Open Access Article
Open Access ArticleAd aurum: tunable transfer of N-heterocyclic carbene complexes to gold surfaces†
Nathaniel L.
Dominique
 a,
Ran
Chen
b,
Alyssa V. B.
Santos
a,
Ran
Chen
b,
Alyssa V. B.
Santos
 b,
Shelby L.
Strausser
c,
Theodore
Rauch
a,
Chandler Q.
Kotseos
b,
Shelby L.
Strausser
c,
Theodore
Rauch
a,
Chandler Q.
Kotseos
 a,
William C.
Boggess
a,
Lasse
Jensen
a,
William C.
Boggess
a,
Lasse
Jensen
 *b,
David M.
Jenkins
*b,
David M.
Jenkins
 *c and
Jon P.
Camden
*c and
Jon P.
Camden
 *a
*a
aDepartment of Chemistry and Biochemistry, University of Notre Dame, Notre Dame, Indiana 46556, USA. E-mail: jon.camden@nd.edu
bDepartment of Chemistry, Penn State University, 101 Chemistry Building, University Park, PA 16802, USA
cDepartment of Chemistry, University of Tennessee, Knoxville, Knoxville, Tennessee 37996, USA
First published on 17th October 2022
Abstract
The exceptional stability of N-heterocyclic carbene (NHC) monolayers on gold surfaces and nanoparticles (AuNPs) is enabling new and diverse applications from catalysis to biomedicine. Our understanding of NHC reactivity at surfaces; however, is quite nascent when compared to the long and rich history of NHC ligands in organometallic chemistry. In this work, well-established transmetalation reactions, previously developed for NHC transfer in homogeneous organometallic systems, are explored to determine how they can be used to create carbene functionalized gold surfaces. Two classes of NHCs, based on imidazole and benzimidazole scaffolds, were tested. The resulting AuNP surfaces were analyzed using X-ray photoelectron spectroscopy (XPS), laser desorption ionization mass spectrometry (LDI-MS), and surface-enhanced Raman spectroscopy (SERS). Reaction of either a Au(I) or Ag(I) isopropyl benzimidazole NHC complex with citrate-capped AuNPs yields, in both cases, a chemisorbed NHC that is bound through a Au adatom. Theoretical calculations additionally illustrate that binding through the Au adatom is favored by more than 10 kcal mol−1, in good agreement with experiments. Surprisingly, reaction of Au(I), Ag(I), and Cu(I) diisopropylphenyl imidazole NHCs do not follow the same pattern. The Cu complex undergoes transmetalation with very little deposition of Cu; whereas, unexpectedly, the Ag complex foregoes transmetalation and instead adducts to the AuNP with retention of the Ag–C bond. Theoretical calculations illustrate that the imidazole ligand affords significant dispersion interactions with the gold surface, which may stabilize binding through the Ag adatom motif, despite its less favorable bonding energies. Taken together these results suggest a unique ability to tune the reactivity by changing the carbene structure and raise critical questions about how established transmetalation reactions in organometallic chemistry can be applied to form NHC functionalized surfaces.
Introduction
Gold nanoparticles (AuNPs) facilitate wide-ranging applications in biological sensing,1 biomedicine,2–6 and catalysis7–10 and these applications rely almost exclusively on chemisorbed molecules to tune the nanoparticle surface chemistry.11 N-heterocyclic carbene (NHC) ligands recently emerged as an alternate ligand for gold surface passivation12–17 with proven stability in conditions where state-of-the-art thiol monolayers fail.18–24 Despite their promise, our understanding of NHC monolayers is still nascent when compared to organometallic chemistry of NHCs. Indeed, NHC ligands have a remarkable history in organometallics with decades of research establishing their reactivity and applications, most notably in chemical catalysis.25–29 Given the rich history of NHCs in organometallic chemistry, borrowing their well-established reactions may usher in new directions for NHC monolayers on AuNPs.Transmetalation reactions in homogeneous organometallic chemistry provide a facile route to transfer NHC ligands to metal atom centers. In particular, silver30–33 and copper34,35 carbene complexes are routinely used to access gold carbene complexes, which then find frequent use as designer catalysts (Fig. 1, top).36 In contrast to the gold complexes, these silver and copper complexes are often air stable and easily prepared via a base-free, one-step synthesis involving Ag2O37–39 or Cu2O,34,35 respectively. Despite the widespread use of transmetalation reactions in organometallic chemistry,40 only one report employs a copper NHC complex for AuNP functionalization;41 however, they were unable to confirm transmetalation occurred and could not rule out the possibility of a carbene binding motif through a copper adatom.42
In this manuscript, we present a comprehensive experimental and theoretical study of transmetalation reactions from silver and copper NHC complexes to create NHC functionalized AuNPs. The nanoparticle surface chemistry is probed with a suite of techniques including X-ray photoelectron spectroscopy (XPS), laser-desorption ionization mass spectrometry (LDI-MS) and surface-enhanced Raman spectroscopy (SERS), all of which were further augmented by theoretical calculations. By tracking these reactions, we elucidate surprising differences in how the NHC complex and ligand structure lead to dramatically different reactivity with the citrate-capped AuNP surfaces. While these results indicate that transmetalation could be an excellent strategy for NHC transfer to Au surfaces, the surface reactions depend on multiple variables when compared to their homogeneous organometallic chemistry counterparts.
