[Pt1Ag37(SAdm)21(Dppp)3Cl6]2+: intercluster transformation and photochemical properties†
Yaru
Zhen
ab,
Shan
Jin
 c,
Xi
Kang
c,
Xi
Kang
 ab,
Chang
Xu
ab,
Chang
Xu
 a,
Cao
Fang
ab,
Daqiao
Hu
a,
Cao
Fang
ab,
Daqiao
Hu
 *ab and
Manzhou
Zhu
*ab and
Manzhou
Zhu
 *ab
*ab
aDepartment of Chemistry and Centre for Atomic Engineering of Advanced Materials, Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials, Anhui University, Hefei 230601, P. R. China. E-mail: hudaqiao@ahu.edu.cn; zmz@ahu.edu.cn
bKey Laboratory of Structure and Functional Regulation of Hybrid Materials of Ministry of Education, Anhui University, Hefei 230601, P. R. China
cInstitutes of Physical Science and Information Technology, Anhui University, Hefei 230601, P. R. China
First published on 20th June 2022
Abstract
Ligand-exchange-induced structure transformation has been exploited as an effective approach to dictate the size and structure of nanoparticles with atomic precision. However, phosphine ligand induced intercluster transformation remains rarely explored. Herein, we report the controllable preparation and the structural elucidation of a [Pt1Ag37(SAdm)21(Dppp)3Cl6]2+ nanocluster (Pt1Ag37; where Dppp is 1,3-bis(diphenyphosphino)propane and HSAdm is 1-adamantanethiol). Notably, the reaction of Pt1Ag37 with PPh3 triggers an intercluster transformation, giving rise to a stable and smaller-sized nanocluster, [Pt1Ag28(SAdm)18(PPh3)4]2+ (Pt1Ag28). Such an intercluster transformation is systematically monitored with ESI-MS and UV-vis. The photochemical properties of Pt1Ag37 and Pt1Ag28 nanoclusters are further investigated. Of note, in the presence of chiral Bdpp diphosphines (Bdpp = 2,4-bis-(diphenylphosphino) pentane), the enantiomeric Pt1Ag37(SAdm)21(R/S-Bdpp)3Cl6 (R/S-Pt1Ag37) are obtained, and such chiral nanoclusters displayed obvious optical activity. The results in this work hopefully shed light on the future preparation of metal nanoclusters for customized applications.
1 Introduction
Atomically precise metal nanoclusters are a class of molecular-like nanomaterials, which can be precisely manipulated with adjustable physicochemical properties at the atomic level.1–11 Such nanoclusters have rich opportunities in both fundamental studies (e.g., structure-dependent properties) and promising applications (e.g., medicine, sensing, catalysis and energy).7,12–15 Several efficient synthesis methods have been proposed to controllably prepare nanoclusters with molecular purity, including size focusing, ligand exchange, and intermolecular assembly.16–21 Among them, the ligand exchange induced structure transformation (LEIST) is a highly versatile strategy for correlating the surface motifs/organic–metal interface with the reactivity of nanoclusters.21–29 In the past decade, the LEIST of thiolated gold nanoclusters has been widely studied.30–33 Jin et al. reported transformation of rod-like biicosahedral Au38(SCH2CH2Ph)24 to a tetrahedral Au36(TBBT)24 nanocluster.34 The transformation from Au23(SCy)16 to Au36(TBBT)24via Au28(TBBT)20 that activated by the presence of bulky 4-tert-butylbenzenethiol ligands was demonstrated by the Mandal group.35 In addition, the transformations of alkynyl-protected gold nanoclusters have also been achieved. The Zang group reported the size conversion of a carboranealkynyl-protected gold nanocluster Au28 into a stable and smaller-sized nanocluster Au23.36 Indeed, the ligand exchange can be partial or complete, with or without altering the metallic kernel.Nowadays, increasing interest is focused on the alloy nanoclusters with synergistic effect due to their potential for fundamental research.8,37–39 With respect to silver-rich alloy nanoclusters, especially, for