Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-assisted Wittig-type olefination of aldehydes with p-toluenesulfonylmethyl isocyanide†
Yi-Chun
Wang‡
a,
Xinyi
Chen‡
a,
Gregory R.
Alvey
b,
Andrey
Shatskiy
 b,
Jian-Quan
Liu
b,
Jian-Quan
Liu
 *ab,
Markus D.
Kärkäs
*ab,
Markus D.
Kärkäs
 *b and
Xiang-Shan
Wang
*b and
Xiang-Shan
Wang
 *a
*a
aSchool of Chemistry and Materials Science, Jiangsu Key Laboratory of Green Synthesis for Functional Materials Jiangsu Normal University, Xuzhou, Jiangsu 221116, China. E-mail: liujq316@jsnu.edu.cn; xswang@jsnu.edu.cn
bDepartment of Chemistry, KTH Royal Institute of Technology, SE-100 44 Stockholm, Sweden. E-mail: karkas@kth.se
First published on 20th June 2022
Abstract
The Wittig reaction is a valuable and powerful tool in organic synthesis, providing a convenient route from aldehydes and ketones to alkenes. Herein, a novel copper-assisted Wittig-type olefination of aldehydes with p-toluenesulfonylmethyl isocyanide (TosMIC) is disclosed, providing a direct and operationally simple approach to (E)-vinyl sulfones under mild conditions, compatible with a multitude of common functional groups. Experimental and computational investigations imply that the reaction proceeds through an intriguing electronically-controlled (3 + 2)/retro-(3 + 2) cycloaddition pathway.
Carbon–carbon bond formation is of fundamental importance in contemporary organic synthesis. It finds application in the synthesis of biologically relevant target molecules, commodity chemicals, and materials.1,2 Despite transition metal-catalyzed carbon–carbon bond formation being a well-established research field,3,4 there is still great appeal in developing novel carbon–carbon coupling reactions, and even more so, for reactions producing carbon–carbon double bonds. Commercially available aldehydes and ketones are versatile building blocks in chemical synthesis and can be employed in a myriad of transformations. The properties of the carbonyl functionality allow for its application in reactions that are key to the synthetic chemists’ arsenal, such as Grignard, Wittig, and aldol reactions, to name a few.5 Of these, the Wittig reaction is one of the most efficient and streamline methodologies for converting the carbon–oxygen double bond of an aldehyde or a ketone into a new carbon–carbon double bond.5,6 It is well-recognized that such protocols firstly undergo an intermolecular (2 + 2) cycloaddition process, in which the phosphine ylide adds to the carbonyl to form a four-membered cyclic intermediate. The intermediate is short-lived and quickly breaks down via a reverse (2 + 2) cycloaddition to generate the alkene.7 Aldehydes and ketones play an irreplaceable role in this reactivity pattern. Therefore, the discovery of unprecedented coupling partners and catalysts for this reactivity is key to realizing new transformations and, subsequently, providing a lucrative springboard for diversification in chemical synthesis.
