Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct synthesis of a stable radical doped electrically conductive coordination polymer†
Yong
Yan
a,
Ning-Ning
Zhang
*b,
Lisa Marie
Tauche
c,
Kavipriya
Thangavel
 c,
Andreas
Pöppl
c,
Andreas
Pöppl
 c and
Harald
Krautscheid
c and
Harald
Krautscheid
 *a
*a
aFakultät für Chemie und Mineralogie, Institut für Anorganische Chemie, Universität Leipzig, Johannisallee 29, 04103 Leipzig, Germany. E-mail: Krautscheid@rz.uni-leipzig.de
bSchool of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng 252059, P. R. China. E-mail: zhangningning@lcu.edu.cn
cFelix Bloch Institute for Solid State Physics, Universität Leipzig, Linnéstraβe 5, 04103 Leipzig, Germany
First published on 12th August 2022
Abstract
Radical coordination polymers exhibit tremendous potential in many areas. However, stable radical ligands are still rare and the syntheses of radical ligand derived coordination polymers are mainly limited to liquid diffusion based methods. Therefore, further exploration of new types of stable radical coordination polymers is strongly desired. Herein, we report on an NDI-based coordination polymer, [K2(ONDI)]∞ (K-ONDI), which is synthesized directly by solvothermal reaction and exhibits radical properties and high stability in air as well as in common organic solvents. The NDI˙− radicals in K-ONDI are supposed to originate from electron transfer to the ONDI2− ligand under solvothermal conditions. Furthermore, benefitting from continuous π-stacking of the ligand in the crystal structure and unpaired electrons from NDI˙−, K-ONDI shows intriguing semiconductive behaviour with an electrical conductivity value of 10−6 S cm−1.
Introduction
Owing to potential applications in the fields of magnetic1 or optical materials,2 catalysts,3 and electrical energy storage,4 radical coordination polymers (RCPs) have attracted huge attention recently. Unfortunately, although RCPs exhibit intriguing potential, their radical states usually are not stable in air because of the high reactivity of ligands with unpaired electrons. In most cases, RCPs are sensitive to oxygen and need handling in inert atmosphere. In order to obtain stable RCPs, radical ligands with good stability have been introduced, e.g., N-oxyl derivatives,3 perchlorotriphenylmethyl,5 and tetracyanoquinodimethane (TCNQ) derivatives.1 However, available and stable radical ligands are still rare until now.6 In addition, it is hard to maintain stability for most radical ligands under solvothermal conditions that are used widely for coordination polymers synthesis. The synthesis methods of RCPs are mainly limited to liquid diffusion.1,5 Exploration of new types of stable RCPs is still a challenge.Besides synthesis from radical ligands, RCPs can also be obtained by reducing or oxidizing redox-active coordination polymers.7 In comparison to the limited number of radical ligands, neutral redox-active molecules that can form radical states under specific conditions are more easily accessible. Naphthalenediimides (NDI) are well known redox-active compounds featuring large planar structures with extended π-electron systems8a,b and have been widely used to design multifunctional coordination polymers, such as photochromic or electrochromic materials, sensors, and energy storage and conversion materials.8c,d Until now, radical character of coordination polymers based on NDI derived ligands was mainly generated by post-synthetic treatment like photo irradiation or reduction by chemical reagents.9 Direct syntheses of NDI based radical coordination polymers are limited to electrochemical method.10 Besides, the radical state of most NDI based coordination polymers does not remain for a long time because of easy re-oxidation of the NDI˙− core when exposed to air,9c which limits their potential applications. Further exploration of direct synthesis of stable radical NDI based coordination polymers is desired.
According to literature,11 coordination bonds and π–π interactions, as two kinds of non-covalent interactions, are favourable for stabilizing radicals. It is well known that in the NDI˙− radical anion, the unpaired electron is delocalized over the naphthalene core structure and even extends into substituents on the nitrogen atoms in some cases.8c,12 If the NDI core can coordinate to metal ions directly, the radical state of NDI˙− might be stabilized. However, in most cases the NDI core was only taken as a redox-active moiety and not directly engaged in coordination to metal ions.7b Recently, we found that the N-hydroxy modified NDI ligand H2ONDI (H2ONDI = 2,7-dihydroxybenzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetraone) can be deprotonated and coordinate to metal ions via the hydroxamate and carbonyl groups of the NDI core.13a Besides, ONDI2− prefers to form π−π stacking structures when assembled with metal ions, which is also favourable for stabilizing radicals. This ligand has hardly been used for the synthesis of RCPs before.13 Herein, we report on the NDI based coordination polymer [K2(ONDI)]∞ (K-ONDI; ONDI2− = 2,7-dioxybenzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetraone) with radical properties that was synthesized directly in a solvothermal reaction. The radical species remain stable even when exposed to air for one month or upon immersing into common organic solvents for five days. Furthermore, the concentration of radicals in K-ONDI can be tuned by adjusting the synthesis temperature, and it thus exhibits interesting semiconductive behaviour with a moderate electrical conductivity reaching about 10−6 S cm−1.