Experimental
We investigated two classes of NHC: (1) benzimidazole-based with isopropyl side groups and (2) imidazole-based with diisopropylphenyl (Dipp) side groups, since these are the two general classes of NHCs previously reported on AuNPs (Fig. 1, bottom).19,22 All the AuNPs in this study were 23 ± 5 nm in diameter and were prepared via citrate reduction of gold aurate salt yielding citrate-capped AuNPs.43 Complexes (1)AuCl, (2)AuCl, (2)AgCl, and (2)CuCl were purchased from commercial vendors while (1)AgBr was synthesized by the method of Ghosh.44 (1)AgBr was synthesized in lieu of (1)AgCl because there are no published syntheses for (1)AgCl in the literature. 1-AuNPs were formed via the addition of (1)AuCl in acetonitrile to citrate-capped gold colloids to produce a final ligand concentration of 14.9 μM by the method of Camden and Jenkins.45 While the detailed mechanism of 1-AuNP formation remains unexplored, we hypothesize that the carbene is deposited via the citrate reduction of the Au(I) complex with release of the halide into solution. Reactions with the other four complexes proceeded in the same manner with final ligand concentrations of 14.9 μM for (1)AgBr and 10 μM for the other three complexes.All resulting AuNPs were characterized by XPS, inductively coupled plasma optical emission spectroscopy (ICP-OES), LDI-MS, and SERS and augmented with theoretical calculations. XPS characterization is a standard technique to distinguish between chemisorption and physisorption of the NHC to the metal surface. The presence of a Au–C bond is revealed primarily by the shifting of the N 1s peak46–48 to a lower binding energy (∼400 eV) when compared to the unbound, positively charged, NHC ligand (∼402 eV).49 Additionally, XPS may elucidate the deposition of Ag or Cu onto the surface during the transfer process. ICP-OES characterization is routinely used to quantify alloys of Ag50 and Cu51 with Au; therefore, we employ ICP-OES to quantify the nanoparticle composition after treatment with Ag and Cu NHC complexes. LDI-MS characterization is a powerful tool to probe the chemical composition of NHC monolayers and reveal reactions at the surface.52 Here, we use LDI-MS to probe the ligand transfer process. SERS is a highly surface sensitive technique capable of measuring the vibrational spectroscopy of ligands on AuNP surfaces.53,54 Our groups previously established SERS techniques as a probe of NHC monolayers on gold surfaces21 and AuNPs45 with the added capability of determining the ligand orientation.55 Here, we use SERS to elucidate whether AuNPs treated with Ag or Cu NHC complexes are distinguishable from AuNPs treated with Au complexes. Any differences in SERS spectra would show that monolayers with different surface orientation or binding motifs form on the surface.
The experimental measurements were further augmented with theoretical calculations of carbene molecules bound to a gold cluster, which is known to well model the NHC-surface interactions.45,55 Briefly, density functional theory employing the BP86 functional56,57 with dispersion correction58 in the Amsterdam density functional program (ADF)59,60 was used to perform geometry optimizations, normal mode calculations, and bonding analysis.
Additional experimental and theoretical details are contained in the ESI.†
Results and discussion
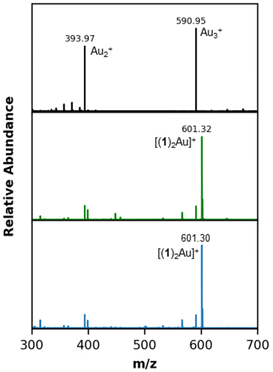
Citrate-capped AuNPs treated with (1)AuCl form 1-AuNP.45 High resolution XPS spectra of this system illustrate the presence of an N 1s peak at 400.5 eV, in excellent agreement with previous studies for ligand 1 immobilized onto Au surfaces (Fig. 2, top).19,45 We then compared these results to AuNPs treated with (1)AgBr, revealing not only the N 1s peak at 400.4 eV, but also a characteristic signal for Ag 3d5/2 in the XPS spectra (Fig. 2, bottom). The Ag 3d5/2 peak appears at 367.8 eV, within the range observed for Ag0 in Ag/Au nanoparticle alloys.61,62 ICP-OES measurements illustrate that, within experimental uncertainty, all (102 ± 14%) of the silver added to the sample is incorporated into the AuNPs after treatment with (1)AgCl (Table S5†). While these data illustrate that chemisorbed carbenes form on AuNPs treated with (1)AgBr, XPS cannot distinguish between carbenes bound to different coinage metals.63 Moreover, STM studies indicate that carbenes may bind through Ag, Cu, or Au adatoms.42LDI-MS characterization of a control sample of gold nanoparticles with acetonitrile and no NHCs contains predominately Au2+ and Au3+ clusters at 394 m/z and 590 m/z, respectively (Fig. 3, top). Treatment of citrate-capped AuNPs with (1)AuCl gives predominantly [(1)2Au]+ at 601 m/z (Fig. 3, middle).52 Most notably, treatment of AuNPs with (1)AgBr gives predominantly [(1)2Au]+ at 601 m/z (Fig. 3, bottom). Notably, no NHC-silver clusters appear in the mass spectra, which suggests that the Au-bound NHC is the dominant surface species, as opposed to the Ag-bound NHC. These LDI–MS results, in concert with XPS data, demonstrates that exposure of citrate-capped AuNPs to (1)AgBr results in a transmetalation reaction.