p-Toluenesulfonylmethyl isocyanide (TosMIC) is a versatile reagent that is widely used for construction of nitrogen-containing heterocyclic motifs, including oxazoles, oxazolidines, thiazoles, pyrroles, indoles, imidazoles, triazoles.8 The structural features of this versatile reagent are of excellent utility when synthesizing organic compounds and show comprehensive chemistry with different functionalities. Recently, we disclosed copper- and silver-catalyzed heteroaromatization protocols proceeding through condensation between propargylic alcohols and TosMIC, thus revealing a conceptually novel reactivity profile of the latter.9 Based on these findings and our continued efforts in copper- and silver-catalyzed reactions involving isocyanides,9,10 we herein report the first example of copper-assisted Wittig-type olefination reaction of aldehydes with TosMIC via sequential (3 + 2) cycloaddition—retro-(3 + 2) cycloaddition (Fig. 1). Notably, this reaction provides a direct and operationally simple approach to (E)-vinyl sulfones with various functional groups under mild conditions from two basic chemicals. Vinyl sulfones are fruitful moieties and are key building blocks in numerous natural products and synthetic functional molecules. Furthermore, they serve as valuable intermediates in chemical synthesis and have even been found to inhibit an array of enzymatic processes, providing unique opportunities for their use in drug design and medicinal chemistry.11,12
Initially, 2-nitrobenzaldehyde (1a) and TosMIC (2) were selected as the model substrates to optimize the reaction conditions. The reaction of 1a (0.6 mmol) and 2 (0.5 mmol) with 2 equivalents of a base (K2CO3, Cs2CO3 or DBU) in N,N-dimethylformamide (DMF) at 25 °C afforded oxazole 4a in high yields (Table 1, entries 1–3). Upon addition of CuI (30 mol%) into the reaction system, a new product, vinylsulfone 3a, was isolated from the reaction in 13% yield (Table 1, entry 4). Encouraged by these results, a survey of copper salts, such as Cu(OAc)2, Cu(OTf)2, CuCl and copper powder, was undertaken for the reaction of 1a and 2 in DMF at 25 °C. Here, CuI offered the highest yield of the olefinic product 3a, while Cu(OAc)2 and Cu(OTf)2 were ineffective towards the formation of 3a under the same conditions (Table 1, entries 5–8). Other transition metal-based catalysts, commonly employed in isocyanide chemistry, such as Ag2CO3 and Pd(OAc)2, also proved ineffective (Table 1, entries 9 and 10). Delightfully, the addition of ligands slightly increased the yield of 3a (Table 1, entries 11–13). Next, increasing the amount of the copper salt to 0.5, 1.0, and 2.0 equivalents afforded 3a in dramatically improved yields (Table 1, entries 14 and 15). Also, the solvent had a significant influence on the transformation. Thus, changing the solvent from DMF to DMSO produced the anticipated olefinic product 3a in up to 90% yield, while THF and CH3CN had a negative effect on the reaction and delivered 3a in diminished yields (Table 1, entries 17, 19 and 20). Finally, conducting the reaction with different bases identified Cs2CO3 as the most productive base additive (Table 1, entries 18–20). Thus, the conditions from Table 1, entries 17 and 20 were considered optimal and employed for further investigations.
| Entry | [Cu] | Base | Ligand | Solvent | Yieldb (%) | |
|---|---|---|---|---|---|---|
| 3a | 4a | |||||
| a Reaction conditions: 1a (91 mg, 0.6 mmol, 1.2 equiv.), 2 (98 mg, 0.5 mmol, 1.0 equiv.), [Cu] (0.3 equiv.), base (2.0 equiv.), ligand (1.2 equiv.), solvent (2.0 mL), rt, 8 h. b Isolated yields. c 0.5 equiv. of CuI. d 1.0 equiv. of CuI. e 2.0 equiv. of CuI. | ||||||
| 1 | — | K2CO3 | — | DMF | 0 | 89 |
| 2 | — | Cs2CO3 | — | DMF | 0 | 92 |
| 3 | — | DBU | — | DMF | 0 | 73 |
