Open Access Article
Open Access ArticleSelective ozone activation of phenanthrene in liquid CO2†
Honghong Shia,
Michael Lundina,
Andrew Danbya,
Eden P. Goc,
Abhimanyu Patilb,
Huaxing Zhou b,
Timothy A. Jackson
b,
Timothy A. Jackson ac and
Bala Subramaniam
ac and
Bala Subramaniam *ad
*ad
aCenter for Environmentally Beneficial Catalysis, University of Kansas, Lawrence, KS 66047, USA. E-mail: bsubramaniam@ku.edu
bExxonMobil Research and Engineering Company, 1545 Rt 22 East, Annandale, New Jersey 08801, USA
cDepartment of Chemistry, University of Kansas, Lawrence, KS 66045, USA
dDepartment of Chemical and Petroleum Engineering, University of Kansas, Lawrence, KS 66045, USA
First published on 23rd December 2021
Abstract
We demonstrate liquid CO2 (8 °C, 4.4 MPa) as a benign medium to perform safe ozonolysis of phenanthrene at near-ambient temperatures. The ozonolysis products consist of several monomeric oxidation products such as diphenaldehyde, diphenic acid and phenanthrenequinone as well as polymeric structures up to 1130 Da. The observed chemical shifts (1H-6.03 ppm, 13C-104.38 ppm) in 2D-NMR spectra of the products confirm the formation of secondary ozonide. Based on the range of observed products, a Criegee-type mechanism is proposed. The ability to deconstruct phenanthrene and produce oxygenated precursors via this technique is particularly of interest in creating new materials from aromatic moieties.
Introduction
Phenanthrene (C14H10) is a polycyclic aromatic hydrocarbon (PAH) present in coal tar and petroleum residues. It is ubiquitous in ambient air, surface water and food products, originating from incomplete fossil fuel and wood combustion.1 Phenanthrene is nearly insoluble in water but soluble in many low polarity organic solvents such as those listed in Table 1. To date, there is limited commercial use of phenanthrene for producing useful chemicals. Catalytic hydrogenation2–4 and cracking5–7 of phenanthrene as a model compound have been reported in the context of developing technologies for upgrading PAHs. Compared to these routes, oxidation is attractive for selectively transforming PAHs to useful chemicals, particularly those possessing functional groups such as aldehyde and/or carboxylic acid. Such oxidation products can be valuable precursors for making a host of new materials with tunable properties for specific applications including easily recyclable polymers and fibers. For instance, diphenic acid has potential commercial value as a plasticizer similar to phthalic anhydride which is obtained from the oxidation of naphthalene,8 and phenanthrenequinone is useful as a photo-initiator during the synthesis of photopolymers.9| Solvent | Solubility (g) at 5 °C | Solubility (g) at 10 °C |
|---|---|---|
| Methanol | 1.8 | 2.4 |
| Light petroleum, b.p. 60–85 °C | 3.2 | 4 |
| Ethanol | 3.26 | 3.77 |
| Glacial acetic acid | — | — |
| Carbon tetrachloride | 9.8 | 12.66 |
| Ether | 20.64 | 23.84 |
| Acetone | 31.02 | 36.54 |
| Chloroform | 29.6 | 34.3 |
| Benzene | 29.86 | 36.66 |
| Carbon disulfide | 45.88 | 54.48 |
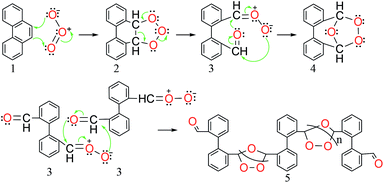
Ozone is an allotrope of oxygen that is highly reactive. Industrial applications of ozone include water treatment,10 bleaching of lignin11 and the Emery processes for making azelaic acid and nonanoic acid.12 The ozonolysis of PAHs, including phenanthrene, has been experimentally studied in various organic solvents.13–16 The reaction pathways and products (Scheme 1) are largely dependent on the choice of solvent used. Participating solvents such as methanol react with the carbonyl oxide (3 in Scheme 1) to form the peracetal compound. By reduction or steam distillation, this peracetal compound was found to form diphenaldehyde, which upon oxidation results in the formation of diphenic acid or phenanthrenequinone. When using alcohol/water mixture as the participating solvent, diphenaldehyde has been reported to form in a single step.