Results and discussion
Crystal structure
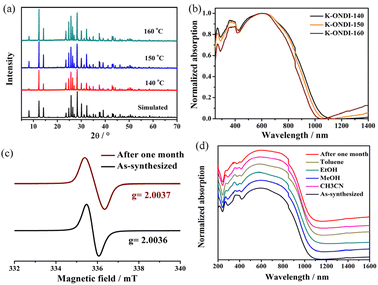
Single crystals of K-ONDI were obtained by solvothermal reaction of H2ONDI with KBF4 in a DMF/MeOH solvent mixture. Crystal structure analysis by X-ray diffraction reveals that K-ONDI crystallizes in space group P21/n with two formula units per unit cell. Each K+ ion is coordinated by seven O atoms from five ligand molecules. Each ONDI2− ligand bridges ten K+ ions through four chelating hydroxamate groups, μ2 coordination of carbonyl oxygen atoms, and μ3 coordination of N−O− oxygen atoms (Fig. 1a and S1†), resulting in a dense 3D metal–organic network (Fig. 1c). This coordination mode of the ligand ONDI2− is observed for the first time.13 Continuous intermolecular π−π interactions between adjacent ONDI2− ligands are found along the crystallographic a axis. The shortest distance between phenyl centroids is 3.582 Å (Fig. 1b); the distance between parallel naphthalene planes is 3.371 Å (Fig. 1b), close to the typical interplanar distance of 3.3 Å of NDI organic derivatives.10,14 Intermolecular π−π interactions between neighbouring ligands can be clearly observed from the calculated gradient isosurfaces according to the method reported by Johnson et al. (Fig. S2†).15 It is worth to mention that NDI based coordination polymers showing π−π stacking structures are rare.10,13a Such a ligand arrangement is beneficial for charge transport.Optical absorption and EPR spectroscopy
Although the colour of H2ONDI is light yellow and that of the mono-deprotonated species is brown, the as-synthesized K-ONDI is dark blue, the solid-state absorption spectrum covers the complete visible range (Fig. 2a). In general, alkaline metal ions do not contribute to optical absorption properties in organic–inorganic hybrids. The huge difference in absorption spectra of the ligand in its protonated state, H2ONDI·DMA, and the coordination polymer implies that a new species with a broad absorption band should be present in K-ONDI.In order to investigate the origin, the optical absorption spectrum of H2ONDI in solution was recorded. The results show that the organic ligand dissolved in DMA displays a broad absorption band around 650 nm (Fig. 2b) accompanied by a colour change from light yellow to light blue when treated with an excess of tetrabutylammonium fluoride (TBAF) as a weak base. The EPR spectrum shows that a more concentrated blue solution (1.0 × 10–2 mol l−1) exhibits an obvious unpaired electron signal (Fig. S3†). According to previous studies, the formation of NDI˙− radical anions can be induced by treatment with Lewis basic anions like F−, CN−, and OH− accompanied by a colour change.16 Therefore, the broad absorption band at 650 nm appearing in solution of H2ONDI could be attributed to the formation of NDI˙− radical anions induced by F−. As for K-ONDI, it not only shows a similar broad absorption band as H2ONDI when treated with 60 eq. TBAF, but also displays a radical signal in its X-band EPR spectrum with a g factor of 2.0042(5) (Fig. 2c), close to values reported for NDI˙− radical anions in DMF solvent17a and in coordination polymers.17b,c A fit of the X-band EPR spectrum revealed a dominating Gaussian line shape with a line width of ΔBGpp = 0.43(2) mT with a small Lorentzian line width contribution of ΔBLpp = 0.16(2) mT. The obtained line widths are in agreement with previous reports of NDI˙− radical anions in coordination polymers.17b,c Moreover, Q-band EPR spectroscopy of K-ONDI revealed a small axial anisotropy of the g tensor with g⊥ = 2.0044(2) and g|| = 2.0026(2) and slightly smaller line widths ΔBGpp = 0.35(2) mT and ΔBLpp = 0.10(2) mT as compared to the X band spectra. An axially symmetric g tensor has been likewise reported for NDI˙− radical anions.18 Therefore, it is reasonable to assign the observed EPR signal and the absorption band around 620 nm in K-ONDI to the NDI˙− radical anions. The absorption bands from 200 nm to 400 nm should be attributed to the π−π* transition of the organic ligand.7a,19 Compared to K-ONDI, the NDI˙− absorption band of H2ONDI in solution is slightly red-shifted, probably caused by the large polarity of the solvent DMA.
Since only the NDI˙− radical signal was detected by EPR and no other redox species were found in the solid state cyclic voltammogram of K-ONDI (Fig. S4†), intramolecular electron transfer can be excluded. According to literature, alkylamines, acting as electron donors, can induce NDI˙− formation by electron transfer.20 Considering that Me2NH is formed by hydrolytic decomposition of DMF,21a the NDI˙− present in K-ONDI may be generated by the alkylamine formed by decomposition of DMF under solvothermal conditions.
