Open Access Article
Open Access ArticleSimple, quick and green isolation of cannabinoids from complex natural product extracts using sustainable mesoporous materials (Starbon®)†
Thomas M.
Attard
ab,
Christopher
Goodwin
a,
Povilas
Nalivaika
a,
Jennifer
Attard
 ac,
Vitaliy L.
Budarin
a,
Alexandra
Lanot
d,
Damien
Bove
b,
James H.
Clark
ac,
Vitaliy L.
Budarin
a,
Alexandra
Lanot
d,
Damien
Bove
b,
James H.
Clark
 a and
Con Robert
McElroy
a and
Con Robert
McElroy
 *a
*a
aDepartment of Chemistry, University of York, York, YO105DD, UK. E-mail: rob.mcelroy@york.ac.uk
bRX Extraction Ltd, Unit 10, Rowan Trade Park, Bradford, BD4 8TQ, UK
cCircular Bioeconomy Research Group, Munster Technological University Kerry, V92 CX88, Ireland
dDepartment of Biology, University of York, York, YO105DD, UK
First published on 25th April 2022
Abstract
The current process to purify cannabidiol (CBD) from C. sativa extract is long and intensive, requiring several steps such as winterification for 48 hours at −45 °C and high-temperature, high vacuum distillation. These processes are capital intensive and generate large amounts of toxic solvent waste. In contrast, the solid phase extraction (SPE) methodology proposed herein will change the way CBD is obtained, doing so in a single step that is fast and reusable. Furthermore, the new process is simple and easily implemented and does not require any intensive operator training. Starbon® A300 was successfully employed as the stationary phase in SPE taking Cannabis sativa extract in hexane to selectively physisorb the cannabinoids onto the surface, followed by ethanol to bring about desorption at up to 93% (by GC-FID). A similar one pot system was also proven, using Fedora hemp stem dust as feedstock, with extraction and adsorption in supercritical CO2 followed by desorption in ethanol.
Introduction
There has been a substantial increase in the global demand for cannabinoids from Cannabis sativa L., especially with the ever-increasing legalisation of medicinal Cannabis; Cannabis for medical use has now been legalised in a host of countries.1 Cannabidiol (CBD) has been the main cannabinoid investigated for medical use due to its non-psychoactive properties and plethora of pharmacological properties in the treatment of neurological and central nervous system (CNS) disorders, consequently possessing significant therapeutic importance.2 The medicinal advances for use of CBD have seen investigations in seizures,3 spasms,4 migraines,5 pain relief,6 anxiety,7 glaucoma,8 anti-nausea,9 anti-bacterial10 and anti-inflammatory purposes.11 It should be noted that the two dominant cannabinoids in the plant are the acidic forms of CBD and tetrahydrocannabinol (THC), i.e. CBDA and THCA, these are normally converted into CBD and THC via a decarboxylation step.12One of the major hurdles in CBD product utilisation has always been separation and purification. While the extraction of cannabinoids is a relatively straightforward process, the conventional purification of cannabinoids is a long and intensive process. The desired cannabinoids, having the specific pharmaceutical/nutraceutical activity, are co-extracted with a plethora of other unwanted hydrophobic compounds, which leads to a number of potential major problems, from unwanted texture and appearance to the lowering of activity/performance of the target molecule.13 This results in the need for separation and purification technology that is highly energy and time-consuming as well as costly. The conventional extraction and purification is a step-wise process, each step increasing the purity of the cannabinoid content and comprises extraction, winterisation (a time-consuming step, taking between 1–3 days to ensure that all waxes and lipids are removed), chlorophyll removal, short path distillation (decarboxylation occurs here if the sample has not already undergone heating) and finally chromatography (flash, high-performance liquid chromatography (HPLC), centrifugal partition chromatography (CPC) etc.).14 These technologies have high capital costs associated with them, are time-consuming processes and often use large volumes of solvent. In recent commercial applications selective cannabinoid purification was achieved using a series of fractionating solvent systems and/or chromatography.15,16 However, both column and liquid chromatographic methods involve long run times, small sample loadings and poor yields. Furthermore, techniques using large volumes of solvents or column effluents have high process mass intensities (PMI)17 using solvents such as dichloromethane or chloroform.