doping Pt heteroatoms into Ag nanoclusters that gives rise to Pt@Ag alloy nanoclusters, several research efforts have been made for investigating the structure–property correlations at the atomic level. Despite this, a limited number of Pt doping sliver-rich nanoclusters were reported, including Pt1Ag24(SPhMe2)18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 protected by thiolates, Pt1Ag14(SR)6(PPh3)8,41 Pt1Ag24(SR)16(PPh3)3,42 Pt1Ag26(2-EBT)18(PPh3)6,18 Pt1Ag28(S-Adm)18(PPh3)4 (Pt1Ag28 for short),43 Pt1Ag31(SR)16(DPPM)3Cl344 coprotected by thiolates and phosphine ligands, Pt5Ag22(C
40 protected by thiolates, Pt1Ag14(SR)6(PPh3)8,41 Pt1Ag24(SR)16(PPh3)3,42 Pt1Ag26(2-EBT)18(PPh3)6,18 Pt1Ag28(S-Adm)18(PPh3)4 (Pt1Ag28 for short),43 Pt1Ag31(SR)16(DPPM)3Cl344 coprotected by thiolates and phosphine ligands, Pt5Ag22(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)32,45 Pt1Ag42(C
CPh)32,45 Pt1Ag42(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPhMe)28,46 stabilized by alkynyl, and so on. Among these nanoclusters, much effort has been devoted to the one-pot synthesis, such as Pt1Ag24(SPhMe2)18, Pt1Ag24(SR)16(PPh3)3, Pt1Ag26(2-EBT)18(PPh3)6, Pt5Ag22(C
CPhMe)28,46 stabilized by alkynyl, and so on. Among these nanoclusters, much effort has been devoted to the one-pot synthesis, such as Pt1Ag24(SPhMe2)18, Pt1Ag24(SR)16(PPh3)3, Pt1Ag26(2-EBT)18(PPh3)6, Pt5Ag22(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)32, and Pt1Ag42(C
CPh)32, and Pt1Ag42(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPhMe)28. Meanwhile, the ligand exchange strategy is a versatile approach to achieve Pt@Ag alloy nanoclusters with novel structures and distinct properties. For example, Pt1Ag28 was prepared by etching Pt1Ag24(SPhMe2)18 with both the HS-Adm and PPh3 ligands40 and when the Pt1Ag28 was reacted with Ag2(Dppm)Cl2, Pt1Ag31 was generated.44 The fabrication of more Pt@Ag alloy nanoclusters is of great significance for further investigating the structure–property correlations in detail, rendering the future preparation of metal nanoclusters or cluster-based nanomaterials with customized structures and properties.
CPhMe)28. Meanwhile, the ligand exchange strategy is a versatile approach to achieve Pt@Ag alloy nanoclusters with novel structures and distinct properties. For example, Pt1Ag28 was prepared by etching Pt1Ag24(SPhMe2)18 with both the HS-Adm and PPh3 ligands40 and when the Pt1Ag28 was reacted with Ag2(Dppm)Cl2, Pt1Ag31 was generated.44 The fabrication of more Pt@Ag alloy nanoclusters is of great significance for further investigating the structure–property correlations in detail, rendering the future preparation of metal nanoclusters or cluster-based nanomaterials with customized structures and properties.
Herein, we report the synthesis of a [Pt1Ag37(SAdm)21(Dppp)3Cl6]2+ (denoted as Pt1Ag37) nanocluster co-protected by 1,3-bis(diphenyphosphino)propane (Dppp) and 1-adamantanethiol (HS-Adm) ligands. The phosphine ligand-exchange-induced structure transformation of Pt1Ag37 to [Pt1Ag28(SAdm)18(PPh3)4]2+ (denoted as Pt1Ag28) nanocluster was fulfilled in the presence of PPh3. The intercluster conversion was monitored by ESI-MS and UV-vis absorption spectra. Furthermore, both Pt1Ag37 and Pt1Ag28 nanoclusters were emissive. Compared to Pt1Ag37, the photoluminescence (PL) lifetime of Pt1Ag28 was largely increased from 17.53 ns to 3.97 μs,47 which might be attributed to different PL mechanism induced by different electronic structure in Pt1Ag37 and Pt1Ag28, respectively.48 Additionally, chiral R/S-Pt1Ag37 nanoclusters were obtained via substituting achiral Dppp with homologous R/S-Bdpp ligands, which showed exciting CD activity and CPL response.
