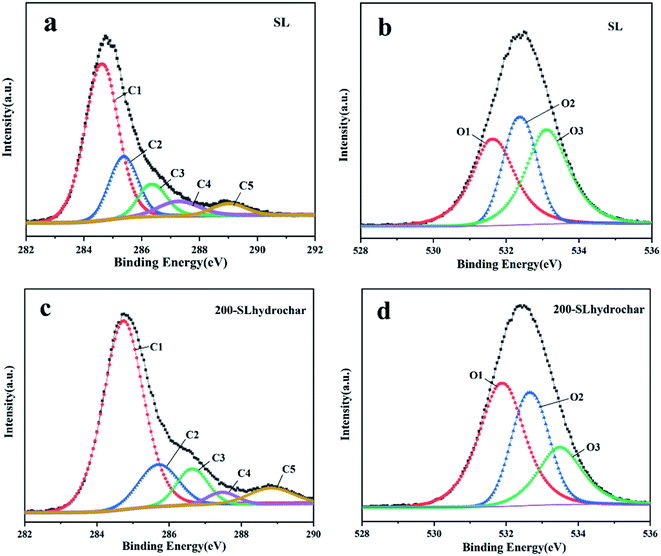
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–H, C–OH in hydroxyl or ether groups, C
C, C–H, C–OH in hydroxyl or ether groups, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carbonyl groups and O
O in carbonyl groups and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O in carboxylic or ester groups, respectively.38 The fitted peaks of the O 1s XPS spectra are approximately located at 531.8 eV (O1), 533.1 eV (O2) and 534.2 eV (O3), corresponding to C
C–O in carboxylic or ester groups, respectively.38 The fitted peaks of the O 1s XPS spectra are approximately located at 531.8 eV (O1), 533.1 eV (O2) and 534.2 eV (O3), corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in ester or anhydride groups, C–O in hydroxyl groups or ether and COO– in carboxylic groups, respectively.39
O in ester or anhydride groups, C–O in hydroxyl groups or ether and COO– in carboxylic groups, respectively.39
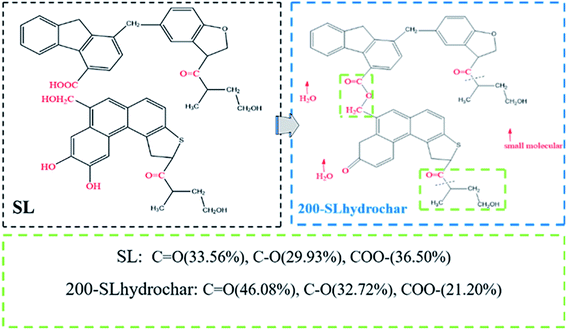
Furthermore, the XPS results revealed the relative percentage of each component in the SL and 200-SLhydrochar, as shown in Table 2. The species and amounts of oxygen-containing functional groups changed and O/C atomic ratio also reduced from 0.394 to 0.328, based on Table 2 and Fig. 6. The C–OH and –COOH might be transformed to C–O–C and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, respectively, according to the foregoing analysis of the FTIR spectra. This is most likely due to intramolecular and intermolecular decarboxylation, dehydration, condensation and aromatization during the HTC process. Therefore, the presumed evolution of surface oxygen-containing functional groups during the hydrothermal carbonization of SL is shown in Fig. 7 based on ultimate analysis, FTIR and XPS characterization analysis results.
C–O, respectively, according to the foregoing analysis of the FTIR spectra. This is most likely due to intramolecular and intermolecular decarboxylation, dehydration, condensation and aromatization during the HTC process. Therefore, the presumed evolution of surface oxygen-containing functional groups during the hydrothermal carbonization of SL is shown in Fig. 7 based on ultimate analysis, FTIR and XPS characterization analysis results.
