Qiang Wang, Zilong Yan and Dong Xing
Org. Chem. Front., 2022,9, 3840-3846
DOI:
10.1039/D2QO00546H,
Research Article
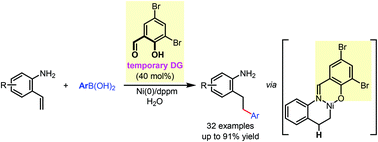
We report a nickel(0)-catalyzed linear-selective hydroarylation of 2-aminostyrenes with arylboronic acids using a bifunctional temporary directing group strategy. In the presence of a catalytic amount of commercially available 3,5-dibromosalicylaldehyde, an aldimine intermediate is formed to interact with the nickel(0) catalyst by both chelation from the imino group and nickel-hydride formation from the phenoxy group. With the imino-assisted six-membered metallacycle formation, excellent linear selectivity has been achieved for this redox-neutral hydroarylation reaction.