To verify this hypothesis, firstly, syntheses of K-ODNI were performed at different temperatures because higher temperature would promote the decomposition of DMF and, thus, produce more alkylamine which would be beneficial for the formation of NDI˙−. The identical PXRD patterns demonstrate phase consistency of products obtained at 140, 150 and 160 °C (Fig. 3a). Also the solid-state UV-vis absorption spectra are very similar and exhibit broad NDI˙− absorption bands at 620 nm (Fig. 3b). Quantitative measurements of the numbers of spins in these three samples by EPR spectroscopy reveal 4.6 × 1014 mg−1 (140 °C), 1.2 × 1015 mg−1 (150 °C), and 4.9 × 1015 mg−1 (160 °C), the increasing concentration of radicals with increasing synthesis temperature (Fig. S5†) is consistent with the hypothesis that the decomposition products of DMF induce electron transfer to NDI leading to NDI˙− radicals.
Further, the solvent DMF was replaced with dimethylacetamide (DMA) and diethylformamide (DEF). DMA and DEF both can produce respective alkylamines by hydrolysis during solvothermal reactions.21 According to the PXRD patterns (Fig. S6a†), and solid-state UV-vis-infrared optical absorption spectra (Fig. S6b†), K-ONDI is obtained also with DMA or DEF. On the other hand, when DMF was replaced with DMSO, no solvothermal reaction could be observed because of the lack of organic bases (Fig. S7†). But in presence of triethylamine (Et3N), K-ONDI was formed and the characteristic band of NDI˙− also appeared (Fig. S8†). This implies that Et3N not only deprotonates the ligand, but also induces the formation of NDI˙−. Since DMF, DMA and DEF can decompose to respective alkylamines under solvothermal conditions and NDI˙− doped K-ONDI can be obtained when using these solvents, it is reasonable to suggest that the formation of NDI˙− radicals in K-ONDI is related to the production of alkylamines by solvent decomposition.
Radical stability
K-ONDI synthesized at 140 °C exhibits good radical stability in air. After exposure to air for one month, the EPR radical signal still remains (Fig. 3c). The characteristic absorption band of the NDI˙− radical also still appears in the solid-state UV-vis-IR absorption spectra (Fig. 3d). In addition, the PXRD patterns show no obvious structural changes after contact to air for one month. On the other hand, K-ONDI also shows good stability in common organic solvents like MeOH, EtOH, CH3CN, and toluene. After immersion in these solvents for five days, no changes were detected by PXRD (Fig. S9†). The absorption spectra of K-ONDI after the immersion process are nearly the same as those of the as-synthesized sample, which demonstrates the NDI˙− radical in K-ONDI is stable even in contact to the solvent. It is worth to mention that such air- and solvent-stable radical coordination polymers based on NDI derivatives are rare.7 Consistent with previous studies,11 the excellent radical stability of K-ONDI combined with its structure features could be attributed to the synergistic interactions of the coordinative bonds of the NDI group and continuous π−π interactions between ligands.Electronic structure calculation
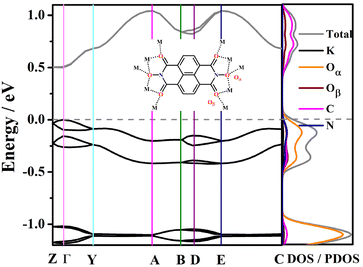
Owing to the existence of continuous intermolecular π–π interactions between ligand molecules, effective orbitals overlap is reasonable to be expected. According to the results of DFT calculations, K-ONDI is a direct semiconductor. The calculated bandgap is 0.5 eV, which is in reasonable agreement with the experimentally determined optical gap of 1.27 eV (Fig. S10a†) because of the underestimation of bandgaps obtained from DFT calculations.22 Combined with the κ point path in the Brillouin zone (Fig. S11†), the conduction band (CB) closest to the Fermi level is relatively flat along Z−Γ and B−D direction (Fig. 4), which indicates there is no effective orbital overlap between K+ ions and organic ligands. The CB closest to the Fermi level shows a dispersion along Γ−Y, A−B, and D−E directions, with the dispersion width reaching about 0.18 eV (Fig. S12†). That means there is weak orbital overlap between adjacent columns of stacked ligands. Noticeably, the CB closest to the Fermi level significantly increases along Y−A direction and decreases along E−C direction. The dispersion widths between Y−A and E−C directions are about 0.36 eV. Such a big band dispersion is rare to be observed among coordination polymers23 and demonstrates there is efficient orbital overlap. That corresponds to continuous π−π interactions along the a axis in the crystal structure. In contrast, the dispersions of the VB closest to the Fermi level are small (Fig. 4 and S12†), that means the electron mobility is expected to be bigger than the hole mobility in K-ONDI. Compared with the obvious VB dispersion in Ca-ONDI and Sr-ONDI,13a we suspect the π−π stacking structure of ligands may influence the band structure and charge carrier mobility.From the analysis of density of states (DOS) and partial density of states (PDOS), the valence bands closest to the Fermi level largely consist of the states of Oα atoms (Fig. 4). The conduction band is mainly composed of the states of C atoms, with a small contribution from Oβ. The contributions of N and K atoms to either occupied or unoccupied states near the Fermi level are nearly negligible.