2 Experimental Methods
Chemicals
Hexachloroplatinic(IV) acid (H2PtCl6·6H2O, 99.99%, metals basis), silver nitrate (AgNO3, 99%, metals basis), 1-adamantanethiol (AdmSH, C10H16S, 99%), 1,3-bis(diphenyphosphino)propane (Dppp, 98%), triphenylphosphine (PPh3, 99%), (2S,4S)-(−)-2,4-bis(diphenylphosphino)pentane ((2S,4S-Bdpp, purity 99%), (2R,4R)-(+)-2,4-bis(diphenylphosphino)pentane (2R,4R-Bdpp, purity 99%), tetraphenylboron sodium (NaBPh4, 98%), and sodium borohydride (NaBH4, 99.9%). Methylene chloride (CH2Cl2, HPLC), methanol (CH3OH, HPLC), acetonitrileand (CH3CN, HPLC) and n-hexane (Hex, HPLC grade).Synthesis of the [Pt1Ag37(Dppp)3(SAdm)21Cl6]2+ nanocluster
AgNO3 (60 mg, 0.36 mmol) was dissolved in 10 mL of CH3OH and 2 mL CH3CN under vigorous stirring. After 5 min, 1,3-bis(diphenyphosphino)propane (Dppp, 20 mg, 0.048 mmol) and 1-adamantane mercaptan (AdmSH, 30 mg, 0.178 mmol) were added. H2PtCl6·6H2O (4 mg, 0.0075 mmol) was dissolved in 5 mL of CH3OH and added to the reaction mixture. After half an hour, a freshly prepared sodium borohydride (NaBH4, 20 mg, 0.14 mmol) was added to methanol solution of 50 μL of NEt3. The solution changed from white to dark brown. This reaction lasted for 5 h at room temperature. The crude product was obtained by rotary evaporation and centrifugation 3 min at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The precipitate was washed with excess n-hexane and collected by centrifugation again. The crystals were crystallized from CH2Cl2/hexane at room temperature and afford red block crystals after 7 days. The yield was calculated as 14.6% based on the Pt element for Pt1Ag37.
000 rpm. The precipitate was washed with excess n-hexane and collected by centrifugation again. The crystals were crystallized from CH2Cl2/hexane at room temperature and afford red block crystals after 7 days. The yield was calculated as 14.6% based on the Pt element for Pt1Ag37.
Transformation from [Pt1Ag37(SAdm)21(Dppp)3Cl6]2+ to [Pt1Ag28(SAdm)18(PPh3)4]2+
5 mg of [Pt1Ag37(SAdm)21(Dppp)3Cl6]2+ (0.5 μmol) was dissolved in 15 mL of CH2Cl2, to which solution the PPh3 ligand (5.2 mg, 20 μmol) was added. The reaction was allowed to proceed for 360 min at room temperature. Time-dependent UV-vis and ESI-MS spectra were monitored.Synthesis of the chiral Pt1Ag37(SAdm)21(R/S-Bdpp)3Cl6 nanocluster
Chiral Pt1Ag37 (SAdm)21(R/S-Bdpp)3Cl6 nanocluster was obtained referring to the preparation of Pt1Ag37(SAdm)21(Dppp)3Cl6, using 18 mg (2S,4S-Bdpp)/(2R,4R-Bdpp) in place of 20 mg of Dppp. All the other experimental conditions were kept unchanged.Fitting of dynamic curves
All the dynamic curves in this study were fitted with the exponential decay function:| y = Ae(−x)/τ + y0, |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 is the half-life (T1/2).
2 is the half-life (T1/2).
