Asymmetric total syntheses of five pyrrole-type Stemona alkaloids†
Abstract
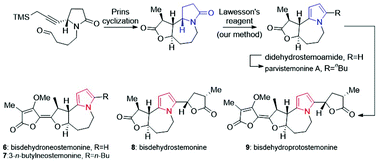
The asymmetric total syntheses of five pyrrole-type Stemona alkaloids and two stereoisomers were accomplished, among which 3-n-butylneostemonine and bisdehydroneostemonine were synthesized for the first time, and the NMR data of bisdehydroneostemonine were revised. Specifically, the 5/7 skeleton of stemoamide was established by employing Prins cyclization, and didehydrostemoamide was obtained from stemoamide using our method of Lawesson's reagent promoted pyrrole synthesis. Using didehydrostemoamide as the common intermediate, five pyrrole Stemona alkaloids were divergently synthesized. This research has enriched the transformation pattern of Stemona alkaloids.


 Please wait while we load your content...
Please wait while we load your content...