| 4 | CuI | K2CO3 | — | DMF | 13 | 83 |
| 5 | Cu(OAc)2 | K2CO3 | — | DMF | 0 | 87 |
| 6 | Cu(OTf)2 | K2CO3 | — | DMF | 0 | 82 |
| 7 | CuCl | K2CO3 | — | DMF | 8 | 86 |
| 8 | Cu powder | K2CO3 | — | DMF | 9 | 36 |
| 9 | Ag2CO3 | K2CO3 | — | DMF | 0 | 97 |
| 10 | Pd(OAc)2 | K2CO3 | — | DMF | 0 | 84 |
| 11 | CuI | K2CO3 | o-Phen | DMF | 21 | 69 |
| 12 | CuI | K2CO3 | L-Proline | DMF | 16 | 72 |
| 13 | CuI | K2CO3 | PPh3 | DMF | 14 | 67 |
| 14c | CuI | K2CO3 | o-Phen | DMF | 39 | 45 |
| 15d | CuI | K2CO3 | o-Phen | DMF | 72 | 24 |
| 16e | CuI | K2CO3 | o-Phen | DMF | 75 | 19 |
| 17 | CuI | K 2 CO 3 | o-Phen | DMSO | 88 | <5 |
| 18d | CuI | K2CO3 | o-Phen | THF | 63 | 28 |
| 19d | CuI | K2CO3 | o-Phen | CH3CN | 54 | 32 |
| 20 | CuI | Cs 2 CO 3 | o-Phen | DMSO | 90 | <5 |
| 21d | CuI | DBU | o-Phen | DMSO | 43 | 21 |
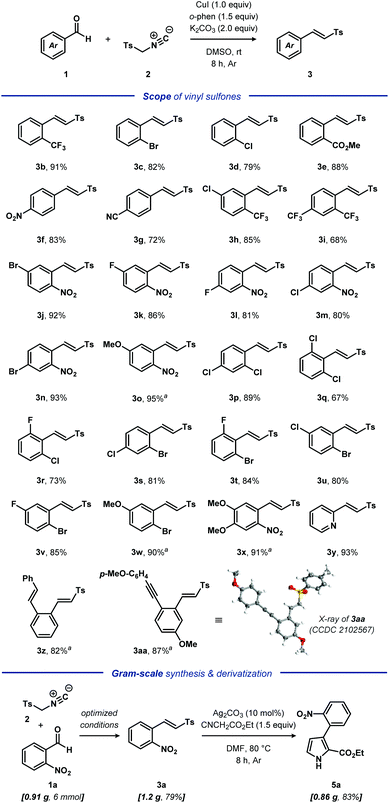
With the optimized reaction conditions in hand, we explored the versatility of the developed protocol (Scheme 1). A set of diversely functionalized aromatic aldehydes 1 were reacted with TosMIC 2, affording the corresponding olefinic products 3 in high yields (Scheme 1). The monosubstituted aromatic aldehydes 1a–1e featuring electron-withdrawing groups on the benzene ring, such as CF3, NO2, Cl, Br, CO2Me and CN, placed at either the ortho- or para-positions were compatible with the developed protocol, leading to the expected products 3a–3g in high to excellent yields (72–91%). Similarly, a range of disubstituted substrates 1h–1w were also efficiently transformed to the corresponding vinyl sulfones 3h–3w in high to excellent yields (68–95%). Notably, a variety of the tolerated synthetically valuable functional groups, including fluoride, chloride and bromide, offer synthetic handles for further functionalization. Gratifyingly, the densely substituted aromatic aldehydes 1x, 1z and 1aa also reacted with 2 to afford the desired products 3x, 3z and 3aa in 91%, 82% and 87% yields, respectively. Notably, substrates 1z and 1aa containing a potentially reactive alkenyl and alkynyl group were productive under the optimized conditions, illustrating the high compatibility of the developed protocol. Furthermore, subjecting the heteroaryl substrate 2-pyridinaldehyde 1y to 2a, delivered the corresponding product 3y with high efficiency. The structure of product 3w was unequivocally confirmed by single crystal X-ray analysis (CCDC 2102567; for details, see the ESI†). Unfortunately, aliphatic aldehydes, benzaldehyde and electron-rich aromatic aldehydes including sensitive functional groups produced the corresponding oxazoles instead of the target vinyl sulfones (for additional details, see the ESI†).
To further explore the synthetic utility and scalability of the established protocol, the reaction of 2-nitrobenzaldehyde 1a and TosMIC 2 was carried out on a 5 mmol scale, providing the expected product 3a in 79% yield (1.2 g). Furthermore, vinyl sulfone 3a was efficiently converted to oligofunctional pyrrole 5 in 83% yield through Bi's method.13 Such oligofunctional pyrroles represent a common structural unit in numerous natural products, potent pharmaceuticals, molecular sensors, and can serve as valuable intermediates in organic synthesis.14 Herein, we have provided a practical method for synthesis of 2,3-disubstituted pyrroles from commodity chemicals.