Starbon® materials (“Starbons”), first developed in 2006, are a bio-based mesoporous material obtained in 3 steps; (1) gelling (2) drying (3) pyrolysis.18 The materials take their name from starch and carbonisation to give Starbon, although they can be produced from any polysaccharide that can be gelled, most notably alginic acid19 from seaweed and pectin20 from fruit waste. Starbons are traditionally classified by a letter and 3 numbers e.g. S300, where the letter defines the starting polysaccharide; S starch, A alginic acid, P pectin, while the number denotes the pyrolysis temperature. The potential to vary the polysaccharide used and the pyrolysis temperature, has allowed for the materials’ properties to be highly tunable to the required application. To date, there have been over 50 Starbon related publications since their discovery, in a variety of fields including catalysis,21 gas capture,22 batteries,23 metal recovery,24 and water treatment.25 Additionally there are two live patents relating to Starbons26,27 with an associated start-up company.28
A significant amount of published work on Starbons exists in the field of separation. Early work demonstrated the uptake of two model dyes from aqueous systems as compared to Norit® activated carbon.29 Both mesoporosity and surface chemistry were found to be far more significant in dye uptake as opposed to surface area, with the highest loading observed being 186 mg g−1. Similar work showed uptake and recovery of phenolic compounds from an aqueous environment.30 In each case the adsorption was carried out in an ideal system of the target phenol in water, with an uptake of between 87 to 139 mg g−1. Desorption was carried out over a 24 hour period in an aqueous system at a pH above 11, with a recovery range of 7–40%. Starbons performed well in the adsorption and controlled desorption of 4 plant growth promoters in aqueous systems.31 Adsorption capacity varied between 76 and 370 mg g−1 while desorption varied between 2 to 47%, depending on the plant growth promoter. A more complex system was investigated, focusing on uptake of a series of phenolic compounds in methanol.32 Each compound was investigated individually for uptake and then tested using a model system of all 10 compounds again in methanol. Results showed some materials had irreversible uptake, while in others, all compounds could be recovered. However, the solid phase extraction (SPE) systems were not tested on real, complex extracts so selective adsorption of the target molecules was not investigated (i.e. whether there would be competition from non-target molecules).
In this work we apply reusable mesoporous Starbons in simple, rapid SPE of cannabinoids from a crude complex extract in a single step giving a product of high purity, with the scope of replacing the number of time-consuming and energy-consuming purification steps highlighted above.
Results
The initial step in this work required the generation of cannabinoid rich complex materials. Flowers from a high cannabinoid Cannabis sativa plants grown in US and dust obtained from the processing of Fedora hemp stems for fibre application were extracted using supercritical carbon dioxide (scCO2) to give a complex mixture in yields of 10.4% and 1.1% respectively. Gas chromatography flame ionisation detector (GC-FID) analysis of the crude extracts indicates multiple classes of compounds including terpenes, long-chain saturated and unsaturated fatty acids, fatty alcohols, aldehydes, n-alkanes, cannabinoids, sterols and wax esters.All Starbons used in this work have been produced at scale on a commercial pilot plant, it is their application as opposed to their synthesis which is being investigated. Alginic acid based Starbons were selected as they are produced at scale more readily than other Starbon materials. Starbons Ltd routinely pyrolysis their material to either 300, 450 or 800 °C as this gives a range of surface properties from hydrophilic to hydrophobic while also ensuring stability as compared to the hydroscopic feedstock (ESI† S1). As such alginic acid derived A300, A450 and A800 Starbon were commercially packed into Instrument Top Sample Preparation (ITSP™) cartridges for use on a Gerstel Multi Purpose Sampler (MPS, ESI† S2). Crude C. sativa extract was taken up in 3 different organic solvents, non-polar hexane, moderately polar ethyl acetate and polar ethanol. These were passed through A450 as a representative Starbon sample and the eluted solution analysed via GC to ascertain adsorption of the cannabinoid target compounds from solution. Good adsorption was observed in hexane, moderate adsorption in ethyl acetate and limited uptake from ethanol. Hexane was therefore selected as the adsorption solvent and ethanol selected as desorption solvent. GC chromatography of these early results shows the selective adsorption and desorption of cannabinoids using Starbon A300.