Electrical conductivity
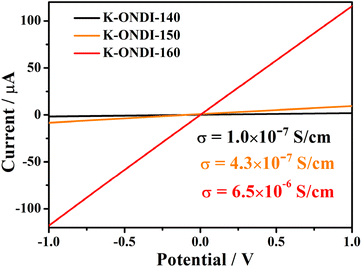
Due to the obvious radical signal and the crystal structure allowing efficient π orbital overlap, the electrical conductivity of K-ONDI (pressed pellets) obtained at different synthesis temperatures was measured using the two-probe method under direct current. The electrical conductivities of K-ONDI synthesized at different temperatures are 1.0 × 10−7 S cm−1 (140 °C), 4.3 × 10−7 S cm−1 (150 °C), and 6.5 × 10−6 S cm−1 (160 °C) (Fig. 5), respectively. This behaviour could be attributed to the increasing number of unpaired electrons (Fig. S5†). It is worth to mention that the electrical conductivity measured from pressed pellets is usually underestimated because of grain boundary resistance and sample anisotropy.24 According to the single crystal structure data and theoretical calculation, the mechanism of electrical conductivity of K-ONDI could be assigned to band-like charge transport based on effective orbitals overlap originating in continuous intermolecular π−π interactions between organic ligands.23b On the other hand, considering the presence of grain boundary resistance in pressed pellets of a polycrystalline samples and possible defects caused by doped NDI˙−, hopping charge transport may also contribute to the electrical conductivity of K-ONDI.24a According to previous studies,25 the population of radical species in materials is important and proportional to their electrical conductivity. Compared to reported radical doped coordination polymers,7a,25K-ONDI exhibits a relatively high electrical conductivity even with less detected unpaired electrons (Table S2†), which probably is caused by an efficient charge transport path based on the π–π stacking structure.Measurements of the temperature dependent electrical conductivities of K-ONDI in the range of 25–80 °C (Fig. S22†) show that the electrical conductivity of K-ONDI increases with increasing temperature. The activation energies estimated from the slope (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) σ vs. 1/T) in the linear range are 0.44, 0.42 and 0.15 eV for K-ONDI synthesized at 140, 150 and 160 °C, respectively.
σ vs. 1/T) in the linear range are 0.44, 0.42 and 0.15 eV for K-ONDI synthesized at 140, 150 and 160 °C, respectively.
Conclusions
In summary, we directly synthesized an NDI based coordination polymer with a significant and adjustable concentration of radical species by solvothermal reaction. This compound displays a strong radical EPR signal of NDI˙− that most likely is generated by electron transfer from amines, formed by decomposition of DMF under solvothermal conditions, to the NDI core of the ligand. Benefitting from its π–π stacking structure and coordination bonds to metal ions, K-ONDI exhibits good radical stability in air and common organic solvents. Due to efficient π orbitals overlap and unpaired electrons of NDI˙−, K-ONDI shows a moderate electrical conductivity. Considering the wide applications of naphthalenediimide derivatives in coordination polymers, we believe that this work shows a new prospective to direct synthesis of radical doped CPs that would arouse interesting magnetic or electrical properties. Besides, this compound displays a broad optical absorption and semiconductive performance, which make it an ideal candidate for photo- or electrocatalysis.Experimental
Materials and methods
All reagents were purchased commercially and used without further purification. 2,7-Dihydroxybenzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetraone (H2ONDI) was synthesized according to a reported procedure.13d H2ONDI·DMA and (Me2NH2)(HONDI) were prepared based on a reported method.13a Elemental analyses were carried out on a VARIO EL analyzer (Elementar). Thermogravimetric (TG) analyses were performed in Al2O3 crucibles on a NETZSCH STA 449 F1 thermal analyzer under Argon (50 ml min−1) atmosphere at a heating rate of 10 K min−1. Powder X-ray diffraction (PXRD) patterns were collected in Debye Scherrer mode on a STOE StadiP diffractometer using Cu-Kα1 radiation (λ = 154.060 pm) at room temperature in the 2θ range of 5–80°. UV-Vis absorption spectra were collected on a Jasco V-670 UV–Vis–NIR spectrometer. IR spectra were measured on KBr pellets using a Bruker TENSOR 27 spectrometer.X-ray crystallography
Single-crystal X-ray diffraction measurements were carried out on a STOE STADIVARI diffractometer equipped with an X-ray micro-source (Cu-Kα, λ = 154.186 pm) and a DECTRIS Pilatus 300k detector. The structure was solved by direct methods and refined using SHELX.26 The hydrogen atoms of the ligand were added geometrically and refined using the riding model. The final structure was refined using a full-matrix least-squares refinement on F2. All crystallographic calculations were performed within the Olex2 crystallographic software.27Cyclic voltammetry
The solid-state cyclic voltammogram of K-ONDI was measured using a three-electrode cell at room temperature. About 5 mg of K-ONDI crystalline powder were milled and dispersed in 1 mL of ethanol. Two drops of the crystalline powder slurry were drop-cast onto the precleaned glassy carbon electrode and dried in air for the fabrication of the working electrode. Pt wire and Ag/Ag+ act as the counter electrode and the reference electrode, respectively. Electrochemical measurements of the sample were carried out using a 0.1M [(n-Bu)4N]PF6 solution in acetonitrile under N2 atmosphere. The reduction potentials of K-ONDI were obtained from the cyclic voltammogram and corrected with respect to the Fc/Fc+ internal standard.Electrical conductivity measurement
The conductivity of pressed pellets of K-ONDI (diameter 13 mm) was measured on a Biologic SP-150 instrument. The direct current–voltage (I–V) curves of K-ONDI pellets sandwiched between two silver gel coated hard plastic sheets (covered with Cu film on one side) electrodes were recorded under ambient conditions from −1 to +1 V. The specific electric conductivity σ is calculated by the expression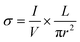 , where L is the thickness and r the radius of the pellet. The values of I and V were obtained from the I–V curve. The temperature dependent conductivity measurement (Fig. S22†) was performed in an oven under ambient atmosphere. The temperature increased from room temperature to 80 °C in 2 h. The activation energy was calculated according to the Arrhenius equation:
, where L is the thickness and r the radius of the pellet. The values of I and V were obtained from the I–V curve. The temperature dependent conductivity measurement (Fig. S22†) was performed in an oven under ambient atmosphere. The temperature increased from room temperature to 80 °C in 2 h. The activation energy was calculated according to the Arrhenius equation: 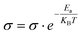 , where KB is the Boltzmann constant and T represents the absolute temperature.