In sharp contrast, a solvent such as chloroform does not react with the carbonyl oxide 3 and has therefore been classified as a non-participating solvent by Bailey and co-workers.18 Schmitt and O'Connor reported the formation of a mono-ozonide during the ozonolysis of phenanthrene in chloroform as solvent (reaction performed in a dry ice-methanol bath at −78 °C).19 The molecular weight (MW) of this ozonide was reported as approximately 223 Da, based on the Rast method that correlates the melting point change of camphor (with and without ozonide product) to the molecular weight of the ozonide. These authors also reported that this ozonide can be converted to dialdehyde by either catalytic reduction or hydrolysis reactions and to diphenic acid by KMnO4 treatment (oxidation). Bailey16,18 and Wibaut19 reported on the ozonolysis of phenanthrene in chloroform as solvent at similar conditions. Both groups reported that the final ozonide product might be polymeric in nature. The molecular weight of the ozonide was reported to be 675 Da by Bailey's group and in the range of 1200–1860 Da by Wibaut's group. This discrepancy hampers accrual of reliable mechanistic insights.
As noted, these previously reported studies of PAHs were typically performed at very low temperatures (e.g., −78 °C in a dry ice-methanol bath), presumably to ensure stability of the energetic intermediates (such as low-molecular-weight ozonides and peroxides) that are known to be unstable even at ambient temperatures. Yet another concern with ozonolysis studies is the propensity of organic solvents, including halogenated solvents such as chloroform, to ozone attack and the formation of phosgene and flammable vapors.20,21
In this work, we employ liquid CO2 instead of organic and halogenated solvents to perform phenanthrene ozonolysis safely at near-ambient temperatures. CO2 is inert to O3 attack and the presence of dense CO2 (a flame retardant) in the vapor phase eliminates flammability. The concept was previously demonstrated for non-aromatic substrates such as fatty acid methyl esters and cyclohexane.22–24 The extent of miscibility of phenanthrene in liquid CO2 was visually confirmed in a view-cell reactor (ESI, pages S2–S3†).
Results and discussion
A reaction cell equipped with an ultrasound transducer (to facilitate mixing of gas and liquid phases) and a differential pressure transducer to measure liquid CO2 level (and thereby its volume) was used in our ozonolysis experiments (ESI, pages S4–S8†). This cell was filled with 10 mL of CO2 at a desired temperature. A blank test performed in this closed-cell reactor showed that >95% of phenanthrene can be recovered with an acetone wash post-reaction, following careful CO2 release by depressurization. An ozonolysis experiment with feed O3:substrate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was performed with a reaction duration of 15 min. The acetone cell wash containing the products was centrifuged, and the clear upper layer was pipetted out carefully until only 1–2 mL of acetone was left in the vial. The vial was then left in the fume hood at room temperature until the acetone was evaporated. A thin layer of white, translucent solid material (“insoluble product”) was observed and weighed (Table 2). The acetone-soluble fraction was analyzed by HPLC (Table 2). As discussed later, the insoluble product was characterized by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF), gel permeation chromatography (GPC) and nuclear magnetic resonance (NMR) analyses.
1 was performed with a reaction duration of 15 min. The acetone cell wash containing the products was centrifuged, and the clear upper layer was pipetted out carefully until only 1–2 mL of acetone was left in the vial. The vial was then left in the fume hood at room temperature until the acetone was evaporated. A thin layer of white, translucent solid material (“insoluble product”) was observed and weighed (Table 2). The acetone-soluble fraction was analyzed by HPLC (Table 2). As discussed later, the insoluble product was characterized by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF), gel permeation chromatography (GPC) and nuclear magnetic resonance (NMR) analyses.
| In ACWa solution (mmol) | Insoluble product (mg) | Xc | Yd | CBe | |||
|---|---|---|---|---|---|---|---|
| Unreacted PAHb | AHb | PAQb | DPAb | ||||
a ACW: acetone cell wash.b PAH: phenanthrene, AH: diphenaldehyde, PAQ: phenanthrenequinone, DPA: diphenic acid; operating conditions: T = 8 °C, P = 975 psi, O3/O2 = 2 mol%, volume of liquid CO2 = 10 mL, initial PAH = 1.12 mmol.c X: conversion (%) of phenanthrene.d  e e  |
|||||||
| 0.72 | 0.02 | 0.008 | 0.01 | 13.9 | 36% | 11% | 68% |
From the results shown in Table 2, it can be inferred that the organic C balance (based on acetone-soluble product fraction) shows a deficit of 32%, suggesting that a significant amount of the products is not either recoverable from the cell or quantifiable by current analytical methods. Diphenaldehyde (0.02 mmol or 4.2 mg) was still found as the main product in the acetone wash. However, the cumulative weight of the soluble products was significantly less than the amount of insoluble product (13.9 mg).