The full program used by the MPS is given in the ESI† (Table 1) but the SPE methodology is as follows. The Starbon is first washed with the adsorption solvent to prep the solid phase in the desired solvent system. The hemp extract is then passed through the Starbon and the solvent collected – this is the adsorption sample. In order to ensure only material that is strongly bound to the Starbon is retained, more adsorption solvent is passed through the material to wash any residual content. The desorption solvent is then passed through the Starbon to remove any physisorbed material and collected – this is the desorption sample (Fig. 1). The Starbon is washed with more desorption solvent to ensure all bound material has been removed from the pore network. The Starbon is then re-conditioned with the desorption solvent for the next run.
| A300 | A300 post adsorption | A300 post desorption | |
|---|---|---|---|
| S BET surface m2 g−1 | 95.1338 | 40.1390 | 90.0233 |
| V BJHp cm3 g−1 | 0.2350 | 0.1620 | 0.2092 |
| Micropore volBJH cm3 g−1 | 0.0182 | 0 | 0.0165 |
| Mesopore volBJH cm3 g−1 | 0.2321 | 0.1726 | 0.2026 |
| DiameterBJH nm | 11.4797 | 11.8821 | 11.2838 |
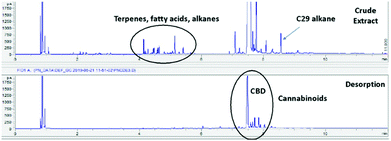 | ||
| Fig. 1 GC chromatograph of HTs: (Crude Extract) Crude C. sativa extract, (Desorption) Ethanol desorption phase after passing through A300. Higher definition figures are available in the ESI.† | ||
The choice of desorption solvents was also investigated. The rational being that a polar solvent was required so that the CBD has greater affinity for the mobile phase than the stationary phase (Starbon). Methanol, ethanol and propanol were trailed as desorption solvents, with hexane as adsorption solvent. Methanol gave 53% CBD recovery, ethanol 84% and propanol 79%. Presumably this result is due to ethanol having enough non-polar content to rapidly exchange with hexane, while still being sufficiently polar to disrupt binding of CBD to the surface (ESI† S3).
A series of repeat adsorption/desorption runs were conducted reusing the same Starbon material and cyclohexanone as an external standard. Interestingly, the first cycle always gave reduced desorption, suggesting a degree of irreversible adsorption and conditioning of the Starbon. This is probably due to the blocking of some smaller pores. Activity was benchmarked against Norit® Activated Carbon (AC). AC exhibited poor CBD uptake, with minimal reduction of the cannabinoid peaks in the adsorption solvent compared to the control. Fig. 2 indicates the superiority of A300 in terms of cannabinoid recovery, with a total extraction of 71.68 ± 4.1%. A800 performed well, while A450 results indicated more cannabinoids travelled directly through the cartridge than preferentially adsorbed onto the Starbon. As the materials increase in carbonisation temperature, they increase in surface area, pore volume and micropore volume. These properties alone do not explain the observed trend as the performance is A300 > A800 > A450. This suggests that the surface chemistry of Starbon is the dominating property.
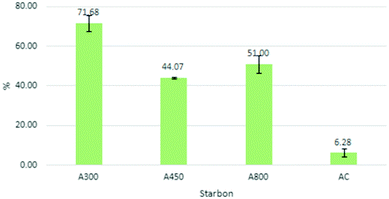 | ||
| Fig. 2 A-series % CBD recovery quantified by external standard, C. sativa extract loaded at 19 mg g−1 adsorbent. | ||
The lower temperature A300 material is more hydrophilic, containing aldehyde, carboxylic acid, ketone and OH functionality. The 450 contains fewer hetero atoms as well as significant unsaturation, while the 800 material is too conductive for an IR to be obtained as it is principally a conjugated aromatic system. This structure temperature relationship has been extensively discussed in the literature,18,19 with a key figure illustrating this reproduced in the ESI† (S1). In all cases the cannabinoids here are in their acidic form, decarboxylation only occurs upon heating. There appear to be stronger polar interactions between the cannabinoids and A300 than with π–π stacking between the aromatic ring of the cannabinoids and the poly-aromatic A800 surface. Also of significance is the speed of uptake, the C. sativa extract had a contact time of just 30 seconds to the material (as determined by the slowest possible add speed on the MPS). If contact time is reduced to just under 2 seconds, adsorption and recovery of cannabinoids is reduced by almost 50% but this still indicates that initial uptake is rapid, followed by a longer period to reach equilibrium (ESI† S4). Desorption speed was also investigated but the contact time of ethanol on the Starbon did not influence the %CBD recovery. This suggests that breaking the cannabinoid-Starbon interaction occurs rapidly in a polar solute.