A series of control experiments were carried out to probe the mechanism of the established transformation (Scheme 2). First, the addition of 2 equivalents of the commonly used radical scavenger 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) to the reaction mixture did not affect the reaction and provided product 3a in 73% isolated yield.15 This result strongly suggests that the disclosed reaction does not proceed through a free-radical pathway. Furthermore, employing oxazole 4a as the substrate under the optimized conditions did not produce the olefinic product 3a, indicating that copper-activated oxazole intermediate is critical to the ring-opening step of the developed reaction.16 Based on the experimental observations and relevant literature precedents,17,18 a plausible mechanism for the formation of vinyl sulfone 3 was proposed (Scheme 2). DFT calculations were performed using the three-parameter exchange–correlation hybrid functional B3LYP19 with D3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 dispersion correction for studying the possible existence of copper-activated isocyanide or copper-activated aldehyde. The results suggest that it is more reasonable for the isocyanide to preferentially coordinate to the copper center, whereas coordination of the aldehyde is not favored in this catalytic system (Fig. S1, for additional details, see the ESI†). Here, the base facilitates abstraction of the α-proton from TosMIC 2, promoting the generation of the copper-coordinated intermediate A,21 which then undergoes (3 + 2) cycloaddition to produce the annulated adduct B (copper-activated aldehyde). Finally, the annulated adduct B undergoes retro-(3 + 2) cycloaddition to give the target product 3 through the elimination of Cu(o-phen)NCO.18 There may be two competing scenarios for forming copper-activated oxazole intermediates: (1) protonation or (2) elimination. Thus, the copper-activated oxazole intermediates with electron-rich aromatic rings are easily protonated to furnish oxazoles, the stability of which makes it challenging to carry out a ring-opening sequence under the optimized reaction conditions. Next, DFT calculations were performed to gain further mechanistic insight into the key ring-opening process (for details, see the ESI†). As shown in Scheme 2, the retro-(3 + 2) cycloaddition processes of Int1-H and 2-nitrobenzoxazole copper intermediate Int1-NO2 were explored, suggesting that Int1-NO2 is significantly more reactive than Int1-H. The reaction barrier for TS1-H is 20.9 kcal mol−1, while the nitrobenzoxazole copper intermediate in TS1-NO2 has a significantly lower barrier of 14.8 kcal mol−1. This contrasts the concerted cleavage of C–N and C–O bond in TS1-H, which in TS1-NO2 is also concomitant with the migration of copper from the carbon to the nitrogen site. Thus, we speculate that the electron-withdrawing nitro group in Int1-NO2 promotes the migration of copper and further reduces the energy barrier of the reaction.
20 dispersion correction for studying the possible existence of copper-activated isocyanide or copper-activated aldehyde. The results suggest that it is more reasonable for the isocyanide to preferentially coordinate to the copper center, whereas coordination of the aldehyde is not favored in this catalytic system (Fig. S1, for additional details, see the ESI†). Here, the base facilitates abstraction of the α-proton from TosMIC 2, promoting the generation of the copper-coordinated intermediate A,21 which then undergoes (3 + 2) cycloaddition to produce the annulated adduct B (copper-activated aldehyde). Finally, the annulated adduct B undergoes retro-(3 + 2) cycloaddition to give the target product 3 through the elimination of Cu(o-phen)NCO.18 There may be two competing scenarios for forming copper-activated oxazole intermediates: (1) protonation or (2) elimination. Thus, the copper-activated oxazole intermediates with electron-rich aromatic rings are easily protonated to furnish oxazoles, the stability of which makes it challenging to carry out a ring-opening sequence under the optimized reaction conditions. Next, DFT calculations were performed to gain further mechanistic insight into the key ring-opening process (for details, see the ESI†). As shown in Scheme 2, the retro-(3 + 2) cycloaddition processes of Int1-H and 2-nitrobenzoxazole copper intermediate Int1-NO2 were explored, suggesting that Int1-NO2 is significantly more reactive than Int1-H. The reaction barrier for TS1-H is 20.9 kcal mol−1, while the nitrobenzoxazole copper intermediate in TS1-NO2 has a significantly lower barrier of 14.8 kcal mol−1. This contrasts the concerted cleavage of C–N and C–O bond in TS1-H, which in TS1-NO2 is also concomitant with the migration of copper from the carbon to the nitrogen site. Thus, we speculate that the electron-withdrawing nitro group in Int1-NO2 promotes the migration of copper and further reduces the energy barrier of the reaction.
