A spider hanging inside a carbon cage: off-center shift and pyramidalization of Sc3N clusters inside C84 and C86 fullerene cages†
Ze
Fu‡
 ,
Min
Guo‡
,
Yang-Rong
Yao‡
,
Qingyu
Meng
,
Yingjing
Yan
,
Qin
Wang
,
Yi
Shen
and
Ning
Chen
,
Min
Guo‡
,
Yang-Rong
Yao‡
,
Qingyu
Meng
,
Yingjing
Yan
,
Qin
Wang
,
Yi
Shen
and
Ning
Chen
 *
*
College of Chemistry, Chemical Engineering and Materials Science, and State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou, Jiangsu 215123, P. R. China. E-mail: chenning@suda.edu.cn
First published on 3rd August 2022
Abstract
Metal nitride cluster fullerenes (NCFs) are the most intensively studied endohedral fullerenes due to their exceptional structural variety. It is commonly understood that in NCFs, small clusters such as Sc3N favor C82 and smaller cages, while large clusters (e.g., Tb3N and Gd3N) favor C84 and larger cages. Endohedral structures with small nitride clusters encaged inside large carbon cages (e.g., C84 and C86), although theoretically probed, have never been experimentally obtained. Herein, we report two novel NCFs, Sc3N@Cs(51365)-C84 and Sc3N@D3(19)-C86, which have been successfully synthesized and characterized using MALDI-TOF mass spectrometry, X-ray single-crystal diffraction and UV–vis–NIR spectroscopy. Crystallographic analysis shows that, while in most previously reported cluster fullerenes, clusters tend to take a central position inside fullerene cages, in these two structures, the Sc3N clusters are shifted to one side of the cage and unexpectedly pyramidalized inside the large cages of C84 and C86, which resembles a spider hanging inside a carbon cage. These observations, together with the stretched Sc–N bonds, suggest that the M3N cluster can self-adjust not only its configuration but also its position relative to fullerenes to optimize the metal–cage distances as well as cluster–cage interactions, thus promoting the stability of endohedral structures. This work provides new insight into the interaction mechanisms between the clusters and carbon cages of endohedral fullerenes.
Introduction
Endohedral metallofullerenes (EMFs) feature unique host–guest molecular structures in which metal ions or metallic clusters are encapsulated in variable carbon cages. Complex metal–cage interactions are formed between endohedral moieties and fullerene cages, which are essential for the stability of these endohedral fullerene compounds.1–3 Endohedral fullerenes have shown great potential in the application of biomedicine, catalysis and molecular electronic devices due to their unique molecular and electronic structures.4–7Among the EMFs, cluster fullerenes (CFs) are the largest family, with variable clusters encapsulated inside fullerenes. Since the discovery of Sc3N@C80 in 1999, in the past two decades, this family has been largely expanded and extensively studied, including metal nitride cluster fullerenes (NCFs), metal carbide cluster fullerenes (CCFs) and metal cyanide cluster fullerenes (CYCFs).8,9 One of the interesting studies for CFs is the cluster configuration variations in cluster fullerenes, which is important for understanding the interactions between clusters and carbon cages.9 Previous studies found that clusters with flexible configurations, such as M2C2, M2O and MCN, can adjust their configurations inside the confined space of fullerene cages to achieve optimized metal–cage interactions, which contributes to the stabilization of host–guest molecular structures.10–13 Factors such as different carbon cage isomers, the size of the carbon cage and the metal ionic radii of encapsulated clusters can all lead to changes in the cluster configuration.14–18 For instance, as the size of the carbon cage decreases, M2C2 clusters change from a nearly linear stretched geometry to a constrained “butterfly” structure, whereas MCN clusters change from a nearly linear shape to a triangular configuration.13,19,20 Moreover, in non-IPR (isolated pentagon rule) carbon cages, clusters can be deformed to obtain stronger interactions with the carbon cage due to the high local strain of the heptagon or fused pentagons, thus stabilizing these carbon cages.21–27 Overall, these flexible encaged clusters can self-adjust their size and shape to achieve optimal metal–cage interactions, which is essential for the stability of cluster fullerene compounds.
NCFs have been the most abundant and most intensively studied endohedral fullerenes in the past decade. Trimetallic nitride clusters have a relatively rigid configuration compared with M2O, M2C2 and MCN clusters. Thus, due to their less flexible configurations, it has been well acknowledged that large nitride clusters tend to be encapsulated inside large carbon cages, while small clusters tend to be encapsulated in small carbon cages to maintain their planarity.28 In addition, large-sized metal nitride clusters tend to be pyramidalized inside small carbon cages.29–31 However, how small rigid clusters interact with large cages (e.g., C84 and C86) has never been experimentally observed and studied. Sc3N is the smallest encapsulated cluster in NCFs, as the ionic radius of Sc (0.75 Å) is much smaller than those of other lanthanides, such as Er (0.89 Å), Tb (0.92 Å) and Gd (0.94 Å). Previous theoretical studies suggest that the Sc3N cluster is off-center inside cages larger than C82, whereas this displacement leads to a less effective cluster–cage interaction, which is likely unstable. Thus, Sc3N@C2n cluster fullerenes with a cage size of C84 and larger are almost impossible to obtain.32 As a result, whether small clusters such as Sc3N, which has a relatively rigid configuration, can be stabilized inside large fullerene cages such as C84 and C86 has remained unknown to date.
Herein, we report the synthesis and isolation of two novel NCFs, Sc3N@Cs(51365)-C84 and Sc3N@D3(19)-C86, which were characterized by MALDI-TOF mass spectrometry, X-ray single-crystal diffraction, UV–vis–NIR spectroscopy and Fourier transform infrared spectroscopy. The detailed structural analysis demonstrates that the clusters show an off-center shift to one side of the cage and unexpected pyramidalization in both C84 and C86.