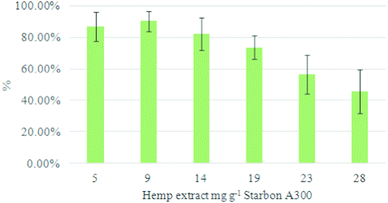
The adsorption capacity of A300 was investigated to see how much CBD could be loaded from the HT extract, thus determining the optimal loading of CBD. This was done by preparing a series of HT extract solutions of varying concentrations (mg mL−1), thus varying the loading of extract to Starbon and determining the percentage recovery of CBD for each concentration. The results are summarised in Fig. 3.
Low concentrations showed high levels of CBD recovery. All runs were repeated five times for reliability, although with the initial run results discarded as they are always significantly poorer. This demonstrates the ease of repeatability of this method and the relative consistency of the A300 for multiple extraction runs. The level of CBD recovery seems to decrease above loadings of 14 mg g−1 at which point more significant levels of cannabinoids are found in both the adsorb and the washing steps. This indicates that the loading of CBD is too high to be completely physisorbed onto the Starbon and so some remains in solution. As such the amount of CBD detected in the adsorption sample increases. The excess of CBD also means more canabinoids are retained in the wetted Starbon without being bound to the surface. This is removed in the first washing step using the adsorption solvent. Results at 19 mg g−1 are almost within error and at this higher loading, reasonable to take forward as an optimised loading.
The crude hemp top extract has been characterised used GC-FID and GC-MS. Key components have been identified and the relevant chromatograms annotated in the ESI.†
The A300 Starbon was scaled up from a 16 mg scale to a 10 g scale to assess potential industrial viability (ESI† S5). This would represent a potential step forward for green extraction processes. The same method and washing steps were carried out manually in line with the MPS sequence. The extraction was run twice to negate the effect of the first extraction being inconsistent, as explained previously. As with the small-scale work, conditioning of the Starbon was vital as the first extraction run gave a poor CBD recovery in the desorption phase. A CBD recovery of 97% was extracted on the second run and quantified via the external standard. This is compared with a value of 71% obtained on the ITSP cartridge. The flow rate of the large-scale experiment was slower than the 10 uLs−1 (30 second contact time) of the small-scale work as only a slight vacuum was applied. This is a potential reason for the higher extraction yield of CBD observed. This suggests that contact time between the extract and the Starbon is greater here than in the small-scale automated work suggesting the system requires time to fully equilibrate. Large scale SPE also allowed sufficient sample for proton and carbon NMR (ESI† S6 and S7) as well as Liquid Injection Field Desorption Ionisation mass spectrometry (LIFDI-MS, S8 and S9, ESI†).
The NMR data confirmed that both the desorbed product is of relatively high purity and that the cannabinoid has principally been isolated in its acidic form, CBDA. All GC data showed CBD due to decarboxylation at the temperatures employed. LIFDI-MS indicates that there are a number of higher molecular weight compounds still present within the desorb sample, but significantly there are no signals related to triglyceride species 869–943 m/z.
The large scale SPE trials also meant sufficient Starbon material was available for analysis by nitrogen porosimetry (Table 1). Upon uptake of cannabinoids and other material that physisorbs onto the surface of A300, all micropores have become blocked and the mesopore volume has also dropped by over 25%, with an overall pore volume decrease of 31%. This is reflected in the drop in surface area and the increase in pore diameter. On desorption the surface area is only 5% lower than the original material. Overall pore volume is now only 11% lower, with a loss of 9% of microporosity and 13% of mesoporosity. The 20% recovered pore volume being attributed to that involved in binding of material that undergoes selective adsorption and desorption. As a greater relative number of larger pores have been lost, the average pore diameter is now smaller than the original material. This data shows that mesopores and micropores are both important in the selective uptake of cannabinoids and also demonstrates a degree of irreversible conditioning in keeping with other experimental data.
Finding a replacement for hexane as the adsorption solvent was investigated due to its classification as “Suspected to be Toxic to Reproduction”.33 As there are not many non-polar, green solvents with sufficient volatility to allow recovery of extracts, scCO2 was investigated as a replacement for hexane as the adsorption solvent.34 For these trials, A300 was used to pack a column for adsorption/desorption of CBD using scCO2 as the adsorption solvent and ethanol as the desorption solvent. A 25 ml column packed with 7 g of A300 was placed between the extractor and the back pressure regulator to allow flow of extractives through the Starbon.