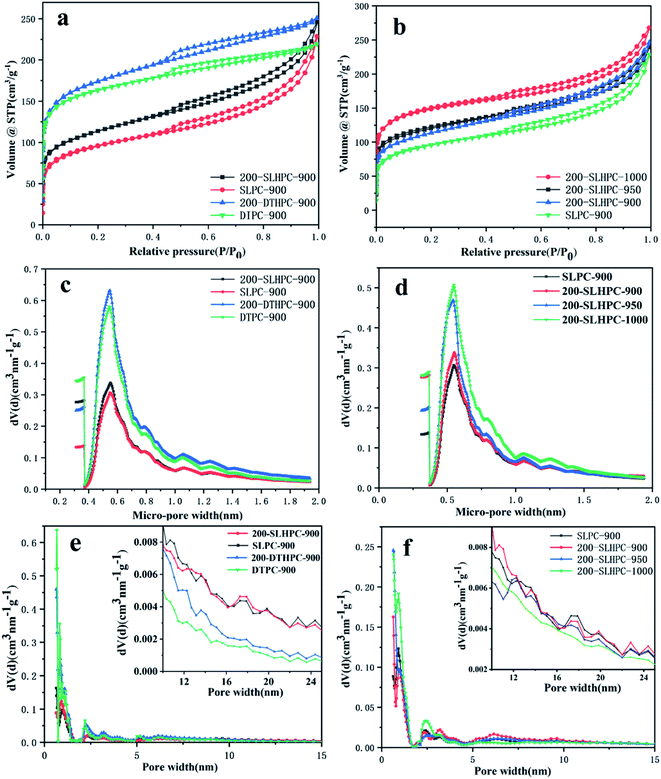
3.3. Textural properties and adsorption performance of HPCs
The pore structure of porous carbon was investigated by N2 adsorption–desorption experiments referring to previous reports.40 The effects of HTC pretreatment and activation temperature on the porous structures of the HPCs are shown in Table 3.| Sample | SBETa (m2 g−1) | Vtb (cm3 g−1) | Smicc (m2 g−1) | Smesd (m2 g−1) | Vmicc (cm3 g−1) | Vmesd (cm3 g−1) | Davee (nm) |
|---|---|---|---|---|---|---|---|
| a SBET: specific surface area from multiple BET method.b Vt: total pore volume at P/P0 = 0.99.c Smic, Vmic: t-method micropore surface area and micropore volume.d Smes, Vmes: difference of SBET, Smic, and Vt, Vmic, respectively.e Dave: average pore diameter. | |||||||
| DTPC-900 | 609.471 | 0.3396 | 551.291 | 58.180 | 0.246 | 0.095 | 2.427 |
| 200-DTHPC-900 | 639.493 | 0.3880 | 581.313 | 58.180 | 0.246 | 0.095 | 2.229 |
| SLPC-900 | 348.218 | 0.3537 | 272.787 | 75.431 | 0.143 | 0.225 | 4.063 |
| 200-SLHPC-900 | 435.351 | 0.3806 | 346.465 | 88.886 | 0.168 | 0.225 | 3.731 |
| 200-SLHPC-950 | 464.972 | 0.371 | 390.413 | 74.559 | 0.183 | 0.198 | 3.313 |
| 200-SLHPC-1000 | 548.991 | 0.414 | 483.170 | 65.821 | 0.223 | 0.199 | 3.017 |
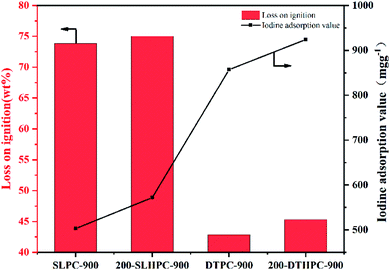
Besides this, the pore development of PCs has also been shown to be reflected by the iodine adsorption value and the loss on ignition (LOI), from a review of many studies.42,43 The LOI and the iodine adsorption value of the four types of PCs are shown in Fig. 9, from which it can be seen that the two low-rank coal-based PCs with HTC pretreatment have higher iodine adsorption values from the 503 mg g−1 of SLPC-900 to the 572 mg g−1 of 200-SLHPC-900 and 857 mg g−1 of DTPC-900 to 924 mg g−1 of 200-DTHPC-900, respectively, indicating that they have enhanced pore structures and good adsorption performance. However, lignite-based PCs have higher LOI values than those of bituminous coal-based PCs, as their degree of metamorphism is relatively low, molecular weight is smaller and their activation reaction is more intense. Furthermore, the higher LOI values of HPCs are due to the partial removal of the side and branched chains of the organic macromolecules during the HTC treatment stage via dehydration, decarboxylation and condensation reactions.