Conclusions
In conclusion, we report a novel copper-assisted Wittig-type olefination reaction of aldehydes with TosMIC under mild reaction conditions, providing a convenient approach to an array of functionalized vinyl sulfones in good to excellent yields. Based on experimental and computational investigations, an intriguing electronically controlled (3 + 2)/retro-(3 + 2) cycloaddition sequence is proposed. Considering the importance of the Wittig reaction and the relevance of vinyl sulfones as building blocks, this methodology will undoubtedly find practical applications in the future. Further investigation into applying this catalytic system to aromatic ketones is currently underway.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by Outstanding Youth Fund of Jiangsu Province (BK20211607), NSFC of China (No. 21702078), and 2021 National and Provincial College Students Innovation and Entrepreneurship Training Program (202110320010Z), the Priority Academic Program Development of Jiangsu Higher Education Institutions, FORMAS (grant no. 2019-01269), Swedish Research Council (grant no. 2020-04764), Olle Engkvist Foundation, Magnus Bergvall Foundation, and KTH Royal Institute of Technology. The Wenner-Gren Foundations are kindly acknowledged for a postdoctoral fellowship to J.-Q. L.References
- For recent reviews see: (a) A. Y. Chan, I. B. Perry, N. B. Bissonnette, B. F. Buksh, G. A. Edwards, L. I. Frye, O. L. Garry, M. N. Lavagnino, B. X. Li, Y. Liang, E. Mao, A. Millet, J. V. Oakley, N. L. Reed, H. A. Sakai, C. P. Seath and D. W. C. MacMillan, Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis, Chem. Rev., 2022, 50, 1485–1542 CrossRef PubMed; (b) J. Zhang, X. Lu, C. Shen, L. Xu, L. Ding and G. Zhong, Recent Advances in Chelation-Assisted Site- And Stereoselective Alkenyl C–H Functionalization, Chem. Soc. Rev., 2021, 50, 3263–3314 RSC; (c) S. Rej, Y. Ano and N. Chatani, Bidentate Directing Groups: An Efficient Tool in C–H Bond Functionalization Chemistry for the Expedient Construction of C–C Bonds, Chem. Rev., 2020, 120, 1778–1877 CrossRef PubMed; (d) R. Blieck, M. Taillefer and F. Monnier, Metal-Catalyzed Intermolecular Hydrofunctionalization of Allenes: Easy Access to Allylic Structures via the Selective Formation of C–N, C–C, and C–O Bonds, Chem. Rev., 2020, 120, 13545–13598 CrossRef CAS PubMed.
- For selected reviews see: (a) X. Tang, W. Wu, W. Zeng and H. Jiang, Copper-Catalyzed Oxidative Carbon–Carbon and/or Carbon–Heteroatom Bond Formation with O2 or Internal Oxidants, Acc. Chem. Res., 2018, 51, 1092–1105 CrossRef CAS PubMed; (b) C.-S. Wang, P. H. Dixneuf and J.-F. Soulé, Photoredox Catalysis for Building C–C Bonds from C(sp2)–H Bonds, Chem. Rev., 2018, 118, 7532–7585 CrossRef CAS PubMed; (c) D. Ravelli, S. Protti and M. Fagnoni, Carbon–Carbon Bond Forming Reactions via Photogenerated Intermediates, Chem. Rev., 2016, 116, 9850–9913 CrossRef CAS PubMed; (d) G. Brahmachari, Design for Carbon–Carbon Bond Forming Reactions Under Ambient Conditions, RSC Adv., 2016, 6, 64676–64725 RSC; (e) C.-L. Sun and Z.-J. Shi, Transition-Metal-Free Coupling Reactions, Chem. Rev., 2014, 114, 9219–9280 CrossRef CAS PubMed.
- Z. Wang, L. Hu, N. Chekshin, Z. Zhuang, S. Qian, J. X. Qiao and J.-Q. Yu, Ligand-Controlled Divergent Dehydrogenative Reactions of Carboxylic Acids via C–H Activation, Science, 2021, 374, 1281–1285 CrossRef CAS PubMed.
- Z. Zhuang and J.-Q. Yu, Lactonization as A General Route to β-C(sp3)–H Functionalization, Nature, 2020, 577, 656–659 CrossRef CAS PubMed.
- Y. Xia and J. Wang, Transition-Metal-Catalyzed Cross-Coupling with Ketones or Aldehydes via N-Tosylhydrazones, J. Am. Chem. Soc., 2020, 142, 10592–10605 CrossRef CAS PubMed.
- S. L. Schwikkard, Comprehensive Organic Functional Group Transformations II, Elsevier, 2nd edn, 2004, vol. 3, pp. 723–759 Search PubMed.