The extractor was loaded with C. sativa dust (HD) (60 grams) and extraction carried out at optimised conditions of 65 °C and 400 bar. HD is an interesting feedstock as the number and complexity of compounds in the extract is significantly higher than that found in HT, including long-chain hydrocarbons, saturated and unsaturated fatty acids, fatty alcohols, fatty aldehydes, wax esters and sterols.35 Also of note, the cannabinoid content in this biomass is significantly lower. As with earlier SPE work, the first extraction, adsorption and desorption results were not used as this conditioned the solid phase. Results from the second run displayed in Fig. 4 clearly show that the majority of volatile components in the extract are not retained by the A300 column and were removed with the scCO2; i.e. little adsorption occurred. It is also evident that under such conditions and with a loading of roughly 94 mg g−1 (extract to A300), the cannabinoids are either overloading the Starbon and/or capacity has been reduced. The desorbed phase showed that the cannabinoids were preferentially adsorbed onto the A300, along with short chain fatty acids.
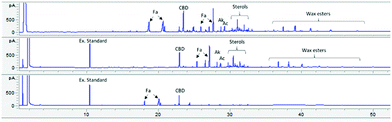 | ||
| Fig. 4 GC-FID chromatograms representing 3 extracts. From top to bottom; Original hemp dust extract, adsorption, desorption phases. Fa – fatty acids, Ak – alkanes, Ac – Alcohols. Higher definition figures are available in the ESI,† full chaticterisation of hemp dust extract is available in the literature.35 | ||
GC-EI-MS analysis conducted on the one pot hemp dust desorbed phase showed the following cannabinoids; Cannabidiol (CBD), Cannabigerol (CBG), Cannabichromene (CBC) and Tetrahydrocannabinol (THC). Interestingly CBG, CBC and THC were not previously observed in scCO2 hemp dust extracts carried out without solid phase extraction (SPE). This confirms that Starbon SPE enriches the cannabinoid content as compared to the crude extract. All cannabinoids appear to have affinity to the Starbon material, although some may do so more preferentially. This also needs to be studied more thoroughly in future work.
The innovation and green credentials of Starbon SPE setup is the application of a renewable, bio-derived mesoporous material that replaces the need for a number of hazardous steps. The industrial process to purify cannabinoids from hemp extract is long and intensive, requiring several steps such as winterification for two days at −45 °C and high-temperature, high vacuum distillation. These processes generate large amounts of toxic solvent waste and are energy intensive. In contrast, the work shown herein uses a single step to obtain cannabinoids that is fast and reusable. It makes use of a lower amounts of ethanol which is a food-grade solvent and has the potential to apply scCO2 as the adsorption solvent.
The work herein shows promise for developing a one-pot system, wherein extraction and purification of cannabinoids from C. sativa occurs simultaneously, reducing significantly the need for extensive post-purification processes.
Experimental
Chemicals
Hexane, deuterated chloroform and cyclohexanone were purchased from Sigma Aldrich. Ethanol and 2-propanol were purchased from Fisher Chemical.Cannabis sativa samples
Cannabis sativa tops (HTs) were provided by RX Extraction. The Cannabis sativa sample was obtained from the US due to the high levels of CBDA (ca. 20% w/w). Hemp dust (HD) was obtained from Harrison Spinks hemp processing facility in North Yorkshire, UK.Starbons
Solid-phase extraction cartridges, obtained from ITSP Solutions Inc., were packed with 16 mg of Starbon. Alginic acid-based Starbons (A-series) were synthesised through Starbons Ltd at the biorenewables development centre (BDC) in Dunnington, York and are derived from kelp. Different Starbons were obtained by varying preparation temperatures: 300, 450 and 800 °C.Purification and isolation of cannabinoid
Analysis of C. sativa extracts Auto sampled adsorption and desorption of crude C. sativa extracts; C. sativa extracts (20 mg) were dissolved in hexane (20 mL) and placed on a Gerstel multipurpose sampler (MPS) purchased from Anatune. Details of the adsorption, desorption system are given in ESI†Table 1. Each repetition on the autosampler consisted of three key steps: adsorption, washing and desorption. The Starbon cartridge was flushed with hexane immediately prior to the run to condition the adsorbent. C. sativa extract (300 μL) was passed through the ITSP cartridge, and the adsorption phase collected. The cartridge was then flushed with hexane (500 μL). The needle was sequentially washed with ethanol, ethyl acetate and hexane (500 μL). Subsequently, ethanol (300 μL) was then passed through the cartridge and collected to give the desorption phase. The cartridge was then flushed with ethanol (500 μL) followed by hexane (500 μL) ready for the next SPE run.A300 scale-up
190 mg of HT extract was dissolved in 166 mL of hexane and passed through a plug of A300 Starbon (10 g) in vacuo and the adsorption phase collected. The cartridge was then washed with 166 mL of hexane. The ethanol desorption solvent (166 mL) was subsequently passed through the Starbon and collected. This was then washed sequentially with ethanol and hexane (166 mL) to prepare for additional runs. The adsorption/desorption run was done twice. The desorption solvent was removed in vacuo to afford products to undergo further analysis. Aliquots of each phase were collected and external standard was added to quantify the results.scCO2 extraction/Starbon