4. Conclusion
Lignite and bitumite have been obviously modified, upgraded, and found to be more inclined to have the physicochemical properties of high-rank coals after hydrothermal carbonization, which promotes subsequent steam activation due to the samples featuring more carbon matrix content and active sites. And the two low-rank coals pretreated using the HTC process are attractive precursors for the preparation of three-dimensional structural HPCs with abundant oxygen-containing species via steam activation. The procured HPCs present a better hierarchical pore structure and excellent adsorption performance in comparison to materials prepared via the normal preparation process in the absence of HTC pretreatment. 200-SLHPC-900 exhibits a better iodine adsorption value of 572 mg g−1 compared with the 503 mg g−1 observed for SLPC-900, while this figure for 200-DTHPC-900 increased by 7.82%, from 857 to 924 mg g−1. This is mainly due to the SSA and pore volume of 200-SLHPC-900 increasing by 25.02% and 2.69% and those of 200-DTHPC-900 increasing by 4.93% and 14.25%, respectively. Meanwhile, the hierarchical porous structure development and pore size distribution could be controlled by the activation temperature. Hence, this mild and novel strategy could not only be used to prepare promising coal-based PCs for use as adsorption materials with good performance but also for achieving environmentally-friendly and high value-added applications using low-rank coal. Future work will focus on exploring the reaction mechanism of the HTC of low-rank coal with the addition of a catalyst.Author contributions
Yufeng Yin: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, writing – original draft, writing – review & editing. Dingcheng Liang: funding acquisition, project administration, resources, investigation, supervision, writing – review & editing. Deqian Liu: formal analysis, writing – review & editing. Qianjun Liu: writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by financial support provided by the National Natural Science Foundation of China (Grant No. 22008255).References
- C. Du, B. Liu, J. Hu and H. Li, Determination of iodine number of activated carbon by the method of ultraviolet-visible spectroscopy, Mater. Lett., 2021, 285, 129137 CrossRef CAS.
- S. Hirunpraditkoon, P. Srinophakun, N. Sombun and E. J. Moore, Synthesis of activated carbon from jatropha seed coat and application to adsorption of iodine and methylene blue, Chem. Eng. Commun., 2015, 202(1), 32–47 CrossRef CAS.
- G. Qu, Y. Han, J. Qi, X. Xing, M. Hou, Y. Sun, X. Wang and G. Sun, Rapid iodine capture from radioactive wastewater by green and low-cost biomass waste derived porous silicon–carbon composite, RSC Adv., 2021, 11(9), 5268–5275 RSC.
- Ö. Şahin, C. Saka, A. A. Ceyhan and O. Baytar, The pyrolysis process of biomass by two-stage chemical activation with different methodology and iodine adsorption, Energy Sources, Part A Recovery, Util. Environ. Eff., 2016, 38(12), 1756–1762 CrossRef.
- X. Long, Y. Chen, Q. Zheng, X. Xie, H. Tang, L. Jiang, J. Jiang and J. Qiu, Removal of iodine from aqueous solution by PVDF/ZIF-8 nanocomposite membranes, Sep. Purif. Technol., 2020, 238, 116488 CrossRef CAS.
- Y. Tachibana, M. Nogami, M. Nomura and T. Suzuki, Simultaneous removal of various iodine species in aqueous solutions of high salt concentrations using novel functional adsorbents, J. Radioanal. Nucl. Chem., 2016, 307(3), 1911–1918 CrossRef CAS.
- S. Ma and H. Zhou, Gas storage in porous metal–organic frameworks for clean energy applications, Chem. Commun., 2010, 46(1), 44–53 RSC.
- R. Dawson, A. I. Cooper and D. J. Adams, Nanoporous organic polymer networks, Prog. Polym. Sci., 2012, 37(4), 530–563 CrossRef CAS.
- G. Zhu, X. Xing, J. Wang and X. Zhang, Effect of acid and hydrothermal treatments on the dye adsorption properties of biomass-derived activated carbon, J. Mater. Sci., 2017, 52(13), 7664–7676 CrossRef CAS.
- D. Liang, Q. Xie, J. Liu, F. Xie, D. Liu and C. Wan, Mechanism of the evolution of pore structure during the preparation of activated carbon from Zhundong high-alkali coal based on gas-solid diffusion and activation reactions, RSC Adv., 2020, 10(55), 33566–33575 RSC.
- Q. Zhuang, J. Cao, Y. Wu, M. Zhao, X. Zhao, Y. Zhao and H. Bai, Heteroatom nitrogen and oxygen co-doped three-dimensional honeycomb porous carbons for methylene blue efficient removal, Appl. Surf. Sci., 2021, 546, 149139 CrossRef CAS.
- C. Zheng, X. Zhou, H. Cao, G. Wang and Z. Liu, Synthesis of porous graphene/activated carbon composite with high packing density and large specific surface area for supercapacitor electrode material, J. Power Sources, 2014, 258, 290–296 CrossRef CAS.
- K. Zhu, K. Egeblad and C. H. Christensen, Mesoporous Carbon Prepared from Carbohydrate as Hard Template for Hierarchical Zeolites, Eur. J. Inorg. Chem., 2007, 2007(25), 3955–3960 CrossRef.