Suspecting that the carbon deficit may be due to combustion of the substrate and/or products, an attempt was made to quantify water in the acetone cell wash of the products (ESI, pages S8 and S9†). The solvent from a control experiment (without ozone) shows a water content of 0.01 wt% while the acetone cell wash of product from the ozonolysis experiment shows a water content of 0.02 wt%. This low-level moisture content (relative to the expected amount of 0.13 wt% for complete substrate combustion) confirms that combustion reactions are insignificant during ozonolysis in liquid CO2 medium.
GPC analysis of the acetone-insoluble product was performed by dissolving it in 5 mL of N,N-dimethylformamide (DMF). As shown in Fig. S5,† large fragments with molecular weight up to 7000 Da were found in the dried insoluble ozonide. To learn if these large molecules have a polymeric structure (repeating unit in the molecule), MALDI-TOF analysis of the insoluble ozonide product present in the acetone as a suspension was performed. The vial containing the suspension was not dried to remove acetone to avoid polymerization during the drying step.
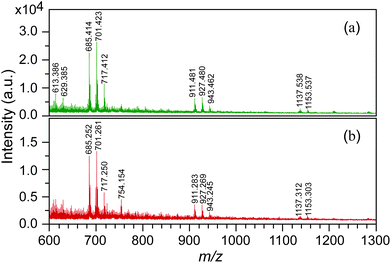
The MALDI-TOF mass spectra of acetone-insoluble products obtained from the high- and low-pressure ozonolysis experiments are shown in Fig. 1(a) and (b). The results from both spectra depict similar peak profiles. In both the spectra, repeating m/z peaks are observed every 226 Da. This finding indicates that the solid product is polymeric in nature and is in agreement with the ozonide structure (monomer value of 226 Da) hypothesized by Bailey et al.18,25 Since sodium salt was used for aiding the ionization, the observed peak at m/z 1153 Da in both spectra corresponds to the sodium adduct of the polymeric ozonide, indicating that the molecular weight of the polymeric ozonide is around 1130 Da. Due to the sample's low concentration in THF, peaks of larger than 1130 Da were too weak to be detected. Based on the formula shown in Scheme 2, we estimate that the number of ozonide units, n (as defined in Scheme 2), for the polymeric ozonide is very close to three.
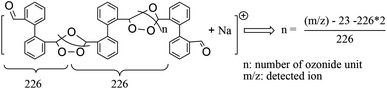 | ||
| Scheme 2 Proposed structure to estimate the number of ozonide units in a detected ion in MALDI-TOF mass spectra. | ||
Based on the results from the MALDI-TOF analysis, the 1H and 13C NMR spectra of the ozonide dimer and oligomer (n = 3) were predicted and shown in Fig. S7.† The chemical shifts at ∼6.5 ppm in the 1H-NMR spectrum and ∼105 ppm in the 13C-NMR spectrum represent the protons and carbons at positions 15 and 19 in the trioxolane ring (a five-membered C–O–O–C–O ring) of the secondary ozonide. These chemical shifts are far from those of other proton and carbon resonances associated with the aromatic ring and aldehyde groups in the oligomer. These resonances are also lacking in the spectra of the other reaction products (diphenaldehyde, diphenic acid and phenanthrenequinone), as ascertained from the spectra of the standards. Therefore, this pair of chemical shifts can be used as a fingerprint to identify the formation of secondary ozonide in the ozonolysis products. Due to the poor solubility of polymeric ozonide in acetone, toluene-d8 was used as deuterated solvent to collect the ozonolysis product for NMR analysis. The 1H- and 2D-NMR results are shown in Fig. S8.†
The 1H NMR spectrum of the recovered ozonolysis product (Fig. S8a†) shows a mixture of unreacted phenanthrene along with the oxidation products. The chemical shifts between 9.5 ppm and 10 ppm are attributed to various aldehyde groups, confirming the formation of diphenaldehyde. In addition, a singlet peak at 6.03 ppm was observed. From the COSY spectrum (Fig. S8b†), only a diagonal-peak was found at 6.03 ppm. The lack of apparent cross-peaks indicates that there is no coupling between pairs of H–H nuclei at this position. The HSQC (Fig. S8c†) confirms the bond of 1H at 6.03 ppm and 13C at 104.38 ppm. The HMBC (Fig. S8d†) detects heteronuclear correlations over longer ranges of about 2–4 bonds. Herein, the longer range of up to 3 carbons was found as peaks at 104 ppm, 128 ppm and 136 ppm, respectively. In summary, the proton at a chemical shift of 6.03 ppm is not coupling with other nearby protons and is directly bonded to a carbon at 104.38 ppm. This proton resonance is also correlated to at least two aromatic carbons at longer distances. Collectively, these observations support the formation of a secondary ozonide with a trioxolane ring (a five-membered C–O–O–C–O ring) in the ozonolysis product. In addition, the chemical shifts (1H-6.03 ppm, 13C-104.38 ppm) are also close to reported values for secondary ozonide.22
In addition to the products listed in Table 2, byproducts with aromatic structures were found from the GC/MS analysis of the acetone soluble solution, with molecular weight ranging from 181 to 219 Da. The total peak area of these byproducts from GC-FID analysis is less than 5%.
Additional product molecules with molecular weight up to 7000 Da were also found in the GPC analysis of the acetone wash solution (Fig. S6†). This finding indicates that part of the polymeric ozonide was dissolved in the acetone solution but not quantified by the GC and HPLC analysis. These unaccounted products will further bridge the organic C deficit.
Based on the experimental findings, we propose the ozonolysis mechanism as shown in Scheme 1. The initial step involves a cycloaddition of ozone to the 9–10 bond in the phenanthrene leading to the formation of primary ozonide 2, which decays readily to the carbonyl oxide 3. In a participating solvent such as MeOH, the Criegee-type carbonyl oxide intermediate is rapidly attacked by solvent; however, this reactivity is not observed in liquid CO2 as solvent. Instead, we propose the formation of two types of secondary ozonide: a polymeric compound 5, formulated on the basis of the monomer molecular weight determination by MALDI-TOF; and a monomeric secondary ozonide 4 which decays to give diphenaldehyde, as observed from the GC/MS results. It is well documented in literature that the ozonolysis of C–C double bond leads to the formation of two aldehydes from the decay of secondary ozonide 4 and the release of hydrogen peroxide (H2O2) via hydrolysis mechanism.26 The diphenaldehyde can be converted to 9,10-phenanthrenequinone in an oxidative dehydrogenation step, which could be a more feasible pathway than its oxidation to form the diphenic acid. The diphenic acid could also be formed from phenanthrenequinone via an oxidative ring-opening route. Experimental evidence has been reported that the oxidative cleavage of C–C bonds in phenanthrenequinone results in the formation of diphenic acid by transfer of lattice oxygen from an appropriate transition metal oxide such as V2O5.27,28
Conclusions
The approach established herein should enable systematic studies of the ozonolysis of various PAHs in liquid CO2 to generate a knowledge base of ozone-initiated oxidation mechanism and value-added products associated with such compounds. Further, as demonstrated elsewhere,21 continuous ozonolysis with controlled residence times will facilitate better tuning of product selectivity compared to batch operation.Author contributions
H. Shi and M. Lundin carried out the ozonation experiments, HPLC, GC-FID, GC/MS and GPC analyses and moisture content measurement. A. Danby contributed to the reaction set-up development and solubility determination of PAH in liquid CO2. E. Go performed the MALDI-TOF experiment and analysed the data. A. Patil and H. Zhou helped with the data interpretation and supervised the project. B. Subramaniam and T. Jackson designed and directed the project. All authors discussed the results and contributed to the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by a grant from ExxonMobil, initiated by Dr Abhimanyu Patil. Tragically, Dr Patil passed away shortly before completion of this project. We dedicate this work to his memory. The authors thank Sarah Neuenswander of the Nuclear Magnetic Resonance Laboratory, University of Kansas, for assistance with NMR experiments. Support for the NMR instrumentation was provided by NIH Shared Instrumentation Grant #S10RR024664 and NSF Major Research Instrumentation Award #1625923. We thank the Synthetic Chemical Biology Core Facility of the University of Kansas for providing the MALDI-TOF service. This core facility was supported in part by NIGMS grants, P20GM113117 and P20GM103638 at NIH.Notes and references
- S. H. Lee and W. C. McCain, in Wildlife Toxicity Assessments for Chemicals of Military Concern, Elsevier, 2015, pp. 591–598 Search PubMed.
- W. Qian, Y. Yoda, Y. Hirai, A. Ishihara and T. Kabe, Appl. Catal., A, 1999, 184, 81–88 CrossRef CAS.
- W. Fu, L. Zhang, D. Wu, M. Xiang, Q. Zhuo, K. Huang, Z. Tao and T. Tang, J. Catal., 2015, 330, 423–433 CrossRef CAS.
- E. Schachtl, L. Zhong, E. Kondratieva, J. Hein, O. Y. Gutiérrez, A. Jentys and J. A. Lercher, ChemCatChem, 2015, 7, 4118–4130 CrossRef CAS.
- P. Yeletsky, T. Reina, O. Bulavchenko, A. Saraev, E. Y. Gerasimov, O. Zaikina, J. Bermúdez, P. Arcelus-Arrillaga, V. Yakovlev and M. Millan, Catal. Today, 2019, 329, 197–205 CrossRef CAS.
- M. Golmohammadi, S. J. Ahmadi and J. Towfighi, J. Supercrit. Fluids, 2016, 113, 136–143 CrossRef CAS.
- M. Zhong, J. Zhai, Y. Xu, L. Jin, Y. Ye, H. Hu, F. Ma and X. Fan, Fuel, 2020, 263, 116763 CrossRef CAS.
- M. A. Salem, M. H. Helel, Y. A. Ammar, M. S. A. El-Gaby, H. K. Thabet and M. A. Gouda, Synth. Commun., 2017, 47, 935–960 CrossRef CAS.
- P. P. A. C. Albuquerque, M. L. Bertolo, L. M. A. Cavalcante, C. Pfeifer and L. F. S. Schneider, J. Esthet. Dent., 2015, 27, S49–S57 CrossRef PubMed.
- R. G. Rice, C. M. Robson, G. W. Miller and A. G. Hill, J. – Am. Water Works Assoc., 1981, 73, 44–57 CrossRef CAS.
- R. Travaini, J. Martín-Juárez, A. Lorenzo-Hernando and S. Bolado-Rodríguez, Bioresour. Technol., 2016, 199, 2–12 CrossRef CAS PubMed.
- Bio-Lubricants Product Brochure by Emery Oleochemicals), https://pdf4pro.com/cdn/bio-lubricants-65bae.pdf, Accessed Dec 20, 2021 Search PubMed.
- P. S. Bailey, Ozonation in organic chemistry V2: Nonolefinic compounds, Elsevier, 2012 Search PubMed.
- A. Lundstedt, M. J. Webb and H. Grennberg, RSC Adv., 2017, 7, 6152–6159 RSC.
- E. J. Moriconi and L. B. Taranko, J. Org. Chem., 1963, 28, 1831–1834 CrossRef CAS.
- J.-J. Yao, Z.-H. Huang and S. J. Masten, Water Res., 1998, 32, 3001–3012 CrossRef CAS.
- H. Henstock, J. Chem. Soc., Trans., 1922, 121, 2124–2128 RSC.
- P. S. Bailey and S. B. Mainthia, J. Org. Chem., 1956, 21, 1335–1336 CrossRef CAS.
- W. J. Schmitt, E. J. Moriconi and W. F. O'Connor, J. Am. Chem. Soc., 1955, 77, 5640–5642 CrossRef CAS.
- D. Kong, D. J. am Ende, S. J. Brenek and N. P. Weston, J. Hazard. Mater., 2003, 102, 155–165 CrossRef CAS PubMed.
- F. L. Greenwood, J. Org. Chem., 1945, 10, 414–418 CrossRef CAS PubMed.
- M. D. Lundin, A. M. Danby, G. R. Akien, T. P. Binder, D. H. Busch and B. Subramaniam, ACS Sustainable Chem. Eng., 2015, 3, 3307–3314 CrossRef CAS.
- M. D. Lundin, A. M. Danby, G. R. Akien, P. Venkitasubramanian, K. J. Martin, D. H. Busch and B. Subramaniam, AIChE J., 2017, 63, 2819–2826 CrossRef CAS.
- X. Chen, D. B. Rice, A. M. Danby, M. D. Lundin, T. A. Jackson and B. Subramaniam, React. Chem. Eng., 2020, 5, 793–802 RSC.
- P. S. Bailey and S. B. Mainthia, J. Org. Chem., 1958, 23, 1089–1092 CrossRef CAS.
- E. Perraudin, H. Budzinski and E. Villenave, Atmos. Environ., 2007, 41, 6005–6017 CrossRef CAS.
- N. Montoya Sánchez and A. de Klerk, Energy Fuels, 2015, 29, 7910–7922 CrossRef.
- N. M. Sánchez and A. De Klerk, Prepr. - Am. Chem. Soc., Div. Energy Fuels, 2014, 59, 558 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra06642k |
| This journal is © The Royal Society of Chemistry 2022 |