, where KB is the Boltzmann constant and T represents the absolute temperature.
EPR measurements
EPR experiments of K-ONDI were performed on Bruker EMXmicro X-band and EMX Q-band spectrometers at room temperature, both equipped with cylindrical cavities. The X-band EPR spectrum of a solution of H2ONDI in DMA was recorded using a flat cell in rectangular TE102 cavity. The EPR spectra were simulated using the MATLAB toolbox EasySpin.28 Spin numbers were determined from the EPR spectra by double integration and comparison with an ultramarine standard sample with known spin number.Computation details
Calculation of intermolecular interactions: the plots of the electron density (ρ) and reduced density gradient (s = 1/(2(3π2)1/3)|∇ρ|/ρ4/3) of K-ONDI was obtained by density functional theory (DFT) calculations.15 Calculations were performed with the B3LYP functional and the 6-311G (d,p) basis set,29 using the Gaussian 16 program.30 The results were analyzed by Multiwfn.31The band structure and DOS were calculated using the CASTEP package.32 The structural model for K-ONDI was directly taken from the single-crystal X-ray diffraction data. The exchange–correlation energy was described by the PBE functional within the GGA.33 The norm conserving pseudopotentials were chosen to modulate the electron–ion interaction.34 The plane-wave cutoff energy was 750 eV, and the threshold of 5 × 10−7 eV was set for the self-consistent field convergence of the total electronic energy. The Fermi level was selected as the reference and set to 0 eV by default. For the DOS/PDOS diagram a line broadening (smearing width) of 0.05 eV was used. Other parameters were set to default values.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from Deutsche Forschungsgemeinschaft (DFG), Universität Leipzig and the graduate school BuildMoNa. This work was also supported by the Open Foundation of State Key Laboratory of Structural Chemistry (20210009). The numerical calculations in this paper have been done on the Scientific Research Cloud Platform in School of Chemistry and Chemical Engineering, Liaocheng University. The authors, K. T. and A. P., gratefully acknowledge funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 813209 entitled PARACAT.Notes and references
-
(a) W. Kosaka, H. Fukunaga and H. Miyasaka, Electron-transferred donor/acceptor ferrimagnet with TC = 91 K in a layered assembly of paddlewheel [Ru2] units and TCNQ, Inorg. Chem., 2015, 54(20), 10001–10006 CrossRef CAS PubMed
; (b) J. Zhang, W. Kosaka, H. Sato and H. Miyasaka, Magnet creation by guest insertion into a paramagnetic charge-flexible layered metal–organic framework, J. Am. Chem. Soc., 2021, 143(18), 7021–7031 CrossRef CAS PubMed
; (c) J. Zhang and W. Kosaka, Kunihisa Sugimoto and H. Miyasaka, Magnetic sponge behaviour via electronic state modulations, J. Am. Chem. Soc., 2018, 140(16), 5644–5652 CrossRef CAS PubMed
.
-
(a) H. Liu, H. Qin, N. Shen, S. Yan, Y. Wang, X. Yin, X. Chen, C. Zhang, X. Dai, R. Zhou, X. Ouyang, Z. Chai and S. Wang, Emergence of a radical-stabilizing metal–organic framework as a radio-photoluminescence dosimeter, Angew. Chem., Int. Ed., 2020, 59, 15209–15214 CrossRef CAS PubMed
; (b) B. Lü, Y. Chen, P. Li, B. Wang, K. Müllen and M. Yin, Stable radical anions generated from a porous perylenediimide metal-organic framework for boosting near-infrared photothermal conversion, Nat. Commun., 2019, 10, 1–8 CrossRef PubMed
.
-
(a) J. L. Zhuang, X. Y. Liu, Y. Zhang, C. Wang, H. L. Mao, J. Guo, X. Du, S. B. Zhu, B. Ren and A. Terfort, Zr-metal–organic frameworks featuring TEMPO radicals: synergistic effect between TEMPO and hydrophilic Zr-node defects boosting aerobic oxidation of alcohols, ACS Appl. Mater. Interfaces, 2019, 11, 3034–3043 CrossRef CAS PubMed
; (b) K. M. Zwolinski and M. J. Chmielewski, TEMPO-appended metal–organic frameworks as highly active, selective, and reusable catalysts for mild aerobic oxidation of alcohols, ACS Appl. Mater. Interfaces, 2017, 9, 33956–33967 CrossRef CAS PubMed
; (c) M. J. Jellen, M. J. Ayodele, A. Cantu, M. D. E. Forbes and M. A. Garcia-Garibay, 2D arrays of organic qubit candidates embedded into a pillared-paddlewheel metal–organic framework, J. Am. Chem. Soc., 2020, 142, 18513–18521 CrossRef CAS PubMed
.
