A self-cleaning hydrophobic MOF-based composite for highly efficient and recyclable separation of oil from water and emulsions†
Abstract
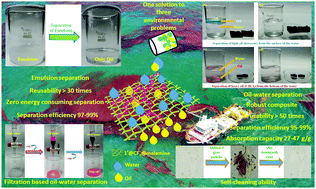
A hydrophobic MOF (1′@CF3) was synthesized by post-synthetic modification to anchor a –CF3 group to the Zr-BDC-OH MOF (1′). The hydrophobic property of the MOF was used for the preparation of a robust hydrophobic composite (1′@CF3@melamine) with melamine sponge. The water contact angle of 1′@CF3@melamine was found to be 145 ± 1°. The hydrophobic composite was demonstrated to separate oil–water mixtures and water-in-oil emulsions even under harsh aquatic environments. The oil–water and emulsion separations were carried out in an easy and fast way without the consumption of energy. The absorption capacity and separation efficiency of the composite for a wide variety of oils were found to be 27–47 g g−1 and 95–99%, respectively. The material showed high recyclability of up to 50 cycles for oil–water separation and for 30 cycles for water-in-oil emulsion separation. In addition, a hydrophobic polymer@1′@CF3-coated glass substrate displayed excellent self-cleaning properties. Furthermore, inexpensive and facile gravity-driven filtration and against gravity-based separation techniques for different oils were developed by employing the composite.


 Please wait while we load your content...
Please wait while we load your content...