Performing SERS on the AuNPs after treatment with (1)AuCl or (1)AgBr furthermore reveals spectra that are quantitatively the same (Fig. 4 and S3†). If transmetalation provides monolayers of different surface orientation, the SERS spectra would reflect these changes. Therefore, the similarity of the SERS spectra suggests that treatment with Ag or Au NHC complexes produces surface bound ligands with indistinguishable surface orientation. These data are in excellent agreement with previous SERS studies of ligand 1 chemisorbed to AuNPs and bound in an upright configuration, showing that each metal complex leads to the same SAM.45,55
 | ||
| Fig. 4 SERS spectra of citrate-capped AuNPs exposed to either (1)AuCl (red) or (1)AgBr (blue). The spectra are indistinguishable within their uncertainties (confidence intervals provided in ESI†) suggesting that the chemisorbed ligands adopt the same orientation regardless of the starting material employed. Spectra are normalized to the band maximum at 1296 cm−1. | ||
We employed density-functional theory (DFT) simulations to compare the bonding energy (BE) and dispersion energy (DE) of carbenes bound through either a gold or silver adatom (Fig. 5). BE calculations were computed using an Au57 cluster with an Ag or Au adatom to illustrate the two possible binding motifs. Ligand 1 has two conformations of the isopropyl side groups: the methyl groups of the isopropyl substituent can point toward or away from the surface.55 Only the “away from” conformation is discussed here as both conformations lead to similar relative energies (Table S3†). The BEs illustrate that the gold adatom binding motif is >10 kcal mol−1 lower energy than the silver adatom binding motif (Fig. 5), in agreement with trends observed for transmetalation reactions in homogeneous organometallic chemistry (Au > Ag).30 Although the DE is a significant part of the BE (40–50%), it is similar for both Au and Ag adatom systems as it is largely determined by the interactions with the underlying Au surface. The significantly lower BE of the Au adatom system is in excellent agreement with the observed transmetalation starting from (1)AgBr complex onto AuNPs.
To explore how different carbene structures react with the gold surface, we repeat the above procedures with an imidazole-based NHC (2) and its respective Au, Ag and Cu complexes. XPS spectra of AuNP surfaces after treatment with the NHC complexes (Fig. 6) reveals the N 1s peak in each spectra is at the energy expected for a chemisorbed carbene (∼400 eV),19 indicating that 2 is chemically bound to the gold surface in all three cases (Fig. 6). As observed for ligand 1, the Ag 3d5/2 peak appears at 367.8 eV (ref. 61 and 62) for AuNPs reacted with (2)AgCl. However, in the case of the reaction with (2)CuCl we observe a small Cu 2p3/2 peak was detected. Using ICP-OES we find that 31 ± 5% of the Ag atoms from (2)AgCl alloy with the AuNPs whereas only 10 ± 1% of the Cu atoms from (2)CuCl alloy with the AuNPs (Table S7†). These measurements illustrate that Cu does not alloy significantly with the AuNP upon transfer. Instead, the reaction of (2)CuCl with AuNPs yields a soluble copper salt which is removed during centrifugation. In summary, all three reactions yield a chemisorbed carbene on the AuNP surface, but they exhibit different degrees of alloying depending on the identity of the metal precursor and ligand.
LDI-MS analysis of the Dipp-NHC nanoparticle systems reveals more complex mass spectra than AuNPs with ligand 1 (Fig. 3), due to the presence of sodium chloride adducts and cyanide adducts (Fig. S7†). The LDI-MS of a citrate-capped AuNP control sample (Fig. 7) revealed predominately Au3+ ions at 590 m/z and a very low background in the region of interest (400–1250 m/z). The mass spectra from AuNPs reacted with (2)AuCl displays ions at 643 m/z and 1196 m/z corresponding to [((2)AuCl)Na]+ and [((2)Au)2CN]+, respectively. In contrast, AuNPs reacted with (2)AgCl yields ions at 495 m/z and 885 m/z arising from [(2)Ag]+ and [(2)2Ag]+, respectively. In contrast to AuNPs treated with (1)AgBr, AuNPs treated with (2)AgCl adopt a distinctly different binding motif: (2)AgCl does not transmetalate and ligand 2 binds to the gold surface through the Ag adatom. Conversely, AuNPs reacted with (2)CuCl give exactly the same spectra as AuNPs reacted with (2)AuCl, suggesting that treatment of AuNPs with either (2)AuCl or (2)CuCl forms the same surface species (Fig. 7). Therefore, reaction with (2)CuCl can be viewed as a partial transmetalation reaction, whereas, (2)AgCl does not transmetalate and Ag is retained at the surface.
SERS spectra obtained from AuNPs treated with Au, Ag or Cu complexes of 2 were also acquired. Similar to the ligand 1 systems, the spectra are indistinguishable within their uncertainties, suggesting that the ligand surface orientation is consistent regardless of whether the NHC source is an Au, Ag or Cu complex (Fig. S4 and S5†). This conclusion is further corroborated by theoretical calculations showing the upright configuration as the most favorable regardless of the adatom (vide infra).