isolation Supercritical CO2 has already been established as a viable methodology for extraction of hydrophobic constituents containing cannabinoids from various C. sativa sources.36 Herein the direct isolation of cannabinoids from hemp dust in one pot is discussed.Lab-scale supercritical fluid extraction of C. sativa biomass
All scCO2 extractions were carried out using a SFE-500 extractor provided by Thar technologies. 80 g of milled C. sativa biomass was placed into the 500 cm3 extraction vessel and connected to the extraction system. Liquid CO2 was then pumped to the required pressure (maintained by an automated back pressure regulator – ABPR) and passed through an inline pre-heater maintained at the desired extraction temperature. The system was run for 2 hours at a flow rate of 30 g min−1 CO2 with continual collection of extract.One pot system
In the one pot system, a stainless steel 10 cm3 column was filled with 7 g of Starbon A300 and connected between the extractor and the ABPR. The system was set to the desired temperature and pressure and run for 2 hours at 30 g min−1 and the extract collected and analysed. Post extraction, ethanol was passed through the column for 10 minutes using the co-solvent modifier pump at a 10 ml min−1 flowrate to give the desorption fraction. The column was reconditioned with scCO2 at 120 bar, 50 °C for 30 minutes at 30 g min−1.Gas chromatography flame ionisation detection (GC-FID)
Samples were quantified by using an Agilent Technologies 7890B GC system and a Hewlett Packard HP 6890 Series GC system. Both GCs were run using flame ionisation detection methods and on identical methods. A Rxi-5HT capillary column (30 m × 250 μm × 0.25 μm nominal) was fitted at constant pressure of 20.16 psi. Helium was the carrier gas used. Both the injector temperature and FID detector temperature were set at 320 °C. 1 μL samples were injected by automated injection, with a split ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The oven temperature profile was as follows: initial temperature of 50 °C, increased to 300 °C at a rate of 30 °C min−1, held at this temperature for 5 mins. Quantification was carried out by means of an external standard (cyclohexanone).
1. The oven temperature profile was as follows: initial temperature of 50 °C, increased to 300 °C at a rate of 30 °C min−1, held at this temperature for 5 mins. Quantification was carried out by means of an external standard (cyclohexanone).
Gas chromatography mass spectrometry (GC-EIMS)
Gas chromatography electronic ionisation mass spectrometry (GC-MS) was run on a PerkinElmer Clarus 500 GC coupled with a Clarus 560 S mass spectrometer. This was run using an Rxi®-5HT 30 m × 250 μm × 0.25 μm at a pressure of 21.4 psi. The carrier gas was helium. The injector temperature is set to 300 oC and the flow rate of 1.0 mL min−1. The method was run at 50 °C starting temperature. The Clarus 500 quadrupole mass spectra were operated in the electron ionisation mode (EI +) at 70 eV, a source temperature of 300 oC, quadrupole in the scan range of 30–1200 amu per second.Porosimetry
Nitrogen-adsorption analysis was carried out using an ASAP 2020 volumetric adsorption analyser from Micrometrics. Measurements were performed at 77 K. Samples were degased at 150 °C, 50 μm Hg for 4 h prior to analysis. The Brunauer–Emmett–Teller (BET) theory was used to determine the surface area, and the Barret–Joyner–Halenda (BJH) equation was applied to determine the mesoporous volume and the pore size.Conclusions
A rapid SPE system using Starbon mesoporous materials has been found to enable rapid and high purity isolation of cannabinoids from complex crude mixtures. Starbons derived from alginic acid pyrolysed to 300 °C gave the best performance, presumably due to surface interactions. The solvent adsorb and desorb system of hexane and ethanol can be potentially replaced with a one pot extraction and separation using scCO2. This shows promise for the development of a one-pot extraction and purification process whereby the cannabinoids are separated from impurities while being extracted from the biomass.Author contributions
Thomas Attard, conceptualisation, supervision, formal analysis, writing – original draft; Christopher Goodwin, investigation, formal analysis, writing – original draft; Povilas Nalivaika, investigation, formal analysis; Jennifer Attard, investigation, formal analysis, writing – review & editing; Vitaliy L. Budarin, methodology; Alexandra Lanot, resources; Damien Bove, resources, funding acquisition; James H. Clark, supervision, writing – review & editing; Con Robert McElroy conceptualisation, supervision, formal analysis, writing – original draft.Conflicts of interest
Dr Rob McElroy is CTO of Starbons Ltd, a start-up from the University of York.Acknowledgements
We would like to thank Mr Karl Heaton, University of York MS centre for running of LIFDI-MS, Mr John Horsefield from Harrison Spinks Beds Ltd for supply of hemp dust, Leeds City Region Enterprise Partnership for part-funding the research.Notes and references
- M. B. Bridgeman and D. T. Abazia, Medicinal cannabis: history, pharmacology, and implications for the acute care setting, Pharm. Ther., 2017, 42, 180 Search PubMed.