-
(a) D. Sheberla, J. C. Bachman, J. S. Elias, C. J. Sun, Y. Shao-Horn and M. Dincă, Conductive MOF electrodes for stable supercapacitors with high areal capacitance, Nat. Mat., 2017, 16, 220–224 CrossRef CAS PubMed
; (b) J. Liu, X. Song, T. Zhang, S. Liu, H. Wen and L. Chen, 2D conductive metal–organic frameworks: an emerging platform for electrochemical energy storage, Angew. Chem., Int. Ed., 2021, 60, 5612–5624 CrossRef CAS PubMed
.
-
(a) D. Maspoch, D. Ruiz-Molina, K. Wurst, N. Doming, M. Cavallini, F. Biscarini, J. Tejada, C. Rovira and J. Veciana, A nanoporous molecular magnet with reversible solvent-induced mechanical and magnetic properties, Nat. Mat., 2003, 2, 190–195 CrossRef CAS PubMed
; (b) D. Maspoch, N. Domingo, D. Ruiz-Molina, K. Wurst, J. M. Hernández, G. Vaughan, C. Rovira, F. Lloret, J. Tejada and J. Veciana, Coexistence of ferro- and antiferromagnetic interactions in a metal–organic radical-based (6,3)-helical network with large channel, Chem. Commun., 2005, 5035–5037 RSC
; (c) A. Datcu, N. Roques, V. Jubera, D. Maspoch, X. Fontrodona, K. Wurst, I. Imaz, G. Mouchaham, J. P. Sutter, C. Rovira and J. Veciana, Three-dimensional porous metal–radical frameworks based on triphenylmethyl radicals, Chem. – Eur. J., 2012, 18, 152–162 CrossRef CAS PubMed
; (d) S. Kimura, M. Uejima, W. Ota, T. Sato, S. Kusaka, R. Matsuda, H. Nishihara and T. Kusamoto, An open-shell, luminescent, two-dimensional coordination polymer with a honeycomb lattice and triangular organic radical, J. Am. Chem. Soc., 2021, 143, 4329–4338 CrossRef CAS PubMed
; (e) S. Kimura, R. Matsuoka, S. Kimura, H. Nishihara and T. Kusamoto, Radical-based coordination polymers as a platform for magnetoluminescence, J. Am. Chem. Soc., 2021, 143, 5610–5615 CrossRef CAS PubMed
.
- T. B. Faust and D. M. D'Alessandro, Radicals in metal–organic frameworks, RSC Adv., 2014, 4, 17498–17512 RSC
.
-
(a) H. C. Wentz, G. Skorupskii, A. B. Bonfim, J. L. Mancuso, C. H. Hendon, E. H. Oriel, G. T. Sazamad and M. G. Campbell, Switchable electrical conductivity in a three-dimensional metal–organic framework via reversible ligand n-doping, Chem. Sci., 2020, 11, 1342–1346 RSC
; (b) M. A. Gordillo, P. A. Benavides, D. K. Panda and S. Saha, The advent of electrically conducting double-helical metal–organic frameworks featuring butterfly-shaped electron-rich π-extended tetrathiafulvalene ligands, ACS Appl. Mater. Interfaces, 2020, 12, 12955–12961 CrossRef CAS PubMed
; (c) H. Y. Wang, J. Y. Ge, C. Hua, C. Q. Jiao, Y. Wu, C. F. Leong, D. M. D'Alessandro, T. Liu and J. L. Zuo, Photo- and electronically switchable spin-crossover Iron(II) metal–organic frameworks based on a tetrathiafulvalene ligand, Angew. Chem., Int. Ed., 2017, 56, 5465–5470 CrossRef CAS PubMed
; (d) D. A. Haynes, L. J. van Laeren and O. Q. Munro, Cobalt porphyrin–thiazyl radical coordination polymers: toward metal–organic electronics, J. Am. Chem. Soc., 2017, 139, 14620–14637 CrossRef CAS PubMed
.
-
(a) M. Al Kobaisi, S. V. Bhosale, K. Latham, A. M. Raynor and S. V. Bhosale, Functional naphthalene diimides: synthesis, properties, and applications, Chem. Rev., 2016, 116, 11685–11796 CrossRef PubMed
; (b) S. V. Bhosale, M. Al Kobaisi, R. W. Jadhav, P. P. Morajkar, L. A. Jones and S. George, Naphthalene diimides: perspectives and promise, Chem. Soc. Rev., 2021, 50, 9845–9998 RSC
; (c) M. Pan, X. M. Lin, G. B. Li and C. Y. Su, Progress in the study of metal–organic materials applying naphthalene diimide (NDI) ligands, Coord. Chem. Rev., 2011, 255, 1921–1936 CrossRef CAS
; (d) Y. Zhou and L. Han, Recent advances in naphthalenediimide-based metal-organic frameworks: structures and applications, Coord. Chem. Rev., 2021, 430, 213665 CrossRef CAS
.