- (a) D. K. Mandal, Stereochemistry and Organic Reactions: Conformation, Configuration, Stereoelectronic Effects and Asymmetric Synthesis, Elsevier, 1st edn, 2021, pp. 375–419 Search PubMed; (b) R. J. Ouellette and J. D. Rawn, Organic Chemistry: Structure, Mechanism, and Synthesis, 2nd edn, Elsevier, 2018, pp. 595–623 Search PubMed; (c) P. A. Byrne and D. G. Gilheany, The Modern Interpretation of the Wittig Reaction Mechanism, Chem. Soc. Rev., 2013, 42, 6670–6696 RSC.
- V. G. Nenajdenko, Isocyanide Chemistry: Applications in Synthesis and Material Science, Wiley-VCH, 1st edn, 2012 Search PubMed.
- (a) X. Chen, A. Shatskiy, J.-Q. Liu, M. D. Kärkäs and X.-S. Wang, Synthesis of Sulfonylated Heterocycles via Copper-Catalyzed Heteroaromatization/Sulfonyl Transfer of Propargylic Alcohols, Chem. – Asian J., 2021, 16, 30–33 CrossRef CAS PubMed; (b) J. Liu, Z. Liu, P. Liao and X. Bi, Modular Synthesis of Sulfonyl Benzoheteroles by Silver-Catalyzed Heteroaromatization of Propargylic Alcohols with p-Toluenesulfonylmethyl Isocyanide (TosMIC): Dual Roles of TosMIC, Org. Lett., 2014, 16, 6204–6207 CrossRef CAS PubMed.
- (a) Y. Chen, A. Shatskiy, J.-Q. Liu, M. D. Kärkäs and X.-S. Wang, Silver-Promoted (4+1) Annulation of Isocyanoacetates with Alkylpyridinium Salts: Divergent Regioselective Synthesis of 1, 2-Disubstituted Indolizines, Org. Lett., 2021, 23, 7555–7560 CrossRef CAS PubMed; (b) L. Lv, Y. Chen, A. Shatskiy, J.-Q. Liu, X. Liu, M. D. Kärkäs and X.-S. Wang, Silver-Catalyzed [3+1+1] Annulation of Nitrones with Isocyanoacetates as an Approach to 1,4,5-Trisubstituted Imidazoles, Eur. J. Org. Chem., 2021, 964–968 CrossRef CAS.
- (a) Y. Fang, Z. Luo and X. Xu, Recent Advances in the Synthesis of Vinyl Sulfones, RSC Adv., 2016, 6, 59661–59676 RSC; (b) A. Megia-Fernandez, F. Hernandez-Mateo and F. Santoyo-Gonzalez, Vinyl Sulfone-Based Ferrocenylation Reagents: Applications in Conjugation and Bioconjugation, Org. Biomol. Chem., 2013, 11, 2586–2596 RSC; (c) J. C. Carretero, R. G. Arrayás, N. D. Buezo, J. L. Garrido, I. Alonso and J. Adrio, Recent Applications of Vinyl Sulfones and Vinyl Sulfoxides in Asymmetric Synthesis, Phosphorus, Sulfur Silicon Relat. Elem., 1999, 153, 259–273 CrossRef.
- (a) E. A. Ilardi, E. Vitaku and J. T. Njardarson, Data-Mining for Sulfur and Fluorine: An Evaluation of Pharmaceuticals to Reveal Opportunities for Drug Design and Discovery: Miniperspective, J. Med. Chem., 2014, 57, 2832–2842 CrossRef CAS PubMed; (b) E. Dunny, W. Doherty, P. Evans, J. P. G. Malthouse, D. Nolan and A. J. S. Knox, Vinyl Sulfone-Based Peptidomimetics as Anti-Trypanosomal Agents: Design, Synthesis, Biological and Computational Evaluation, J. Med. Chem., 2013, 56, 6638–6650 CrossRef CAS PubMed; (c) D. C. Meadows, T. Sanchez, N. Neamati, T. W. North and J. Gervay-Hague, Ring Substituent Effects on Biological Activity of Vinyl Sulfones as Inhibitors of HIV-1, Bioorg. Med. Chem., 2007, 15, 1127–1137 CrossRef CAS PubMed; (d) D. C. Meadows and J. Gervay-Hague, Vinyl Sulfones: Synthetic Preparations and Medicinal Chemistry Applications, Med. Res. Rev., 2006, 26, 793–814 CrossRef CAS PubMed.