DFT calculations are employed to probe the binding of ligand 2 with a Au57 cluster via an Au, Ag or Cu adatom. The BE comparison indicates that transmetalation reactions should be favorable for both the Ag and Cu complexes (Fig. 8); however, transmetalation was only observed starting from (2)CuCl. To explore this apparent disagreement and gain insight into reactivity differences between silver complexes of ligand 1 and 2, we computed the DE of the carbene-metal interactions. These calculations show that for ligand 2 the dispersion contribution to the BE is around 70–80%, compared to the 40–50% for ligand 1. These large differences in DE arise from the sterically bulky Dipp side groups of 2 which likely enables more significant dispersion interaction with the Au surface than the isopropyl groups of ligand 1. Therefore, the strong bonding through dispersion interactions might enable the kinetic product Ag-adatom system to form rather than the thermodynamically favorable Au-adatom system.
Glorius and Fuchs recently explored the influence of side groups on the surface mobility of carbenes in an STM study,64 where they demonstrate that the surface mobility of ligand 2 is dramatically lower than imidazole ligands with less sterically bulky side groups.64 These results, in concert with our DFT calculations and experiments, illustrate the differences between reactions at NHC surfaces and their analogous homogeneous organometallic reactions. For example, ligand transfer of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 or 2
44 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 from an Ag atom to an Au atom is routine in organometallic chemistry; however, here we find that only Ag complexes of ligand 1 perform ligand transfer to gold, likely due to surface specific variables (DE and surface mobility) favoring an Ag adatom motif for ligand 2. Therefore, we must conclude that the reactivity of carbene complexes with citrate-capped gold nanoparticles relies heavily on the carbene side groups and choice of metal complex.
32 from an Ag atom to an Au atom is routine in organometallic chemistry; however, here we find that only Ag complexes of ligand 1 perform ligand transfer to gold, likely due to surface specific variables (DE and surface mobility) favoring an Ag adatom motif for ligand 2. Therefore, we must conclude that the reactivity of carbene complexes with citrate-capped gold nanoparticles relies heavily on the carbene side groups and choice of metal complex.
Conclusions
We evaluated how a common reaction in organometallic chemistry, transmetalation, provides a facile route to transfer NHC ligands to citrate-capped AuNPs via Ag and Cu complexes, but discovered an unexpected reaction dependence on the NHC structure and the precursor metal ion. For both Dipp-imidazole and isopropyl-benzimidazole Ag complexes, the silver metal alloyed to the AuNP during the transmetalation reaction. However, by switching from the isopropyl-benzimidazole Ag complex to the Dipp-imidazole Ag complex, the NHC does not transmetalate to the nanoparticle. Instead, an exotic NHC-Ag adatom structure is formed on the AuNP surface, which may provide an alternate route to thiol based methods for doping Ag atoms onto the AuNP surface.65 In the case of the Dipp-NHC–Cu complex the NHC transfer process retains significant transmetallation character, but a small amount of copper is still retained at the nanoparticle surface. Our results demonstrate that detailed surface measurements must be combined with ligand design to achieve the desired reactivity and NHC surface functionality.This work demonstrates that transmetalation is a viable route towards the creation of NHC surfaces and raises critical questions of how the rich history of homogeneous organometallic NHC chemistry may influence the burgeoning field of NHC surface chemistry. By transferring homogeneous organometallic chemistry reactions to metal surfaces, old reactions may find renewed significance.
Author contributions
N. L. D., S. L. S., T. R., C. Q. K. and W. C. B. carried out experiments and data analysis; R. C. and A. V. B. S. carried out theoretical calculations; L. J., D. M. J. and J. P. C. conceptualized the project; and N. L. D., D. M. J. and J. P. C. prepared the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Science Foundation under grant numbers CHE-2108330 (N. L. D. and J. P. C.), CHE-2108328 (D. M. J.) and CHE-2106151 (R. C. and L. J.) and DGE 1255832 (A. V. B. S.). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.N. L. D. gratefully acknowledges the Berthiaume Institute at Notre Dame for summer fellowship funding. N. L. D. and J. P. C. thank the Notre Dame Mass Spectrometry and Proteomics Facility for use of the Bruker UltrafleXtreme and Bruker Impact II instruments; Alexander Mukasyan and Tatyana Orlova of the Notre Dame Integrated Imaging Facility for use of the Magellan 400 SEM; Mike Brueseke and Jon Loftus of the Notre Dame CEST facility for use of the BIC NanoBrook Omni instrument and ICP-OES instrument; and Anna Matzner and Ian Lightcap of the Notre Dame Materials Characterization Facility for use of the PHI VersaProbe II instrument.
References
- K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Gold nanoparticles in chemical and biological sensing, Chem. Rev., 2012, 112(5), 2739–2779, DOI:10.1021/cr2001178 . Epub 20120202.
- C. J. Murphy, A. M. Gole, J. W. Stone, P. N. Sisco, A. M. Alkilany, E. C. Goldsmith and S. C. Baxter, Gold nanoparticles in biology: beyond toxicity to cellular imaging, Acc. Chem. Res., 2008, 41(12), 1721–1730, DOI:10.1021/ar800035u.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, The golden age: gold nanoparticles for biomedicine, Chem. Soc. Rev., 2012, 41(7), 2740–2779, 10.1039/c1cs15237h . Epub 20111122.