- D. Liang, Q. Xie, C. Wan, G. Li and J. Cao, Evolution of structural and surface chemistry during pyrolysis of Zhundong coal in an entrained-flow bed reactor, J. Anal. Appl. Pyrolysis, 2019, 140, 331–338 CrossRef CAS.
- Y. Wu, X. Zhao, J. Cao, S. He, Z. Hao, H. Yu, G. Sun and X. Wei, Porous Carbons Derived from Lignite Mixed with Zn2+-Doped Lignin for Electric Double-Layer Capacitor, Int. J. Electrochem. Sci., 2017, 12(9), 8132–8147 CrossRef CAS.
- Y. Wu, J. Cao, X. Zhao, Z. Hao, Q. Zhuang, J. Zhu, X. Wang and X. Wei, Preparation of porous carbons by hydrothermal carbonization and KOH activation of lignite and their performance for electric double layer capacitor, Electrochim. Acta, 2017, 252, 397–407 CrossRef CAS.
- Q. Zhuang, J. Cao, Z. Hao, X. Wan, Y. Wu, Z. Ni, X. Zhao and X. Wei, Oxygen-rich Hierarchical Porous Carbon Derived from Coal Tar Pitch for Superior Electric Double Layer Capacitor Application, Int. J. Electrochem. Sci., 2018, 13(9), 8440–8453 CrossRef CAS.
- R. Atchudan, T. N. J. I. Edison, S. Perumal and Y. R. Lee, Green synthesis of nitrogen-doped graphitic carbon sheets with use of Prunus persica for supercapacitor applications, Appl. Surf. Sci., 2017, 393, 276–286 CrossRef CAS.
- C. Dai, J. Wan, J. Yang, S. Qu, T. Jin, F. Ma and J. Shao, H3PO4 solution hydrothermal carbonization combined with KOH activation to prepare argy wormwood-based porous carbon for high-performance supercapacitors, Appl. Surf. Sci., 2018, 444, 105–117 CrossRef CAS.
- M. Demir, Z. Kahveci, B. Aksoy, N. K. R. Palapati, A. Subramanian, H. T. Cullinan, H. M. El-Kaderi, C. T. Harris and R. B. Gupta, Graphitic Biocarbon from Metal-Catalyzed Hydrothermal Carbonization of Lignin, Ind. Eng. Chem. Res., 2015, 54(43), 10731–10739 CrossRef CAS.
- J. Deng, T. Xiong, H. Wang, A. Zheng and Y. Wang, Effects of Cellulose, Hemicellulose, and Lignin on the Structure and Morphology of Porous Carbons, ACS Sustainable Chem. Eng., 2016, 4(7), 3750–3756 CrossRef CAS.
- S. A. Nicolae, H. Au, P. Modugno, H. Luo, A. E. Szego, M. Qiao, L. Li, W. Yin, H. J. Heeres, N. Berge and M. Titirici, Recent advances in hydrothermal carbonisation: from tailored carbon materials and biochemicals to applications and bioenergy, Green Chem., 2020, 22(15), 4747–4800 RSC.
- C. Falco, J. P. Marco-Lozar, D. Salinas-Torres, E. Morallón, D. Cazorla-Amorós, M. M. Titirici and D. Lozano-Castelló, Tailoring the porosity of chemically activated hydrothermal carbons: Influence of the precursor and hydrothermal carbonization temperature, Carbon, 2013, 62, 346–355 CrossRef CAS.
- A. Jain, S. Jayaraman, R. Balasubramanian and M. P. Srinivasan, Hydrothermal pre-treatment for mesoporous carbon synthesis: enhancement of chemical activation, J. Mater. Chem. A, 2014, 2(2), 520–528 RSC.
- M. Li, W. Li and S. Liu, Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose, Carbohydr. Res., 2011, 346(8), 999–1004 CrossRef CAS PubMed.
- B. Hu, K. Wang, L. Wu, S. H. Yu, M. Antonietti and M. M. Titirici, Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass, Adv. Mater., 2010, 22(7), 813–828 CrossRef CAS PubMed.
- Z. Hao, J. Cao, X. Zhao, Y. Wu, J. Zhu, Y. Dang, Q. Zhuang and X. Wei, Preparation of porous carbon spheres from 2-keto-l-gulonic acid mother liquor by oxidation and activation for electric double-layer capacitor application, J. Colloid Interface Sci., 2018, 513, 20–27 CrossRef CAS PubMed.