-
(a) L. Han, L. Qin, L. P. Xu, Y. Zhou, J. L. Sun and X. D. Zou, A novel photochromic calcium-based metal–organic framework derived from a naphthalene diimide chromophore, Chem. Commun., 2013, 49, 406–408 RSC
; (b) B. Garai, A. Mallick and R. Banerjee, Photochromic metal–organic frameworks for inkless and erasable printing, Chem. Sci., 2016, 7, 2195–2200 RSC
; (c) H. C. Wentz and M. G. Campbell, Fluoride detection with a redox-active naphthalene diimide metal–organic framework, Polyhedron, 2018, 154, 309–313 CrossRef CAS
.
-
(a) L. Qu, H. Iguchi, S. Takaishi, F. Habib, C. F. Leong, D. M. D'Alessandro, M. Yamashita, H. Abe, E. Nishibori and M. Yamashita, Porous molecular conductor: electrochemical fabrication of through-space conduction pathways among linear coordination polymers, J. Am. Chem. Soc., 2019, 141, 6802–6806 CrossRef CAS PubMed
; (b) S. Koyama, T. Tanabe, S. Takaishi, M. Yamashita and H. Iguchi, Preliminary chemical reduction for synthesizing a stable porous molecular conductor with neutral metal nodes, Chem. Commun., 2020, 56, 13109–13112 RSC
.
- B. Tang, J. Zhao, J. F. Xu and X. Zhang, Tuning the stability of organic radicals: from covalent approaches to non-covalent approaches, Chem. Sci., 2020, 11, 1192–1204 RSC
.
- S. V. Bhosale, C. H. Jani and S. J. Langford, Chemistry of naphthalene diimides, Chem. Soc. Rev., 2008, 37, 331–342 RSC
.
-
(a) Y. Yan, S. Henfling, N.–N. Zhang and H. Krautscheid, Semiconductive coordination polymers with continuous π–π interactions and defined crystal structures, Chem. Commun., 2021, 57, 10407–10410 RSC
; (b) M. A. Houghton, A. Bilyk, M. M. Harding, P. Turner and T. W. Hambley, Effect of guest molecules, metal ions and linker length on the assembly of chiral [2 + 2] metallomacrocycles: solution studies and crystal structures, J. Chem. Soc., Dalton Trans., 1997, 2725–2733 RSC
; (c) L. Zhang, L. Lin, D. Liu, Y. J. Lin, Z. H. Li and G. X. Jin, Stacking interactions induced selective conformation of discrete aromatic arrays and Borromean rings, J. Am. Chem. Soc., 2017, 139, 1653–1660 CrossRef CAS PubMed
; (d) D. Liu, Y. Lu, Y. J. Lin and G. X. Jin, Donor–acceptor [2]-and [3] catenanes assembled from versatile pre-organized Cp*Rh/Ir-directed pseudorotaxane tectons, Chem. – Eur. J., 2019, 25, 14785–14789 CrossRef CAS PubMed
.
- Y. Matsunaga, K. Goto, K. Kubono, K. Sako and T. Shinmyozu, Photoinduced color change and photomechanical effect of naphthalene diimides bearing alkylamine moieties in the solid state, Chem. – Eur. J., 2014, 20, 7309–7316 CrossRef CAS PubMed
.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. T. Yang, Revealing Noncovalent Interactions, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed
.
- S. Saha, Anion-induced electron transfer, Acc. Chem. Res., 2018, 51, 2225–2236 CrossRef CAS PubMed
.
-
(a) G. Andric, J. F. Boas, A. M. Bond, G. D. Fallon, K. P. Ghiggino, C. F. Hogan, J. A. Hutchison, M. A.-P. Lee, S. J. Langford, J. R. Pilbrow, G. J. Troup and C. P. Woodward, Spectroscopy of naphthalene diimides and their anion radicals, Aust. J. Chem., 2004, 57, 1011–1019 CrossRef CAS
; (b) J. Z. Liao, C. Wu, X. Y. Wu, S. Q. Deng and C. Z. Lu, Exceptional photosensitivity of a polyoxometalate-based charge-transfer hybrid material, Chem. Commun., 2016, 52, 7394–7397 RSC
; (c) J. Z. Liao, J. F. Chang, L. Meng, H. L. Zhang, S. S. Wang and C. Z. Lu, Lone pair-π interaction-induced generation of photochromic coordination networks with photoswitchable conductance, Chem. Commun., 2017, 53, 9701–9704 RSC
.
- Y. Beldjoudi, A. Narayanan, I. Roy, T. J. Pearson, M. M. Cetin, M. T. Nguyen, M. D. Krzyaniak, F. M. Alsubaie, M. R. Wasielewski, S. I. Stupp and J. F. Stoddart, Supramolecular tessellations by a rigid naphthalene diimide triangle, J. Am. Chem. Soc., 2019, 141, 17783–17795 CrossRef CAS PubMed
.
