“Breathing” organic cation to stabilize multiple structures in low-dimensional Ge-, Sn-, and Pb-based hybrid iodide perovskites†
Abstract
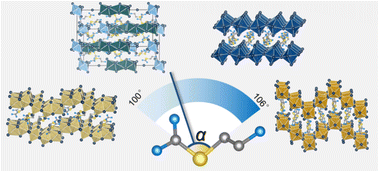
Low-dimensional hybrid inorganic–organic perovskites are excellent candidates for stable optoelectronic devices. The dimensionality of these perovskites depends largely on the organic and inorganic compositions, as well as the synthetic conditions. Here, we report five new hybrid iodides, (ETU)4Ge5I18, (ETU)GeI4, (ETU)SnI4, (ETU)PbI4, and (ETU)3Pb2I10 using only one type of organic cation, namely, S-(2-aminoethyl)isothiouronium (ETU). (ETU)GeI4 and (ETU)SnI4 belong to the (110)-oriented structure-type with “3 × 3” sawtooth corrugated layers and crystallize in a structure with the orthorhombic space group Pbca. (ETU)4Ge5I18 crystallizes in a structure with the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , featuring a 2D layered structure with combinations of corner, edge, and face-sharing [GeI6] octahedra. For the Pb-based series, (ETU)PbI4 has the conventional (100) – oriented 2D type whereas (ETU)3Pb2I10 has a unique 0D structure. Remarkably, the unstable 2D orange-phase (ETU)PbI4 transforms to a stable 0D yellow phase (ETU)3Pb2I10, accompanied by the reduction of the C–S–C angle of the organic cation ETU. The optical band gaps are largely regulated by the diverse types of structure and are in the range of 1.8 eV to 2.8 eV. (ETU)SnI4 is the only material showing notable photoluminescence at room-temperature. Our work showcases the flexibility of the organic cation in determining the structural dimensionality and provides a new strategy in generating new hybrid materials.
, featuring a 2D layered structure with combinations of corner, edge, and face-sharing [GeI6] octahedra. For the Pb-based series, (ETU)PbI4 has the conventional (100) – oriented 2D type whereas (ETU)3Pb2I10 has a unique 0D structure. Remarkably, the unstable 2D orange-phase (ETU)PbI4 transforms to a stable 0D yellow phase (ETU)3Pb2I10, accompanied by the reduction of the C–S–C angle of the organic cation ETU. The optical band gaps are largely regulated by the diverse types of structure and are in the range of 1.8 eV to 2.8 eV. (ETU)SnI4 is the only material showing notable photoluminescence at room-temperature. Our work showcases the flexibility of the organic cation in determining the structural dimensionality and provides a new strategy in generating new hybrid materials.
- This article is part of the themed collection: Inorganic Chemistry Frontiers Emerging Investigator Series 2022–2023


 Please wait while we load your content...
Please wait while we load your content...