- N. S. Abadeer and C. J. Murphy, Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles, J. Phys. Chem. C, 2016, 120(9), 4691–4716, DOI:10.1021/acs.jpcc.5b11232.
- P. Singh, S. Pandit, V. R. S. S. Mokkapati, A. Garg, V. Ravikumar and I. Mijakovic, Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer, Int. J. Mol. Sci., 2018, 19(7), 1979, DOI:10.3390/ijms19071979 . Epub 20180706.
- J. B. Vines, J.-H. Yoon, N.-E. Ryu, D.-J. Lim and H. Park, Gold Nanoparticles for Photothermal Cancer Therapy, Front. Chem., 2019, 7, 167, DOI:10.3389/fchem.2019.00167.
- M. S. Ide and R. J. Davis, The important role of hydroxyl on oxidation catalysis by gold nanoparticles, Acc. Chem. Res., 2014, 47(3), 825–833, DOI:10.1021/ar4001907 . Epub 20131121.
- M. Stratakis and H. Garcia, Catalysis by Supported Gold Nanoparticles: Beyond Aerobic Oxidative Processes, Chem. Rev., 2012, 112(8), 4469–4506, DOI:10.1021/cr3000785.
- J. Lou-Franco, B. Das, C. Elliott and C. Cao, Gold Nanozymes: From Concept to Biomedical Applications, Nano-Micro Lett., 2021, 13, 10, DOI:10.1007/s40820-020-00532-z.
- W. Hou and S. B. Cronin, A Review of Surface Plasmon Resonance-Enhanced Photocatalysis, Adv. Funct. Mater., 2013, 23(13), 1612–1619, DOI:10.1002/adfm.201202148.
- A. Heuer-Jungemann, N. Feliu, I. Bakaimi, M. Hamaly, A. Alkilany, I. Chakraborty, A. Masood, M. F. Casula, A. Kostopoulou, E. Oh, K. Susumu, M. H. Stewart, I. L. Medintz, E. Stratakis, W. J. Parak and A. G. Kanaras, The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles, Chem. Rev., 2019, 119(8), 4819–4880, DOI:10.1021/acs.chemrev.8b00733 . Epub 20190328.
- A. V. Zhukhovitskiy, M. J. MacLeod and J. A. Johnson, Carbene Ligands in Surface Chemistry: From Stabilization of Discrete Elemental Allotropes to Modification of Nanoscale and Bulk Substrates, Chem. Rev., 2015, 115(20), 11503–11532, DOI:10.1021/acs.chemrev.5b00220 . Epub 20150922.
- S. Engel, E. C. Fritz and B. J. Ravoo, New trends in the functionalization of metallic gold: from organosulfur ligands to N-heterocyclic carbenes, Chem. Soc. Rev., 2017, 46(8), 2057–2075, 10.1039/c7cs00023e.
- C. A. Smith, M. R. Narouz, P. A. Lummis, I. Singh, A. Nazemi, C.-H. Li and C. M. Crudden, N-Heterocyclic, Carbenes in Materials Chemistry, Chem. Rev., 2019, 119(8), 4986–5056, DOI:10.1021/acs.chemrev.8b00514 . Epub 20190402.
- Y. An, J. Yu and Y. Han, Recent Advances in the Chemistry of N–Heterocyclic Carbene Functionalized Metal Nanoparticles and Their Applications, Chin. J. Chem., 2019, 37(1), 76–87, DOI:10.1002/cjoc.201800450.
- P. Bellotti, M. Koy, M. N. Hopkinson and F. Glorius, Recent advances in the chemistry and applications of N-heterocyclic carbenes, Nat. Rev. Chem., 2021, 5(10), 711–725, DOI:10.1038/s41570-021-00321-1.
- S. R. Thomas and A. Casini, N-Heterocyclic, carbenes as “smart” gold nanoparticle stabilizers: State-of-the art and perspectives for biomedical applications, J. Organomet. Chem., 2021, 938(121743), 121743, DOI:10.1016/j.jorganchem.2021.121743.
- C. Vericat, M. E. Vela, G. Benitez, P. Carro and R. C. Salvarezza, Self-assembled monolayers of thiols and dithiols on gold: new challenges for a well-known system, Chem. Soc. Rev., 2010, 39(5), 1805–1834, 10.1039/b907301a . Epub 20100224.
- C. M. Crudden, J. H. Horton, I. I. Ebralidze, O. V. Zenkina, A. B. McLean, B. Drevniok, Z. She, H. B. Kraatz, N. J. Mosey, T. Seki, E. C. Keske, J. D. Leake, A. Rousina-Webb and G. Wu, Ultra stable self-assembled monolayers of N-heterocyclic carbenes on gold, Nat. Chem., 2014, 6(5), 409–414, DOI:10.1038/nchem.1891 . Epub 20140323.
- C. M. Crudden, J. H. Horton, M. R. Narouz, Z. J. Li, C. A. Smith, K. Munro, C. J. Baddeley, C. R. Larrea, B. Drevniok, B. Thanabalasingam, A. B. McLean, O. V. Zenkina, I. I. Ebralidze, Z. She, H. B. Kraatz, N. J. Mosey, L. N. Saunders and A. Yagi, Simple direct formation of self-assembled N-heterocyclic carbene monolayers on gold and their application in biosensing, Nat. Commun., 2016, 7, 7, DOI:10.1038/ncomms12654 . Epub 20160902.
- J. F. DeJesus, M. J. Trujillo, J. P. Camden and D. M. Jenkins, N-Heterocyclic, Carbenes as a Robust Platform for Surface-Enhanced Raman Spectroscopy, J. Am. Chem. Soc., 2018, 140(4), 1247–1250, DOI:10.1021/jacs.7b12779 . Epub 20180122.
- R. W. Y. Man, C. H. Li, M. W. A. MacLean, O. V. Zenkina, M. T. Zamora, L. N. Saunders, A. Rousina-Webb, M. Narnbo and C. M. Crudden, Ultrastable Gold Nanoparticles Modified by Bidentate N-Heterocyclic Carbene Ligands, J. Am. Chem. Soc., 2018, 140(5), 1576–1579, DOI:10.1021/jacs.7b08516 . Epub 20180124.
- N. A. Nosratabad, Z. C. Jin, L. Du, M. Thakur and H. Mattoussi, N-Heterocyclic, Carbene-Stabilized Gold Nanoparticles: Mono-Versus Multidentate Ligands, Chem. Mater., 2021, 33(3), 921–933, DOI:10.1021/acs.chemmater.0c03918.
- L. M. Sherman, M. D. Finley, R. K. Borsari, N. Schuster-Little, S. L. Strausser, R. J. Whelan, D. M. Jenkins and J. P. Camden, N-Heterocyclic, Carbene Ligand Stability on Gold Nanoparticles in Biological Media, ACS Omega, 2022, 7(1), 1444–1451, DOI:10.1021/acsomega.1c06168 . Epub 20211217.
- W. A. Herrmann, N-heterocyclic carbenes: a new concept in organometallic catalysis, Angew. Chem., Int. Ed., 2002, 41(8), 1290–1309, DOI:10.1002/1521-3773(20020415)41:8<1290::aid-anie1290>3.0.co;2-y.
- S. Díez-González, N. Marion and S. P. Nolan, N-heterocyclic carbenes in late transition metal catalysis, Chem. Rev., 2009, 109(8), 3612–3676, DOI:10.1021/cr900074m.
- G. C. Vougioukalakis and R. H. Grubbs, Ruthenium-based heterocyclic carbene-coordinated olefin metathesis catalysts, Chem. Rev., 2010, 110(3), 1746–1787, DOI:10.1021/cr9002424.
- X. Bugaut and F. Glorius, Organocatalytic umpolung: N-heterocyclic carbenes and beyond, Chem. Soc. Rev., 2012, 41(9), 3511–3522, 10.1039/c2cs15333e . Epub 20120229.
- M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, An overview of N-heterocyclic carbenes, Nature, 2014, 510(7506), 485–496, DOI:10.1038/nature13384.
- H. M. J. Wang and I. J. B. Lin, Facile synthesis of silver(I)-carbene complexes. Useful carbene transfer agents, Organometallics, 1998, 17(5), 972–975, DOI:10.1021/om9709704.
- I. J. B. Lin and C. S. Vasam, Silver(I) N-heterocyclic Carbenes, Comments Inorg. Chem., 2004, 25(3–4), 75–129, DOI:10.1080/02603590490883652.
- S. T. Liu, C. I. Lee, C. F. Fu, C. H. Chen, Y. H. Liu, C. J. Elsevier, S. M. Peng and J. T. Chen, N-Heterocyclic, Carbene Transfer from Gold(I) to Palladium(II), Organometallics, 2009, 28(24), 6957–6962, DOI:10.1021/om900776j.
- Z. Lu, S. A. Cramer and D. M. Jenkins, Exploiting a dimeric silver transmetallating reagent to synthesize macrocyclic tetracarbene complexes, Chem. Sci., 2012, 3(10), 3081–3087, 10.1039/c2sc20628e.
- M. R. L. Furst and C. S. J. Cazin, Copper N-heterocyclic carbene (NHC) complexes as carbene transfer reagents, Chem. Commun., 2010, 46(37), 6924–6925, 10.1039/c0cc02308f . Epub 20100823.
- F. Nahra, A. Gómez-Herrera and C. S. J. Cazin, Copper(i)-NHC complexes as NHC transfer agents, Dalton Trans., 2017, 46(3), 628–631, 10.1039/c6dt03687b.
- C. C. Chintawar, A. K. Yadav, A. Kumar, S. P. Sancheti and N. T. Patil, Divergent Gold Catalysis: Unlocking Molecular Diversity through Catalyst Control, Chem. Rev., 2021, 121(14), 8478–8558, DOI:10.1021/acs.chemrev.0c00903 . Epub 20210208.
- X. Hu, I. Castro-Rodriguez and K. Meyer, Copper complexes of nitrogen-anchored tripodal N-heterocyclic carbene ligands, J. Am. Chem. Soc., 2003, 125(40), 12237–12245, DOI:10.1021/ja036880+.
- X. Hu, Y. Tang, P. Gantzel and K. Meyer, Silver Complexes of a Novel Tripodal N-Heterocyclic Carbene Ligand: Evidence for Significant Metal−Carbene π-Interaction, Organometallics, 2003, 22(4), 612–614, DOI:10.1021/om020935j.
- X. Hu, I. Castro-Rodriguez, K. Olsen and K. Meyer, Group 11 Metal Complexes of N-Heterocyclic Carbene Ligands: Nature of the MetalCarbene Bond, Organometallics, 2004, 23(4), 755–764, DOI:10.1021/om0341855.
- T. Scattolin and S. P. Nolan, Synthetic Routes to Late Transition Metal-NHC Complexes, Trends Chem., 2020, 2(8), 721–736, DOI:10.1016/j.trechm.2020.06.001.
- S. Thanneeru, K. M. Ayers, M. Anuganti, L. Zhang, C. V. Kumar, G. Ung and J. He, N-Heterocyclic, carbene-ended polymers as surface ligands of plasmonic metal nanoparticles, J. Mater. Chem., 2020, 8(7), 2280–2288, 10.1039/C9TC04776J.
- E. A. Doud, M. S. Inkpen, G. Lovat, E. Montes, D. W. Paley, M. L. Steigerwald, H. Vazquez, L. Venkataraman and X. Roy, In Situ Formation of N-Heterocyclic Carbene-Bound Single-Molecule Junctions, J. Am. Chem. Soc., 2018, 140(28), 8944–8949, DOI:10.1021/jacs.8b05184 . Epub 20180703.
- P. C. Lee and D. Meisel, Adsorption and surface-enhanced Raman of dyes on silver and gold sols, J. Phys. Chem., 1982, 86(17), 3391–3395, DOI:10.1021/j100214a025.
- L. Ray, V. Katiyar, S. Barman, M. J. Raihan, H. Nanavati, M. M. Shaikh and P. Ghosh, Gold(I) N-heterocyclic carbene based initiators for bulk ring-opening polymerization of l-lactide, J. Organomet. Chem., 2007, 692(20), 4259–4269, DOI:10.1016/j.jorganchem.2007.06.033.
- J. F. DeJesus, L. M. Sherman, D. J. Yohannan, J. C. Becca, S. L. Strausser, L. F. P. Karger, L. Jensen, D. M. Jenkins and J. P. Camden, A Benchtop Method for Appending Protic Functional Groups to N-Heterocyclic Carbene Protected Gold Nanoparticles, Angew. Chem., Int. Ed., 2020, 59(19), 7585–7590, DOI:10.1002/anie.202001440 . Epub 20200312.
- M. Koy, P. Bellotti, M. Das and F. Glorius, N-Heterocyclic, carbenes as tunable ligands for catalytic metal surfaces, Nat. Catal., 2021, 4(5), 352–363, DOI:10.1038/s41929-021-00607-z.
- D. T. H. Nguyen, M. Bélanger-Bouliga, L. R. Shultz, A. Maity, T. Jurca and A. Nazemi, Robust Water-Soluble Gold Nanoparticles via Polymerized Mesoionic N-Heterocyclic Carbene–Gold(I) Complexes, Chem. Mater., 2021, 33(24), 9588–9600, DOI:10.1021/acs.chemmater.1c02899.
- C. Y. Wu, W. J. Wolf, Y. Levartovsky, H. A. Bechtel, M. C. Martin, F. D. Toste and E. Gross, High-spatial-resolution mapping of catalytic reactions on single particles, Nature, 2017, 541(7638), 511–515, DOI:10.1038/nature20795 . Epub 20170111.
- A. Ruhling, K. Schaepe, L. Rakers, B. Vonhoren, P. Tegeder, B. J. Ravoo and F. Glorius, Modular Bidentate Hybrid NHC-Thioether Ligands for the Stabilization of Palladium Nanoparticles in Various Solvents, Angew. Chem., Int. Ed., 2016, 55(19), 5856–5860, DOI:10.1002/anie.201508933 . Epub 20160405.
- T. Kim, Q. Zhang, J. Li, L. Zhang and J. V. Jokerst, A Gold/Silver Hybrid Nanoparticle for Treatment and Photoacoustic Imaging of Bacterial Infection, ACS Nano, 2018, 12(6), 5615–5625, DOI:10.1021/acsnano.8b01362 . Epub 20180514.
- R. He, Y.-C. Wang, X. Wang, Z. Wang, G. Liu, W. Zhou, L. Wen, Q. Li, X. Wang, X. Chen, J. Zeng and J. G. Hou, Facile synthesis of pentacle gold-copper alloy nanocrystals and their plasmonic and catalytic properties, Nat. Commun., 2014, 5, 4327, DOI:10.1038/ncomms5327 . Epub 20140707.
- N. L. Dominique, S. L. Strausser, J. E. Olson, W. C. Boggess, D. M. Jenkins and J. P. Camden, Probing N-Heterocyclic Carbene Surfaces with Laser Desorption Ionization Mass Spectrometry, Anal. Chem., 2021, 93(40), 13534–13538, DOI:10.1021/acs.analchem.1c02401 . Epub 20210928.
- X. Gu, M. J. Trujillo, J. E. Olson and J. P. Camden, SERS Sensors: Recent Developments and a Generalized Classification Scheme Based on the Signal Origin, Annu. Rev. Anal. Chem., 2018, 11(1), 147–169, DOI:10.1146/annurev-anchem-061417-125724 . Epub 20180316.