Results and discussion
Synthesis and isolation of Sc3N@C2n (2n = 84 and 86)
Scandium-based endohedral fullerenes were synthesized using a modified Krätschmer–Huffman (direct-current) DC arc-discharge method. Graphite rods packed with Sc2O3/graphite powder (with a molar ratio of Sc/C = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) were annealed and then vaporized in an arcing chamber under a 200 Torr helium and 4 Torr nitrogen atmosphere. The collected raw soot was extracted with carbon disulfide (CS2) for 24 h. Multiple-stage high-performance liquid chromatography (HPLC) separation processes were employed to isolate and purify Sc3N@C84 and Sc3N@C86 (Fig. S1 and S2, ESI†). The purity of the isolated compounds was confirmed by the observation of single peaks using HPLC and high-resolution positive-ion-mode matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (Fig. S3† and Fig. 1). The mass spectra of purified Sc3N@C84 and Sc3N@C86 show single peaks at m/z = 1157.053 and 1180.970, respectively, which are similar to theoretical simulations. Furthermore, the isotopic distributions in the experiment were found to be quite similar to the theoretical prediction.
15) were annealed and then vaporized in an arcing chamber under a 200 Torr helium and 4 Torr nitrogen atmosphere. The collected raw soot was extracted with carbon disulfide (CS2) for 24 h. Multiple-stage high-performance liquid chromatography (HPLC) separation processes were employed to isolate and purify Sc3N@C84 and Sc3N@C86 (Fig. S1 and S2, ESI†). The purity of the isolated compounds was confirmed by the observation of single peaks using HPLC and high-resolution positive-ion-mode matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (Fig. S3† and Fig. 1). The mass spectra of purified Sc3N@C84 and Sc3N@C86 show single peaks at m/z = 1157.053 and 1180.970, respectively, which are similar to theoretical simulations. Furthermore, the isotopic distributions in the experiment were found to be quite similar to the theoretical prediction.
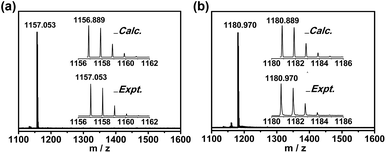 | ||
| Fig. 1 Positive mode MALDI-TOF mass spectra of purified (a) Sc3N@C84 and (b) Sc3N@C86. Insets: experimental and theoretical isotopic distributions for (a) Sc3N@C84 and (b) Sc3N@C86. | ||
Molecular structures of Sc3N@C84 and Sc3N@C86
Sc3N@C2n (2n = 84 and 86) was cocrystallized with NiII(OEP) (OEP = 2, 3, 7, 8, 12, 13, 17, and 18-octaethylporphyrin dianion) to obtain crystals suitable for X-ray measurements. The molecular structures of Sc3N@C84 and Sc3N@C86 were unambiguously determined using single-crystal X-ray diffraction analysis as Sc3N@Cs(51365)-C84 and Sc3N@D3(19)-C86, respectively. Fig. 2a and b shows the cocrystal structures formed by these NCFs with the NiII(OEP) moiety. The closest distances between the nickel atom and the carbon atoms on the cage were measured as 3.029 and 2.981 Å for Sc3N@Cs(51365)-C84 and Sc3N@D3(19)-C86, respectively, suggesting substantial π–π interactions between the fullerene cage and the porphyrin moiety.Crystallographic analysis shows that the cocrystals crystallized in the triclinic space group of P1 for Sc3N@Cs(51365)-C84 and the monoclinic space group of C2/m for Sc3N@D3(19)-C86. The Cs(51365)-C84 carbon cage and the internal nitrogen atom are fully ordered, while the encapsulated metal atoms exhibit a slight disorder. For Sc1A, Sc2A and Sc3A, which constitute the major Sc3N sites, the occupancy is 0.746(4). The minor Sc3N sites comprise Sc1B, Sc2B, and Sc3B with an occupancy of 0.254(4) (Fig. S4a†). For Sc3N@D3(19)-C86, the fullerene cage shows two orientations due to the crystallographic mirror of the C2/m space group. A complete fullerene cage with an occupancy of 0.5 is formed by combining one-half of one orientation and the mirror-related half of the other orientation. The scandium atoms have six crystallographic sites, with 0.313 occupancy for Sc in the major cluster (Sc1, Sc2 and Sc3) and 0.18 occupancy for Sc in the minor cluster (Sc4, Sc5 and Sc6). Moreover, six additional metal sites (Sc1m, Sc2m, Sc3m, Sc4m, Sc5m and Sc6m) are generated via the crystallographic mirror plane (Fig. S4b†). Both of the major Sc3N clusters in both C84 and C86 show a relatively high occupancy, which allows a more precise analysis of their configurations and their interactions with the fullerene cages.
Interaction between Sc3N clusters and large carbon cages
Fig. 2c and d shows the relative positions of the major Sc3N sites to the corresponding cage portions. In Sc3N@Cs(51365)-C84, one of the three Sc atoms is located under the conjunction of fused pentagons, and the other two Sc atoms are located below a [5,5,6] junction ([5] and [6] refer to pentagon and hexagon, respectively). The shortest average distances between the three Sc atoms and the carbon cage are 2.303(10) Å (Sc2A–C5 and Sc2A–C1), 2.276(8) Å (Sc1A–C51 and Sc1A–C30) and 2.306(8) Å (Sc3A–C59 and Sc3A–C38), respectively. Regarding Sc3N@D3(19)-C86, Sc1 is located below a [5,6] junction with the shortest average metal–cage distance of 2.230(15) Å (Sc1–C74 and Sc1-C73). Sc2 and Sc3 are located under a [6,6] junction and a [6,6,6] junction with the shortest average metal–cage distances of 2.167(13) Å (Sc2–C58 and Sc2–C36) and 2.131(16) Å (Sc3–C44 and Sc3–C43), respectively. In general, these Sc–C distances are similar to the Sc–C distances between Sc3N clusters and smaller cages, such as Sc3N@C2n (2n = 68, 78, 80, 82; for details please see Table 1).| Sc3N@Cs(51365)-C84 | Sc3N@D3(19)-C86 | Sc3N@D3(6140)-C68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38,39 38,39 |
Sc3N@D3h(5)-C78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
|
|---|---|---|---|---|
| Distance (Å) | ||||
| M1–N | 2.083(6) | 2.106(7) | 2.022(3) | 1.988(7) |
| M2–N | 2.120(6) | 2.005(5) | 1.974(4) | 1.983(15) |
| M3–N | 1.982(6) | 2.076(6) | 1.961(4) | 2.125(5) |
| Metal–Ca | ||||
| M1–C | 2.225(8)–2.602(8) | 2.188(15)–2.632(17) | 2.247(5)–2.387(5) | 2.058(3)–2.443(3) |
| M2–C | 2.276(10)–2.413(9) | 2.146(13)–2.503(18) | 2.222(5)–2.381(5) | 2.024(16)–2.440(17) |
| M3–C | 2.266(7)–2.661(9) | 2.088(15)–2.612(12) | 2.237(5)–2.380(5) | 2.075(4)–2.440(5) |
| Angles (°) | ||||
| ∑(M–N–M) | 357.9 | 357.2 | 359.8 | 360.0 |
Sc3N@D5h(6)-C80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
Sc3N@Ih(7)-C80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
Sc3N@C2v(39718)-C82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
Sc3N@Cs(39663)-C82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
|
|---|---|---|---|---|
| a Range of distances between the metal atom and the nearest six carbon atoms. | ||||
| Distance (Å) | ||||
| M1–N | 2.014(2) | 2.011(19) | 2.007(4) | 2.112(3) |
| M2–N | 2.031(2) | 1.966(12) | 2.078(3) | 2.052(4) |
| M3–N | 2.041(2) | 1.95(3) | 2.078(3) | 2.038(3) |
| Metal–Ca | ||||
| M1–C | 2.255(3)–2.512(3) | 2.188(10)–2.508(13) | 2.269(17)–2.607(23) | 2.316(6)–2.444(6) |
| M2–C | 2.269(3)–2.624(3) | 2.148(10)–2.516(13) | 2.190(17)–2.613(11) | 2.272(6)–2.553(5) |
| M3–C | 2.232(3)–2.537(3) | 2.18(3)–2.43(3) | 2.117(15)–2.452(11) | 2.256(5)–2.578(6) |
| Angles (°) | ||||
| ∑(M–N–M) | 359.9 | 360.0 | 359.9 | 359.5 |
The Sc–N distances of the Sc3N cluster in the Cs(51365)-C84 cage are 2.083(6) Å for Sc1A–N, 2.120(6) Å for Sc2A–N, and 1.981(6) Å for Sc3A–N. The longer bond length of Sc2A–N can be well explained by the fused pentagons coordinated to Sc2A, which strongly interact with Sc2A and withdraw the electron density from the Sc–N unit, thus weakening the Sc–N bond interaction. Similar phenomena have been observed in other non-IPR nitride cluster fullerenes, such as M3N@C2(22010)-C78 (M = Gd, Tb and Ho),24,25 M3N@Cs(39663)-C82 (M = Gd and Sc),26,27 and M3N@Cs(51365)-C84 (M = Gd, Tb, Er, Tm, Lu and Sc).33–36
Unlike the non-IPR cage of Cs(51365)-C84, there are no fused pentagons in the cage of D3(19)-C86. However, unexpectedly, one of the Sc–N bonds, Sc1–N, also has a longer distance of 2.106(7) Å, compared with 2.005(5) Å for Sc2–N and 2.076(6) Å for Sc3–N, suggesting that Sc1–N is stretched out to facilitate a closer metal–cage contact in IPR D3(19)-C86. Notably, the stretching of M–N bonds in M3N@D3(19)-C86 (M = Gd and Tb) has not been observed.31,37 Thus, this stretched configuration of Sc3N inside D3(19)-C86 suggests that small encapsulated metallic clusters (Sc3N) can adapt their configurations not only inside non-IPR fullerene cages, as generally acknowledged but also in larger IPR fullerene cages, such as C86, to facilitate a closer interaction with the carbon atoms on fullerene cages, which is beneficial for the stabilization of the whole EMF molecule.
Pyramidalization of Sc3N clusters inside large cages
Fig. 2e and f show the out-of-plane position of nitrogen atoms in the Sc3N clusters inside Cs(51365)-C84 and D3(19)-C86, respectively. The Sc–N–Sc angles in the cages of Cs(51365)-C84 and D3(19)-C86 are 357.9° and 357.2°, respectively, suggesting that the Sc3N clusters do not adapt to a fully planar configuration. Moreover, the nitrogen atom is 0.175 Å out of the Sc3 plane in Sc3N@Cs(51365)-C84 and 0.197 Å out of the Sc3 plane in Sc3N@D3(19)-C86, indicating a slight pyramidalization of Sc3N clusters. This observation is rather unexpected as Sc3N is the smallest metallic nitride cluster in the NCF family and always adapts a planar configuration inside fullerene cages, ranging from C68 to C82, as shown in Table 1 and Fig. 4 and S5†8,27,38–42 In fact, to date, the pyramidalization of clusters has only been observed for large clusters, such as Y3N and Gd3N, encaged inside cages smaller than C82. The main cause for this pyramidalization of metallic clusters is the forced squeezing of the large-sized clusters in the small carbon cages, such as M3N@Ih(7)-C80 (M = Y, Gd and Tb).29–31 A general expectation would be that small clusters would not suffer from the compression of the carbon cage and thus would easily adapt to a fully planar configuration. This result, however, validates that small clusters can also be pyramidalized in large cages. Thus, it suggests that the driving force for the pyramidalization of encapsulated clusters is not merely the compressing effect of the cage but, more generally, the self-adaptation of clusters, which facilitates an appropriate interaction between the metal ion and the carbon atoms on the cage.Off-center shift of the Sc3N clusters inside large cages
Fig. 3a shows the positions of clusters in the carbon cages of M3N@Cs(51365)-C84 (M = Gd, Tb, Er, Tm, Lu and Sc).33–36 It can be clearly observed that in the previously reported NCFs, such as M3N@Cs(51365)-C84 (M = Gd, Tb, Er, Tm and Lu), the M3N clusters are all located at the center of the fullerene carbon cages. In comparison, the Sc3N cluster notably shifts from the center of the carbon cage to one side of the Cs(51365)-C84 cage. The same off-center location of the inner Sc3N cluster is observed for Sc3N@D3(19)-C86. For comparison, all of the larger clusters, such as Gd3N and Tb3N, are located at the center of the carbon cage, and the nitrogen atoms are located on the 3-fold axis of the D3(19)-C86 cage (Fig. 3c).31,37In addition, Fig. 3b shows the relative positions for the symmetry plane of the Cs(51365)-C84 cage (marked in red) and the planes of the metal nitride clusters (marked in green) in M3N@Cs(51365)-C84 (M = Gd, Tb, Er, Tm, Lu and Sc).33–36 It shows that inside C84 cages, the dihedral angles between the planes of clusters and the symmetry plane are in the range of 22.2°–26.7°, whereas the dihedral angle between the Sc3N plane and the symmetry plane is 50.2°, suggesting its considerable shift from the center. An even greater off-center shift is observed for Sc3N@D3(19)-C86. The dihedral angle in this case, formed by the plane that bisects the lateral hexagon (marked in red) and the plane in which the Sc3N cluster is located (marked in green), notably increases to 72.7° in Sc3N@D3(19)-C86 (Fig. 3d). For M3N@D3(19)-C86 (M = Gd and Tb), in which the larger Gd3N and Tb3N clusters are located at the center of the cages, the corresponding dihedral angles are only in the range of 20.9–21.2°.31,37
On the other hand, Fig. 4 and Fig. S6† show that the distances between the Sc-triangle planes and the center of gravity of the carbon cages are in the range of 0.005–0.110 Å from C68 to C82. However, the distances of Sc3N@C84 and Sc3N@C86 increase significantly to 0.473 Å and 0.504 Å, respectively, which also indicates the dramatic off-center shift of the Sc3N clusters in the C84 and C86 carbon cages.8,27,38–42
Discussion
Previously, it was generally acknowledged that relatively small nitride clusters, such as Sc3N, favor C80 and smaller cages; for large clusters such as Nd3N, encapsulation into the C88 or larger cages is energetically preferred.28 These theories have also been verified by many experimental results.8,38–40,42–45 Interestingly, NCFs in which smaller clusters are encapsulated inside large cages, such as Sc3N@C2n with cages larger than C82, have never been observed before. On the other hand, theoretical calculations on NCFs predicted the off-center shift of the Sc3N clusters in large carbon cages such as C84, C88 and C96.28,32 Meanwhile, they also pointed out that this shift could result in a less effective cluster–cage interaction; thus, the Sc3N-based NCFs with cage sizes of C84 and larger might not be very stable, which was consistent with the absence of experimental reports of these structures.32 The above crystallographic analysis, however, validates the stabilization of Sc3N@C84 and Sc3N@C86. Moreover, it reveals the off-center shift and, surprisingly, the slight pyramidalization of the Sc3N clusters in these large cages. These observations, together with the stretched Sc–N bonds, suggest that in large cages, due to the small size, the Sc3N cluster favors an off-center location and attaches to one side of the carbon cage like a spider. In addition, it can also adjust its configuration to be slightly pyramidalized to further optimize the Sc–N distances and metal–cage interactions, ultimately forming stable host–guest molecular compounds.UV–vis–NIR spectroscopic characterization
The purified samples of Sc3N@Cs(51365)-C84 and Sc3N@D3(19)-C86 dissolved in carbon disulfide (CS2) were characterized using UV–vis–NIR absorption spectroscopy, as shown in Fig. 5. The spectrum of Sc3N@Cs(51365)-C84 shows two minor shoulder peaks (480 and 669 nm, respectively) with an onset at approximately 1350 nm, resulting in an optical band gap of 0.92 eV. The absorption spectrum is almost identical to the previously reported spectra of M3N@Cs(51365)-C84 (M = Er, Lu, Gd, Tb and Tm),33–36 suggesting that they have the same cage symmetries and charge transfer patterns. In the spectrum of Sc3N@D3(19)-C86, two shoulder peaks at 496 and 691 nm are observed, which are similar to the absorption spectrum of Tb3N@D3(19)-C86,31 indicating that they have the same isomeric structures. The absorption onset is measured at approximately 1400 nm, and the optical band gap is determined to be 0.88 eV. In addition, the optical band gaps of Sc3N@Cs(51365)-C84 and Sc3N@D3(19)-C86 are smaller than those of the other reported members of the NCF family with the same carbon cages.31,33–36 Such small optical band gaps of Sc3N@Cs(51365)-C84 and Sc3N@D3(19)-C86 suggest that a further increase in the large carbon cage size is not favorable for Sc3N cluster fullerene formation, which is consistent with theoretical calculations.32Conclusions
In summary, two novel nitride cluster fullerenes, Sc3N@C2n (2n = 84 and 86), have been successfully synthesized and characterized by MALDI-TOF mass spectrometry, single-crystal XRD and UV–vis–NIR spectroscopy. The molecular structures of the two NCFs were identified as Sc3N@Cs(51365)-C84 and Sc3N@D3(19)-C86 using single crystal X-ray diffraction analysis. Crystallographic analysis shows that, while in most previously reported cluster fullerenes, clusters intend to take a central position inside fullerene cages, in these two structures, the Sc3N clusters are notably shifted to one side of the cage and unexpectedly pyramidalized, which resembles a spider attached to a web. These observations, together with the stretched Sc–N bonds, suggest that the endohedral M3N cluster can self-adjust not only its size and configuration, but also its position relative to fullerenes to optimize the metal–cage distances as well as cluster–cage interactions, thus promoting stability with large cages. This work not only validates the stability of endohedral structures with large carbon cages encapsulating small nitride clusters, but also, more importantly, reveals the unexpected flexibility of cluster–cage interactions, which provides new insight into the interaction mechanisms between the metal clusters and carbon cages of EMFs.Experimental
Synthesis and isolation of Sc3N@C2n (2n = 84 and 86)
Carbon soot containing scandium NCFs was synthesized by a direct-current arc discharge method. Graphite rods packed with Sc2O3 and graphite powder (1.02 g of Sc2O3 powder and 1.97 g of graphite powder per rod and a molar ratio of Sc/C = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) were vaporized in an arcing chamber under a 200 Torr helium atmosphere with 4 Torr N2. The collected raw soot was extracted with carbon disulfide (CS2) for 24 h. The separation and purification of Sc3N@C2n (2n = 84 and 86) were achieved using multistage HPLC procedures. Multiple HPLC columns, including a Buckyprep-M column (25 × 250 mm, Cosmosil, Nacalai Tesque Inc.), a Buckyprep-D column (10 × 250 mm, Cosmosil, Nacalai Tesque, Japan), a 5PBB column (10 × 250 mm, Cosmosil, Nacalai Tesque, Japan), and a Buckprep column (10 × 250 mm, Cosmosil, Nacalai Tesque, Japan), were used in the procedures (further details are described in the ESI†).
15) were vaporized in an arcing chamber under a 200 Torr helium atmosphere with 4 Torr N2. The collected raw soot was extracted with carbon disulfide (CS2) for 24 h. The separation and purification of Sc3N@C2n (2n = 84 and 86) were achieved using multistage HPLC procedures. Multiple HPLC columns, including a Buckyprep-M column (25 × 250 mm, Cosmosil, Nacalai Tesque Inc.), a Buckyprep-D column (10 × 250 mm, Cosmosil, Nacalai Tesque, Japan), a 5PBB column (10 × 250 mm, Cosmosil, Nacalai Tesque, Japan), and a Buckprep column (10 × 250 mm, Cosmosil, Nacalai Tesque, Japan), were used in the procedures (further details are described in the ESI†).
Spectroscopic studies
A positive-ion mode matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometer (Bruker, Germany) was used for mass characterization. The UV–vis–NIR spectra of purified Sc3N@C2n (2n = 84 and 86) were recorded in CS2 solution with a Cary 5000 UV–vis–NIR spectrophotometer (Agilent, USA).X-ray crystallographic study
The black block crystals of Sc3N@C2n (2n = 84 and 86) were obtained by slow diffusion of the carbon disulfide solution of the corresponding metallofullerene compounds into a benzene solution of [NiII(OEP)]. The single-crystal X-ray data of Sc3N@C84 and Sc3N@C86 were collected at 120 K on a diffractometer (APEX II; Bruker Analytik GmbH) equipped with a CCD collector. The multiscan method was used for absorption correction. The structures were solved using intrinsic phasing methods46 and refined on F2 using full-matrix least-squares with the SHELXL 2018 crystallographic software package.47 Hydrogen atoms were inserted at calculated positions and constrained with isotropic thermal parameters.Crystal data for Sc3N@Cs(51365)-C84·[NiII(OEP)]: Mr = 1749.19, 0.12 mm × 0.1 mm × 0.07 mm, triclinic, P1 (no. 2), a = 14.6460(18) Å, b = 14.9090(19) Å, c = 19.743(3) Å, α = 85.084(7)°, β = 88.542(7)°, γ = 62.548(7)°, V = 3811.0(9) Å3, Z = 2, ρcalcd = 1.524 g cm−3, μ(Ga Kα) = 3.152 mm−1, θ = 1.954–52.000, T = 120(2) K, R1 = 0.1245, and wR2 = 0.3112 for all data; R1 = 0.1038 and wR2 = 0.2927 for 9662 reflections (I > 2.0σ(I)) with 1199 parameters. The goodness-of-fit indicator was 1.057. The maximum residual electron density was 1.735 e Å−3.
Crystal data for Sc3N@D3(19)-C86·[NiII(OEP)]·C6H6: Mr = 1851.32, 0.1 mm × 0.08 mm × 0.06 mm, monoclinic, C2/m (no. 12), a = 26.259(3) Å, b = 17.9994(19) Å, c = 17.8301(16) Å, α = 90°, β = 108.472(4)°, γ = 90°, V = 7993.0(14) Å3, Z = 4, ρcalcd = 1.538 g cm−3, μ(Ga Kα) = 3.028 mm−1, θ = 2.273–53.906, T = 120(2) K, R1 = 0.1430, and wR2 = 0.2667 for all data; R1 = 0.0904 and wR2 = 0.2305 for 4504 reflections (I > 2.0σ(I)) with 1244 parameters. The goodness-of-fit indicator was 1.057. The maximum residual electron density was 0.758 e Å−3.
The crystallographic data for these two structures have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the deposition numbers 2178354 and 2178355.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
N. C. thanks the National Science Foundation China (NSFC nos 52172051 and 91961109), the Natural Science Foundation of Jiangsu Province (BK20200041) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- X. Lu, L. Feng, T. Akasaka and S. Nagase, Current status and future developments of endohedral metallofullerenes, Chem. Soc. Rev., 2012, 41, 7723–7760 RSC.
- A. A. Popov, S. Yang and L. Dunsch, Endohedral fullerenes, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed.
- L. Bao, P. Peng and X. Lu, Bonding inside and outside fullerene cages, Acc. Chem. Res., 2018, 51, 810–815 CrossRef CAS PubMed.
- W. Li, F. Qu, L. Liu, Z. Zhang, J. Liang, Y. Lu, J. Zhang, L. Wang, C. Wang and T. Wang, A metallofullertube of Ce2@C100 with a carbon nanotube segment: synthesis, single-molecule conductance and supramolecular assembly, Angew. Chem., 2022, 61, e202116854 CAS.
- A. R. Puente Santiago, M. F. Sanad, A. Moreno-Vicente, M. A. Ahsan, M. R. Cerón, Y.-R. Yao, S. T. Sreenivasan, A. Rodriguez-Fortea, J. M. Poblet and L. Echegoyen, A new class of molecular electrocatalysts for hydrogen evolution: Catalytic activity of M3N@C2n (2n = 68, 78, and 80) fullerenes, J. Am. Chem. Soc., 2021, 143, 6037–6042 CrossRef CAS PubMed.
- C. Zhou, M. Zhen, M. Yu, X. Li, T. Yu, J. Liu, W. Jia, S. Liu, L. Li, J. Li, Z. Sun, Z. Zhao, X. Wang, X. Zhang, C. Wang and C. Bai, Gadofullerene inhibits the degradation of apolipoprotein B100 and boosts triglyceride transport for reversing hepatic steatosis, Sci. Adv., 2020, 6, eabc1586 CrossRef CAS PubMed.
- K. Zhang, C. Wang, M. Zhang, Z. Bai, F.-F. Xie, Y.-Z. Tan, Y. Guo, K.-J. Hu, L. Cao, S. Zhang, X. Tu, D. Pan, L. Kang, J. Chen, P. Wu, X. Wang, J. Wang, J. Liu, Y. Song, G. Wang, F. Song, W. Ji, S.-Y. Xie, S.-F. Shi, M. A. Reed and B. Wang, A Gd@C82 single-molecule electret, Nat. Nanotechnol., 2020, 15, 1019–1024 CrossRef CAS PubMed.
- S. Stevenson, G. Rice, T. Glass, K. Harich, F. Cromer, M. R. Jordan, J. Craft, E. Hadju, R. Bible, M. M. Olmstead, K. Maitra, A. J. Fisher, A. L. Balch and H. C. Dorn, Small-bandgap endohedral metallofullerenes in high yield and purity, Nature, 1999, 401, 55–57 CrossRef CAS.
- S. Yang, T. Wei and F. Jin, When metal clusters meet carbon cages: endohedral clusterfullerenes, Chem. Soc. Rev., 2017, 46, 5005–5058 RSC.
- Q. Tang, L. Abella, Y. Hao, X. Li, Y. Wan, A. Rodríguez-Fortea, J. M. Poblet, L. Feng and N. Chen, Sc2O@C2v(5)-C80: Dimetallic oxide cluster inside a C80 fullerene cage, Inorg. Chem., 2015, 54, 9845–9852 CrossRef CAS PubMed.
- Q. Tang, L. Abella, Y. Hao, X. Li, Y. Wan, A. Rodríguez-Fortea, J. M. Poblet, L. Feng and N. Chen, Sc2O@C3v(8)-C82: A missing isomer of Sc2O@C82, Inorg. Chem., 2016, 55, 1926–1933 CrossRef CAS PubMed.
- T. Yang, Y. Hao, L. Abella, Q. Tang, X. Li, Y. Wan, A. Rodríguez-Fortea, J. M. Poblet, L. Feng and N. Chen, Sc2O@Td(19151)-C76: Hindered cluster motion inside a tetrahedral carbon cage probed by crystallographic and computational studies, Chem. – Eur. J., 2015, 21, 11110–11117 CrossRef CAS PubMed.
- Q. Deng and A. A. Popov, Clusters encapsulated in endohedral metallofullerenes: How strained are they?, J. Am. Chem. Soc., 2014, 136, 4257–4264 CrossRef CAS PubMed.
- C.-H. Chen, K. B. Ghiassi, M. R. Cerón, M. A. Guerrero-Ayala, L. Echegoyen, M. M. Olmstead and A. L. Balch, Beyond the butterfly: Sc2C2@C2v(9)-C86, an endohedral fullerene containing a planar, twisted Sc2C2 unit with remarkable crystalline order in an unprecedented carbon cage, J. Am. Chem. Soc., 2015, 137, 10116–10119 CrossRef CAS PubMed.
- S. Hu, P. Zhao, W. Shen, M. Ehara, Y. Xie, T. Akasaka and X. Lu, Crystallographic characterization of Er2C2@C80–88: Cluster stretching with cage elongation, Inorg. Chem., 2020, 59, 1940–1946 CrossRef CAS PubMed.
- F. Liu, C.-L. Gao, Q. Deng, X. Zhu, A. Kostanyan, R. Westerström, S. Wang, Y.-Z. Tan, J. Tao, S.-Y. Xie, A. A. Popov, T. Greber and S. Yang, Triangular monometallic cyanide cluster entrapped in carbon cage with geometry-dependent molecular magnetism, J. Am. Chem. Soc., 2016, 138, 14764–14771 CrossRef CAS PubMed.
- R. Guan, M. Chen, J. Xin, X.-M. Xie, F. Jin, Q. Zhang, S.-Y. Xie and S. Yang, Capturing the missing carbon cage isomer of C84 via mutual stabilization of a triangular monometallic cyanide cluster, J. Am. Chem. Soc., 2021, 143, 8078–8085 CrossRef CAS PubMed.
- J. Xin, F. Jin, R. Guan, M. Chen, X.-M. Xie, Q. Zhang, S.-Y. Xie and S. Yang, Ancient pigment to treasure: Prussian blue as a cheap solid cyanide/nitrogen dual-source affording the high-yield syntheses of pricey endohedral clusterfullerenes, Inorg. Chem. Front., 2021, 8, 1719–1726 RSC.
- J. Zhang, T. Fuhrer, W. Fu, J. Ge, D. W. Bearden, J. Dallas, J. Duchamp, K. Walker, H. Champion, H. Azurmendi, K. Harich and H. C. Dorn, Nanoscale fullerene compression of an yttrium carbide cluster, J. Am. Chem. Soc., 2012, 134, 8487–8493 CrossRef CAS PubMed.
- W. Shen, Z. Hu, P. Yu, Z. Wei, P. Jin, Z. Shi and X. Lu, An experimental and theoretical study of LuNC@C76,82 revealing a cage-cluster selection rule, Inorg. Chem. Front., 2020, 7, 4563–4571 RSC.
- H. W. Kroto, The stability of the fullerenes Cn, with n = 24, 28, 32, 36, 50, 60 and 70, Nature, 1987, 329, 529–531 CrossRef CAS.
- Y. Zhang, K. B. Ghiassi, Q. Deng, N. A. Samoylova, M. M. Olmstead, A. L. Balch and A. A. Popov, Synthesis and structure of LaSc2N@Cs(hept)-C80 with one heptagon and thirteen pentagons, Angew. Chem., Int. Ed., 2015, 54, 495–499 CAS.
- C.-H. Chen, L. Abella, M. R. Cerón, M. A. Guerrero-Ayala, A. Rodríguez-Fortea, M. M. Olmstead, X. B. Powers, A. L. Balch, J. M. Poblet and L. Echegoyen, Zigzag Sc2C2 carbide cluster inside a [88]fullerene cage with one heptagon, Sc2C2@Cs(hept)-C88: A kinetically trapped fullerene formed by C2 insertion?, J. Am. Chem. Soc., 2016, 138, 13030–13037 CrossRef CAS PubMed.
- C. M. Beavers, M. N. Chaur, M. M. Olmstead, L. Echegoyen and A. L. Balch, Large metal ions in a relatively small fullerene cage: The structure of Gd3N@C2(22010)-C78 departs from the isolated pentagon rule, J. Am. Chem. Soc., 2009, 131, 11519–11524 CrossRef CAS PubMed.
- S. Stevenson, A. J. Rothgeb, K. R. Tepper, J. Duchamp, H. C. Dorn, X. B. Powers, M. Roy, M. M. Olmstead and A. L. Balch, Isolation and crystallographic characterization of two, nonisolated pentagon endohedral fullerenes: Ho3N@C2(22010)-C78 and Tb3N@C2(22010)-C78, Chem. – Eur. J., 2019, 25, 12545–12551 CrossRef CAS PubMed.
- B. Q. Mercado, C. M. Beavers, M. M. Olmstead, M. N. Chaur, K. Walker, B. C. Holloway, L. Echegoyen and A. L. Balch, Is the isolated pentagon rule merely a suggestion for endohedral fullerenes? The structure of a second egg-shaped endohedral fullerene—Gd3N@Cs(39663)-C82, J. Am. Chem. Soc., 2008, 130, 7854–7855 CrossRef CAS PubMed.
- M. Guo, X. Li, Y.-R. Yao, J. Zhuang, Q. Meng, Y. Yan, X. Liu and N. Chen, A non-isolated pentagon rule C82 cage stabilized by a stretched Sc3N cluster, Chem. Commun., 2021, 57, 4150–4153 RSC.
- X. Aparicio-Anglès, N. Alegret, A. Clotet, A. Rodríguez-Fortea and J. M. Poblet, Endohedral metallofullerenes containing lanthanides: A robust yet simple computational approach, J. Phys. Chem. C, 2013, 117, 12916–12921 CrossRef.
- S. Stevenson, J. P. Phillips, J. E. Reid, M. M. Olmstead, S. P. Rath and A. L. Balch, Pyramidalization of Gd3N inside a C80 cage. The synthesis and structure of Gd3N@C80, Chem. Commun., 2004, 2814–2815, 10.1039/B412338G.
- L. Echegoyen, C. J. Chancellor, C. M. Cardona, B. Elliott, J. Rivera, M. M. Olmstead and A. L. Balch, X-Ray crystallographic and EPR spectroscopic characterization of a pyrrolidine adduct of Y3N@C80, Chem. Commun., 2006, 2653–2655, 10.1039/B604011J.
- T. Zuo, C. M. Beavers, J. C. Duchamp, A. Campbell, H. C. Dorn, M. M. Olmstead and A. L. Balch, Isolation and structural characterization of a family of endohedral fullerenes including the large, chiral cage fullerenes Tb3N@C88 and Tb3N@C86 as well as the Ih and D5h isomers of Tb3N@C80, J. Am. Chem. Soc., 2007, 129, 2035–2043 CrossRef CAS PubMed.
- A. A. Popov and L. Dunsch, Structure, stability, and cluster-cage interactions in nitride clusterfullerenes M3N@C2n (M = Sc, Y; 2n = 68–98): A density functional theory study, J. Am. Chem. Soc., 2007, 129, 11835–11849 CrossRef CAS PubMed.
- C. M. Beavers, T. Zuo, J. C. Duchamp, K. Harich, H. C. Dorn, M. M. Olmstead and A. L. Balch, Tb3N@C84: An improbable, egg-shaped endohedral fullerene that violates the isolated pentagon rule, J. Am. Chem. Soc., 2006, 128, 11352–11353 CrossRef CAS PubMed.
- T. Zuo, K. Walker, M. M. Olmstead, F. Melin, B. C. Holloway, L. Echegoyen, H. C. Dorn, M. N. Chaur, C. J. Chancellor, C. M. Beavers, A. L. Balch and A. J. Athans, New egg-shaped fullerenes: Non-isolated pentagon structures of Tm3N@Cs(51365)-C84 and Gd3N@Cs(51365)-C84, Chem. Commun., 2008, 1067–1069, 10.1039/B716037B.
- W.-Q. Shen, L.-P. Bao, S.-F. Hu, X.-J. Gao, Y.-P. Xie, X.-F. Gao, W.-H. Huang and X. Lu, Isolation and crystallographic characterization of Lu3N@C2n (2n = 80–88): Cage selection by cluster size, Chem. – Eur. J., 2018, 24, 16692–16698 CrossRef CAS PubMed.
- S. Hu, P. Zhao, W. Shen, P. Yu, W. Huang, M. Ehara, Y. Xie, T. Akasaka and X. Lu, Crystallographic characterization of Er3N@C2n (2n = 80, 82, 84, 88): The importance of a planar Er3N cluster, Nanoscale, 2019, 11, 13415–13422 RSC.
- M. N. Chaur, X. Aparicio-Anglès, B. Q. Mercado, B. Elliott, A. Rodríguez-Fortea, A. Clotet, M. M. Olmstead, A. L. Balch, J. M. Poblet and L. Echegoyen, Structural and electrochemical property correlations of metallic nitride endohedral metallofullerenes, J. Phys. Chem. C, 2010, 114, 13003–13009 CrossRef CAS.
- S. Stevenson, P. W. Fowler, T. Heine, J. C. Duchamp, G. Rice, T. Glass, K. Harich, E. Hajdu, R. Bible and H. C. Dorn, A stable non-classical metallofullerene family, Nature, 2000, 408, 427–428 CrossRef CAS PubMed.
- M. M. Olmstead, H. M. Lee, J. C. Duchamp, S. Stevenson, D. Marciu, H. C. Dorn and A. L. Balch, Sc3N@C68: Folded pentalene coordination in an endohedral fullerene that does not obey the isolated pentagon rule, Angew. Chem., Int. Ed., 2003, 42, 900–903 CrossRef CAS PubMed.
- T. Cai, L. Xu, M. R. Anderson, Z. Ge, T. Zuo, X. Wang, M. M. Olmstead, A. L. Balch, H. W. Gibson and H. C. Dorn, Structure and enhanced reactivity rates of the D5h Sc3N@C80 and Lu3N@C80 metallofullerene isomers: The importance of the pyracylene motif, J. Am. Chem. Soc., 2006, 128, 8581–8589 CrossRef CAS PubMed.
- T. Wei, S. Wang, F. Liu, Y. Tan, X. Zhu, S. Xie and S. Yang, Capturing the long-sought small-bandgap endohedral fullerene Sc3N@C82 with low kinetic stability, J. Am. Chem. Soc., 2015, 137, 3119–3123 CrossRef CAS PubMed.
- M. M. Olmstead, A. de Bettencourt-Dias, J. C. Duchamp, S. Stevenson, D. Marciu, H. C. Dorn and A. L. Balch, Isolation and structural characterization of the endohedral fullerene Sc3N@C78, Angew. Chem., Int. Ed., 2001, 40, 1223–1225 CrossRef CAS PubMed.
- S. Yang, A. A. Popov and L. Dunsch, Violating the isolated pentagon rule (IPR): The endohedral non-IPR C70 cage of Sc3N@C70, Angew. Chem., Int. Ed., 2007, 46, 1256–1259 CrossRef CAS PubMed.
- M. N. Chaur, F. Melin, B. Elliott, A. Kumbhar, A. J. Athans and L. Echegoyen, New M3N@C2n endohedral metallofullerene families (M = Nd, Pr, Ce; n = 40–53): Expanding the preferential templating of the C88 cage and approaching the C96 cage, Chem. – Eur. J., 2008, 14, 4594–4599 CrossRef CAS PubMed.
- M. N. Chaur, R. Valencia, A. Rodríguez-Fortea, J. M. Poblet and L. Echegoyen, Trimetallic nitride endohedral fullerenes: Experimental and theoretical evidence for the M3N6+@C2n6− model, Angew. Chem., Int. Ed., 2009, 48, 1425–1428 CrossRef CAS PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: A complete structure solution, refinement and analysis program, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- G. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2178354 and 2178355. For ESI and crystallographic data in CIF or another electronic format see DOI: https://doi.org/10.1039/d2qi01318e |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2022 |