- J. Wu, J. Wang, J. Liu, Y. Yang, J. Cheng, Z. Wang, J. Zhou and K. Cen, Moisture removal mechanism of low-rank coal by hydrothermal dewatering: Physicochemical property analysis and DFT calculation, Fuel, 2017, 187, 242–249 CrossRef CAS.
- Z. Liu, F. Zhang and J. Wu, Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment, Fuel, 2010, 89(2), 510–514 CrossRef CAS.
- M. Xu, Q. Huang, R. Sun and X. Wang, Simultaneously obtaining fluorescent carbon dots and porous active carbon for supercapacitors from biomass, RSC Adv., 2016, 6(91), 88674–88682 RSC.
- X. Zhang, C. Zhang, P. Tan, X. Li, Q. Fang and G. Chen, Effects of hydrothermal upgrading on the physicochemical structure and gasification characteristics of Zhundong coal, Fuel Process. Technol., 2018, 172, 200–208 CrossRef.
- X. Li, J. Hayashi and C. Li, FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal, Fuel, 2006, 85(12–13), 1700–1707 CrossRef CAS.
- Y. Yi, P. Wang, G. Fan, Z. Wang, S. Chen, T. Xue and Y. Wen, Hierarchically Porous Carbon Microsphere Doped with Phosphorus as a High Conductive Electrocatalyst for Oxidase-like Sensors and Supercapacitors, ACS Sustainable Chem. Eng., 2020, 8(26), 9937–9946 CrossRef CAS.
- H. Wang, H. Deng, Y. He, L. Huang, D. Wei, T. Hao, S. Wang, L. Jin and L. Zhang, Facile and sustainable synthesis of slit-like microporous N-doped carbon with unexpected electrosorption performance, Chem. Eng. J., 2020, 396, 125249 CrossRef CAS.
- Y. Shen and N. Zhang, A facile synthesis of nitrogen-doped porous carbons from lignocellulose and protein wastes for VOCs sorption, Environ. Res., 2020, 189, 109956 CrossRef CAS PubMed.
- C. Falco, F. Perez Caballero, F. Babonneau, C. Gervais, G. Laurent, M. Titirici and N. Baccile, Hydrothermal Carbon from Biomass: Structural Differences between Hydrothermal and Pyrolyzed Carbons via 13C Solid State NMR, Langmuir, 2011, 27(23), 14460–14471 CrossRef CAS PubMed.
- M. Sevilla and R. Mokaya, Energy storage applications of activated carbons: supercapacitors and hydrogen storage, Energy Environ. Sci., 2014, 7(4), 125–128 RSC.
- C. Qin, S. Wang, Z. Wang, K. Ji, S. Wang, X. Zeng, X. Jiang and G. Liu, Hierarchical porous carbon derived from Gardenia jasminoides Ellis flowers for high performance supercapacitor, J. Energy Storage, 2021, 33, 601–610 Search PubMed.
- H. Dong, M. Li, Y. Jin, Y. Wu, C. Huang and J. Yang, Preparation of Graphene-Like Porous Carbons With Enhanced Thermal Conductivities From Lignin Nano-particles by Combining Hydrothermal Carbonization and Pyrolysis, Front. Energy Res., 2020, 8, 14801–14810 Search PubMed.
- X. Yu, J. Lu, C. Zhan, R. Lv, Q. Liang, Z. Huang, W. Shen and F. Kang, Synthesis of activated carbon nanospheres with hierarchical porous structure for high volumetric performance supercapacitors, Electrochim. Acta, 2015, 182, 908–916 CrossRef CAS.
- X. Zhao, Key Laboratory Of Coal Processing And Efficient Utilization Ministry Of Education, C. U. O. M., Preparation and Characterization of Activated Carbons from Oxygen-rich Lignite for Electric Double-layer Capacitor, Int. J. Electrochem. Sci., 2018, 2800–2816 CrossRef CAS.
- A. Mianowski, M. Owczarek and A. Marecka, Surface Area of Activated Carbon Determined by the Iodine Adsorption Number, Energy Sources, Part A Recovery, Util. Environ. Eff., 2007, 29(9), 839–850 CrossRef CAS.
- B. Guo, C. Hou, L. Fan and Z. Sun, Effect of Extraction Temperature on Hyper-coal Structure and Electrochemistry of Coal-Based Activated Carbon, Chin. J. Inorg. Chem., 2018, 34(9), 1615–1624 CAS.
- X. Zhao, S. Huang, J. Cao, X. Wei, K. Magarisawa and T. Takarada, HyperCoal-derived porous carbons with alkaline hydroxides and carbonate activation for electric double-layer capacitors, Fuel Process. Technol., 2014, 125, 251–257 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |