21-Carba-23-oxaporphyrinoids and 21-oxo-21-carba-23-oxaporphyrinoids: macrocyclic π-conjugation involving the carbonyl moiety†
Abstract
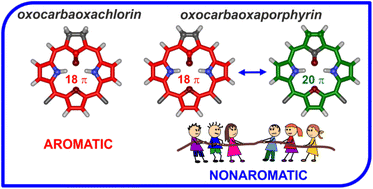
A rational synthesis using the carba analogues of tripyrrane and 1,3-bis(arylhydroxymethyl)furan as synthons resulted in the formation of 21-carba-23-oxaporphyrin and 21-carba-23-oxachlorin. The molecular design preserves all essential virtues of the 21-oxaporphyrin architecture allowing for incorporation of a cyclopentadiene moiety into the macrocyclic framework. Protonation of the inner core carbon atom reveals its adjustable (trigonal versus tetrahedral) geometry. Oxygenation of 21-carba-23-oxachlorin and 21-carba-23-oxaporphyrin afforded aromatic 21-oxo-21-carba-23-oxachlorin and nonaromatic 21-oxo-21-carba-23-oxaporphyrin, respectively. NMR spectroscopy reveals the predominance of the keto tautomer over the hydroxy one. The aromaticity of 21-oxo-21-carba-23-oxachlorin, with a built-in cyclopentanone moiety, resulted from the predomination of the 18 π-electron delocalization assisted by the dipolar resonance contributors. In the case of 21-oxo-21-carba-23-oxaporphyrin incorporating cyclopentadienone, three major canonical structures imply the 20 π-electron antiaromaticity, 18 π-electron aromaticity, or local 4 π-electron antiaromaticity of the cyclopentadienone unit, which seem to contribute to the overall nonaromatic features. DFT optimized molecular models were used to evaluate the nucleus-independent chemical shifts, which are −12.9 for 21-oxo-21-carba-23-oxachlorin and −0.9 for 21-oxo-21-carba-23-oxaporphyrin consistent with magnetic properties reflected by 1H NMR spectra.


 Please wait while we load your content...
Please wait while we load your content...