- J. Liu, Z. Fang, Q. Zhang, Q. Liu and X. Bi, Silver-Catalyzed Isocyanide-Alkyne Cycloaddition: A General and Practical Method to Oligosubstituted Pyrroles, Angew. Chem., Int. Ed., 2013, 52, 6953–6957 CrossRef CAS PubMed.
- H. Fan, J. Peng, M. T. Hamann and J.-F. Hu, Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms, Chem. Rev., 2008, 108, 264–287 CrossRef CAS PubMed.
- (a) X. Huang, X. Cong, P. Mi and X. Bi, Azomethine-Isocyanide [3+2] Cycloaddition to Imidazoles Promoted by Silver and DBU, Chem. Commun., 2017, 53, 3858–3861 RSC; (b) J. Liu, Z. Liu, P. Liao, L. Zhang, T. Tu and X. Bi, Silver-Catalyzed Cross-Coupling of Isocyanides and Active Methylene Compounds by a Radical Process, Angew. Chem., Int. Ed., 2015, 54, 10618–10622 CrossRef CAS PubMed.
- J. Li, X. Liu, J. Deng, Y. Huang, Z. Pan, Y. Yu and H. Cao, Electrochemical Diselenylation of Indolizines via Intermolecular C–Se Formation with 2-Methylpyridines, α-Bromoketones and Diselenides, Chem. Commun., 2020, 56, 735–738 RSC.
- (a) W.-K. Yuan, T. Cui, W. Liu, L.-R. Wen and M. Li, When Ethyl Isocyanoacetate Meets Isatins: A 1, 3-Dipolar/Inverse 1, 3-Dipolar/Olefination Reaction for Access to 3-Ylideneoxindoles, Org. Lett., 2018, 20, 1513–1516 CrossRef CAS PubMed; (b) Z. Hu, J. Dong, Y. Men, Z. Lin, J. Cai and X. Xu, Silver-Catalyzed Chemoselective [4+2] Annulation of Two Isocyanides: A General Route to Pyridone-Fused Carbo- and Heterocycles, Angew. Chem., Int. Ed., 2017, 56, 1805–1809 CrossRef CAS PubMed; (c) Z. Hu, J. Dong and X. Xu, Silver-Catalyzed [3+2] Cycloaddition of Azomethine Ylides with Isocyanides for Imidazole Synthesis, Adv. Synth. Catal., 2017, 359, 3585–3591 CrossRef CAS; (d) Y. Gao, Z. Hu, J. Dong, J. Liu and X. Xu, Chemoselective Double Annulation of Two Different Isocyanides: Rapid Access to Trifluoromethylated Indole-Fused Heterocycles, Org. Lett., 2017, 19, 5292–5295 CrossRef CAS PubMed; (e) X. Zhang, X. Wang, Y. Gao and X. Xu, Silver-Catalyzed Formal [3+2]-Cycloaddition of α-Trifluoromethylated Methyl Isocyanides: A Facile Stereoselective Synthesis of CF3-Substituted Heterocycles, Chem. Commun., 2017, 53, 2427–2430 RSC.
- Z. Wang, L. Li, X. Zhang and X. Bi, Silver-Catalyzed Unstrained C(CO)-Alkyl Bond Scission via, [3+2]/retro-[3+2] Cycloaddition of Ketones with N-Isocyanoiminotriphenylphosphorane, Sci. China: Chem., 2021, 64, 1157–1163 CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A Consistent and Accurate ab initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- (a) A. D. Becke, Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior, J. Chem. Phys., 1993, 98, 5648–3100 CrossRef CAS; (b) A. D. Becke, Density-Functional Thermochemistry, III. The Role of Exact Exchange, Phys. Rev. A, 1988, 38, 3098–5652 CrossRef CAS PubMed; (c) C. Lee, W. Yang and R. G. Parr, Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- S. Kamijo, C. Kanazawa and Y. Yamamoto, Copper- or Phosphine-Catalyzed Reaction of Alkynes with Isocyanides. Regioselective Synthesis of Substituted Pyrroles Controlled by the Catalyst, J. Am. Chem. Soc., 2005, 127, 9260–9266 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: 1H, 13C and 19F NMR spectra, and experimental and computational details. CCDC 2102567. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qo00472k |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2022 |