- D. J. Sholler, L. Schoene and T. R. Spindle, Therapeutic Efficacy of Cannabidiol (CBD): a Review of the Evidence From Clinical Trials and Human Laboratory Studies, Curr. Addict. Rep., 2020, 7, 405–412 CrossRef PubMed.
- P. H. Patra, et al., Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models, Epilepsia, 2019, 60, 303–314 CrossRef CAS PubMed.
- S. A. Hussain, D. J. Dlugos, M. R. Cilio, N. Parikh, A. Oh and R. Sankar, Synthetic pharmaceutical grade cannabidiol for treatment of refractory infantile spasms: A multicenter phase-2 study, Epilepsy Behav., 2020, 102, 106826 CrossRef PubMed.
- L. L. Mechtler, F. M. Gengo and V. H. Bargnes, Cannabis and Migraine: It’s Complicated, Curr. Pain Headache Rep., 2021, 25, 16 CrossRef PubMed.
- S. Boyaji, J. Merkow, R. N. M. Elman, A. D. Kaye, R. J. Yong and R. D. Urman, The Role of Cannabidiol (CBD) in Chronic Pain Management: An Assessment of Current Evidence, Curr. Pain Headache Rep., 2020, 24, 4 CrossRef PubMed.
- J. W. Skelley, C. M. Deas, Z. Curren and J. Ennis, Use of cannabidiol in anxiety and anxiety-related disorders, J. Am. Pharm. Assoc., 2020, 60, 253–261 CrossRef PubMed.
- A. Vallée, Y. Lecarpentier and J.-N. Vallée, Cannabidiol and the Canonical WNT/β-Catenin Pathway in Glaucoma, Int. J. Mol. Sci., 2021, 22, 3798 CrossRef PubMed.
- E. M. Rock, et al., Evaluation of repeated or acute treatment with cannabidiol (CBD), cannabidiolic acid (CBDA) or CBDA methyl ester (HU-580) on nausea and/or vomiting in rats and shrews, Psychopharmacology, 2020, 237, 2621–2631 CrossRef CAS PubMed.
- M. Aqawi, R. V. Sionov, R. Gallily, M. Friedman and D. Steinberg, Anti-Bacterial Properties of Cannabigerol Toward Streptococcus mutans, Front. Microbiol., 2021, 12, 656471 CrossRef.
- F. Sunda and A. Arowolo, A molecular basis for the anti-inflammatory and anti-fibrosis properties of cannabidiol, FASEB J., 2020, 34, 14083–14092 CrossRef CAS PubMed.
- T. Moreno, P. Dyer and S. Tallon, Cannabinoid Decarboxylation: A Comparative Kinetic Study, Ind. Eng. Chem. Res., 2020, 59, 20307–20315 CrossRef CAS.
- R. Brenneisen, Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents, in: Marijuana and the Cannabinoids, ed. M. A. ElSohly, Humana Press, 2007 Search PubMed.
- M. Valizadehderakhshan, A. Shahbazi, M. Kazem-Rostami, M. S. Todd, A. Bhowmik and L. Wang, Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review, Agriculture, 2021, 11, 384 CrossRef.
- X. N. Roura, Methods of purifying cannabinoids, compositions and kits thereof, Patent US20180000879A1, 2017 Search PubMed.
- X. N. Roura, Methods of purifying cannabinoids using liquid: liquid chromatography, Patent US11034639B2, 2019 Search PubMed.