-
(a) J. Z. Liao, H. L. Zhang, S. S. Wang, J. P. Yong, X. Y. Wu, R. Yu and C. Z. Lu, Multifunctional radical-doped polyoxometalate-based host–guest material: photochromism and photocatalytic activity, Inorg. Chem., 2015, 54, 4345–4350 CrossRef CAS PubMed
; (b) K. Fuku, M. Miyata, S. Takaishi, T. Yoshida, M. Yamashita, N. Hoshino, T. Akutagawa, H. Ohtsu, M. Kawano and H. Iguchi, Emergence of electrical conductivity in a flexible coordination polymer by using chemical reduction, Chem. Commun., 2020, 56, 8619–8622 RSC
.
-
(a) A. Mallick, B. Garai, M.
A. Addicoat, P. S. Petkov, T. Heine and R. Banerjee, Solid state organic amine detection in a photochromic porous metal organic framework, Chem. Sci., 2015, 6, 1420–1425 RSC
; (b) S. H. Lee, B. M. Oh, C. Y. Hong, S. K. Jung, S. H. Park, G. G. Jeon, Y. W. Kwon, S. Jang, Y. Lee, D. Kim, J. H. Kim and O. P. Kwon, Gas-induced ion-free stable radical anion formation of organic semiconducting solids as highly gas-selective probes, ACS Appl. Mater. Interfaces, 2019, 11, 35904–35913 CrossRef CAS PubMed
.
-
(a) A. D. Burrows, K. Cassar, R. M. W. Friend, M. F. Mahon, S. P. Rigbyb and J. E. Warren, Solvent hydrolysis and templating effects in the synthesis of metal-organic frameworks, CrystEngComm, 2005, 7, 548–550 RSC
; (b) R. Seetharaj, P. V. Vandana, P. Arya and S. Mathew, Dependence of solvents, pH, molar ratio and temperature in tuning metal organic framework architecture, Arabian J. Chem., 2019, 12, 295–315 CrossRef CAS
.
-
(a) K. Burke, Perspective on density functional theory, J. Chem. Phys., 2012, 136, 150901 CrossRef PubMed
; (b) M. Grüning, A. Marini and A. Rubio, Density functionals from many-body perturbation theory: the band gap for semiconductors and insulators, J. Chem. Phys., 2006, 124, 154108 CrossRef PubMed
.
-
(a) A. Pathak, J. W. Shen, M. Usman, L. F. Wei, S. Mendiratta, Y. S. Chang, B. Sainbileg, C.-M. Ngue, R. S. Chen, M. Hayashi, T. T. Luo, F. R. Chen, K. H. Chen, T. W. Tseng, L. C. Chen and K. L. Lu, Integration of a (–Cu–S–)n plane in a metal–organic framework affords high electrical conductivity, Nat. Commun., 2019, 10, 1721–1728 CrossRef PubMed
; (b) C. H. Hendon, A. J. Rieth, M. D. Korzyński and M. Dincă, Grand challenges and future opportunities for metal–organic frameworks, ACS Cent. Sci., 2017, 3, 554–563 CrossRef CAS PubMed
.
-
(a) L. S. Xie, G. Skorupskii and M. Dincă, Electrically conductive metal–organic frameworks, Chem. Rev., 2020, 120, 8536–8580 CrossRef CAS PubMed
; (b) G. Skorupskii, B. A. Trump, T. W. Kasel, C. M. Brown, C. H. Hendon and M. Dincă, Efficient and tunable one-dimensional charge transport in layered lanthanide metal–organic frameworks, Nat. Chem., 2020, 12, 131–136 CrossRef CAS PubMed
.
- S. Zhang, D. K. Panda, A. Yadav, W. Zhou and S. Saha, Effects of intervalence charge transfer interaction between π-stacked mixed valent tetrathiafulvalene ligands on the electrical conductivity of 3D metal–organic frameworks, Chem. Sci., 2021, 12, 13379–13391 RSC
.
- G. M. Sheldrick, A short history of SHELX, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed
.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: a complete structure solution, refinement and analysis program, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS
.
- S. Stoll and A. Schweiger, EasySpin, a comprehensive software package for spectral simulation and analysis in EPR, J. Magn. Reson., 2006, 178, 42–55 CrossRef CAS PubMed
.
- C. T. Lee, W. T. Yang and R. G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed
.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed
.
- T. Lu and F. W. Chen, Multiwfn: a multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed
.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. I. J. Probert, K. Refson and M. C. Payne, First principles methods using CASTEP, Z. Kristallogr. – Cryst. Mater., 2005, 220, 567–570 CrossRef CAS
.
-
(a) B. Hammer, L. B. Hansen and J. K. Norskov, Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 7413–7421 CrossRef
; (b) J. P. Perdew and Y. Wang, Accurate and simple analytic representation of the electron-gas correlation energy, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 13244–13249 CrossRef PubMed
.
-
(a) D. R. Hamann, M. Schlüter and C. Chiang, Norm-conserving pseudopotentials, Phys. Rev. Lett., 1979, 43, 1494–1497 CrossRef CAS
; (b) J. S. Lin, A. Qteish, M. C. Payne and V. Heine, Optimized and transferable nonlocal separable ab initio pseudopotentials, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 4174–4180 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Additional tables and Fig. S1–S22. CCDC 2153468. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi01180h |
| This journal is © the Partner Organisations 2022 |