- J. Langer, D. Jimenez de Aberasturi, J. Aizpurua, R. A. Alvarez-Puebla, B. Auguié, J. J. Baumberg, G. C. Bazan, S. E. J. Bell, A. Boisen, A. G. Brolo, J. Choo, D. Cialla-May, V. Deckert, L. Fabris, K. Faulds, F. J. García de Abajo FJ, R. Goodacre, D. Graham, A. J. Haes, C. L. Haynes, C. Huck, T. Itoh, M. Käll, J. Kneipp, N. A. Kotov, H. Kuang, E. C. Le Ru, H. K. Lee, J.-F. Li, X. Y. Ling, S. A. Maier, T. Mayerhöfer, M. Moskovits, K. Murakoshi, J.-M. Nam, S. Nie, Y. Ozaki, I. Pastoriza-Santos, J. Perez-Juste, J. Popp, A. Pucci, S. Reich, B. Ren, G. C. Schatz, T. Shegai, S. Schlücker, L.-L. Tay, K. G. Thomas, Z.-Q. Tian, R. P. Van Duyne, T. Vo-Dinh, Y. Wang, K. A. Willets, C. Xu, H. Xu, Y. Xu, Y. S. Yamamoto, B. Zhao and L. M. Liz-Marzán, Present and Future of Surface-Enhanced Raman Scattering, ACS Nano, 2020, 14(1), 28–117, DOI:10.1021/acsnano.9b04224 . Epub 20191008.
- M. J. Trujillo, S. L. Strausser, J. C. Becca, J. F. DeJesus, L. Jensen, D. M. Jenkins and J. P. Camden, Using SERS To Understand the Binding of N-Heterocyclic Carbenes to Gold Surfaces, J. Phys. Chem. Lett., 2018, 9(23), 6779–6785, DOI:10.1021/acs.jpclett.8b02764 . Epub 20181115.
- A. D. Becke, Density-functional exchange-energy approximation with correct asymtoticbehavior, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 3098–3100 CrossRef CAS PubMed.
- J. P. Perdew, Density-functional approximation for the correlation energy of the inhomogeneous, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 8822–8824 CrossRef PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the Damping Function in Dispersion Corrected Density Functional Theory, J. Comput. Chem., 2011, 1457 Search PubMed.
- V. Gt, F. M. Bickelhaupt, E. J. Baerends, C. F. Guerra, S. Gisbergen, J. G. Snijders and T. Ziegler, Chemistry with ADF, J. Comput. Chem., 2001, 931 Search PubMed.
- R. Rüger, M. Franchini, T. Trnka, A. Yakovlev, E. van Lenthe, P. Philipsen, T. van Vuren, B. Klumpers and T. Soini, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, 2022, vol. 1, Available from: https://www.scm.com Search PubMed.
- R. J. Chimentão, I. Cota, A. Dafinov, F. Medina, J. E. Sueiras, J. L. G. de la Fuente, J. L. G. Fierro, Y. Cesteros and P. Salagre, Synthesis of silver-gold alloy nanoparticles by a phase-transfer system, J. Mater. Res., 2006, 21(1), 105–111, DOI:10.1557/jmr.2006.0014.
- L. J. Torres-Pacheco, A. Osornio-Villa, N. A. García-Gómez, A. Olivas, R. Valdez, M. Guerra-Balcázar, L. Álvarez-Contreras and N. Arjona, Effect of AuM (M: Ag, Pt & Pd) bimetallic nanoparticles on the sorbitol electro-oxidation in alkaline medium, Fuel, 2020, 274, 117864, DOI:10.1016/j.fuel.2020.117864.
- L. Jiang, B. Zhang, G. Médard, A. P. Seitsonen, F. Haag, F. Allegretti, J. Reichert, B. Kuster, J. V. Barth and A. C. Papageorgiou, N-Heterocyclic, carbenes on close-packed coinage metal surfaces: bis-carbene metal adatom bonding scheme of monolayer films on Au, Ag and Cu, Chem. Sci., 2017, 8(12), 8301–8308, 10.1039/c7sc03777e . Epub 20170928.
- G. Q. Wang, A. Ruhling, S. Amirjalayer, M. Knor, J. B. Ernst, C. Richter, H. J. Gao, A. Timmer, H. Y. Gao, N. L. Doltsinis, F. Glorius and H. Fuchs, Ballbot-type motion of N-heterocyclic carbenes on gold surfaces, Nat. Chem., 2017, 9(2), 152–156, DOI:10.1038/nchem.2622 . Epub 20161003.
- E. C. Fritz, C. Nimphius, A. Goez, S. Wurtz, M. Peterlechner, J. Neugebauer, F. Glorius and B. J. Ravoo, Sequential surface modification of au nanoparticles: from surface-bound Ag(I) complexes to Ag(0) doping, Chemistry, 2015, 21(12), 4541–4545, DOI:10.1002/chem.201406396 . Epub 20150204.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of citrate-capped gold nanoparticles as well as additional data and experimental details for SERS, LDI-MS, XPS, ICP-OES and theoretical simulations of carbene-AuNP systems. See DOI: https://doi.org/10.1039/d2qi01941h |
| This journal is © the Partner Organisations 2022 |