- C. Jimenez-Gonzalez, C. S. Ponder, Q. B. Broxterman and J. B. Manley, Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry To Drive More Sustainable Processes, Org. Process Res. Dev., 2011, 15, 912–917 CrossRef CAS.
- V. Budarin, et al., Starbons: New Starch-Derived Mesoporous Carbonaceous Materials with Tunable Properties, Angew. Chem., Int. Ed., 2006, 45, 3782–3786 CrossRef CAS PubMed.
- R. J. White, V. Budarin, R. Luque, J. H. Clark and D. J. Macquarrie, Tuneable porous carbonaceous materials from renewable resources, Chem. Soc. Rev., 2009, 38, 3401 RSC.
- R. J. White, V. L. Budarin and J. H. Clark, Pectin-Derived Porous Materials, Chem. – Eur. J., 2010, 16, 1326–1335 CrossRef CAS PubMed.
- A. S. Matharu, S. Ahmed, B. Almonthery, D. J. Macquarrie, Y.-S. Lee and Y. Kim, Starbon/High-Amylose Corn Starch-Supported N-Heterocyclic Carbene-Iron(III) Catalyst for Conversion of Fructose into 5-Hydroxymethylfurfural, ChemSusChem, 2018, 11, 716–725 CrossRef CAS PubMed.
- R. A. Milescu, et al., The role of surface functionality of sustainable mesoporous materials Starbon® on the adsorption of toxic ammonia and sulphur gasses, Sustainable Chem. Pharm., 2020, 15, 100230 CrossRef.
- S. Kim, et al., Green electrode processing using a seaweed-derived mesoporous carbon additive and binder for LiMn2O4 and LiNi1/3Mn1/3Co1/3O2 lithium ion battery electrodes. Sustainable, Energy Fuels, 2019, 3, 450–456 CAS.
- A. Muñoz García, et al., Starch-derived carbonaceous mesoporous materials (Starbon®) for the selective adsorption and recovery of critical metals, Green Chem., 2015, 17, 2146–2149 RSC.
- M. A. Tony, H. L. Parker and J. H. Clark, Treatment of laundrette wastewater using Starbon and Fenton's reagent, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2016, 51, 974–979 CrossRef PubMed.
- V. Budarin, J. H. Clark, R. Luque, D. J. Macquarrie, K. Milkowski and R. J. White, Carbonaceous materials, Patent US8790548B2, 2014 Search PubMed.
- R. J. White, J. H. Clark, V. L. V. Budarin and D. J. Macquarrie, Polysaccharide derived materials, Patent US9457338B2, 2016 Search PubMed.
- https://www.starbons.com/. (accessed 28/11/21).
- H. L. Parker, A. J. Hunt, V. L. Budarin, P. S. Shuttleworth, K. L. Miller and J. H. Clark, The importance of being porous: polysaccharide-derived mesoporous materials for use in dye adsorption, RSC Adv., 2012, 2, 8992 RSC.
- H. L. Parker, V. L. Budarin, J. H. Clark and A. J. Hunt, Use of Starbon for the Adsorption and Desorption of Phenols, ACS Sustainable Chem. Eng., 2013, 1, 1311–1318 CrossRef CAS.
- J. M. Shannon, J. H. Clark, M. Ingrid De Heer, T. Ekblad and A. S. Matharu, Kinetic and Desorption Study of Selected Bioactive Compounds on Mesoporous Starbons: A Comparison with Microporous-Activated Carbon, ACS Omega, 2018, 3, 18361–18369 CrossRef CAS.
- V. G. Zuin, et al., Polysaccharide-derived mesoporous materials (Starbon®) for sustainable separation of complex mixtures, Faraday Discuss., 2017, 202, 451–464 RSC.
- https://echa.europa.eu/substance-information/-/substanceinfo/100.003.435. (accessed 28/11/21).
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, CHEM21 selection guide of classical-and less classical-solvents, Green Chem., 2016, 18, 288–296 RSC.
- T. M. Attard, C. Bainier, M. Reinaud, A. Lanot, S. J. McQueen-Mason and A. J. Hunt, Utilisation of supercritical fluids for the effective extraction of waxes and Cannabidiol (CBD) from hemp wastes, Ind. Crops Prod., 2018, 112, 38–46 CrossRef CAS.
- S. Qamar, Y. J. M. Torres, H. S. Parekh and J. Robert Falconer, Extraction of medicinal cannabinoids through supercritical carbon dioxide technologies: A review, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2021, 1167, 122581 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qm00028h |
| This journal is © the Partner Organisations 2022 |