DFT calculations
The structures of PtAg nanocluster is fully optimized by using density functional theory (DFT) method at B3LYP/def2SVP49,50 level of theory with Grimme D3 corrections51 and verified to be true minima by frequency check. The cubane groups in the ligands of synthesized cluster are replaced by CH3 groups in order to reduce the computational cost. UV absorption simulations are performed at the same level by time-dependent density functional theory (TD-DFT)52,53 method. All calculations are carried out in Gaussian 1654 and Multiwfn55 package, and the Kohn–Sham orbitals are visualized in the Chemcraft program.56Characterization
All UV-vis spectra of the nanoclusters were recorded using an Agilent 8453, and the samples were dissolved in CH2Cl2 whose background correction was made using a CH2Cl2 blank. Thermogravimetric analysis (TGA) was carried out using a thermogravimetric analyzer (DTG-60H, Shimadzu Instruments, Inc.) with 5 mg of the nanocluster in a SiO2 pan at a heating rate of 10 K min−1 from 323 to 1073 K. X-ray photoelectron spectroscopy (XPS) measurements were performed using a Thermo ESCALAB 250 configured with a monochromated Al Kα (1486.8 eV) 150 W X-ray source, 0.5 mm circular spot size, a flood gun to counter charging effects, and an analysis chamber base pressure lower than 1 × 10−9 mbar, and the data were collected with FAT of 20 eV. Electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS) measurements were performed on a MicrOTOF-QIII high-resolution mass spectrometer. Photoluminescence spectra were measured using a HORIBA FluoroMax+ spectrofluorometer with the same optical density (OD) of ∼0.05. Absolute quantum yields were measured with dilute solutions of nanoclusters (0.05 OD absorption at 410 nm) on a HORIBA FluoroMax+. Transmission electron microscopy (TEM) was conducted on a JEM-2100 microscope with an accelerating voltage of 200 kV. Data collection for single crystal X-ray diffraction was carried out using a Stoe Stadivari diffractometer under a liquid nitrogen flow at 170 K, using graphite-monochromatized Cu Kα radiation (λ = 1.54186 Å). Data reductions and absorption corrections were performed using SAINT and SADABS programs, respectively. CD spectra were recorded with a BioLogic MOS-500 CD spectropolarimeter in a 0.1 cm path length quartz cell. The spectra were recorded in diluted solutions of dichloromethane and the signal of the blank solvent was subtracted. PL intensity and polarization spectra were measured on a JASCO CPL-300 circularly polarized luminescence spectrophotometer.3 Results and discussion
Synthesis and characterization of Pt1Ag37 nanocluster
The Pt1Ag37 nanocluster was synthesized via a one-pot procedure. Red block crystals were obtained after the cultivation in DCM/Hex for seven days (Fig. S1a†). TEM image showed that the Pt1Ag37 was uniform with an average size of 1.69 nm (Fig. S1b†). The UV-vis spectra of Pt1Ag37 in CH2Cl2 displayed two strong peaks centered at 408 nm and 475 nm, and two weak shoulder peaks at 327 and 435 nm, in good agreement with the time-dependent density functional theory (TDDFT) calculated spectrum (Fig. 1a). Based on computed excitation energies and Kohn–Sham (KS) orbitals, we further assigned the orbital transition modes of four distinct absorption peaks which were shown in Fig. S2.† To verify the formula of Pt1Ag37, the electrospray ionization mass spectrometry (ESI-MS) was performed that showed an intense peak at m/z of 4574.8 Da, corresponding to the characteristic peak of [Pt1Ag37(SAdm)21(Dppp)3Cl6]2+ (Fig. 1b), with a weak peak at 3891.16 Da corresponding to the [Pt1Ag32(SAdm)16(Dppp)2Cl6 (CH2Cl2)4(CH3CN)2]2+, with a loss of Ag5S5 motif and one Dppp ligand of Pt1Ag37. The experimental isotopic distributions matched perfectly with the simulation model. The free electron count of Pt1Ag37 was calculated as 8e (i.e., 37 (Ag)-21 (SR)-6 (Cl)-2 (charge) = 8e), the same as that of the reported Pt1Ag24(SR)16(PPh3)3,42 Pt1Ag26(2-EBT)18(PPh3)6,18 and Pt1Ag28(SAdm)18(PPh3)443 nanoclusters. The X-ray photoelectron spectroscopy (XPS) result revealed the presence of Pt, Ag, P, Cl, C and S in the nanocluster (Fig. 1c). An expanded view of the Pt 4f spectrum showed two peaks centered at 71.08 eV and 74.41 eV, corresponding to characteristic peaks of Pt 4f7/2 and Pt 4f5/2 for Pt0, respectively (Fig. S3†).57,58 Thermogravimetric analysis (TGA) was also performed with a total weight loss of 53.94 wt% (Fig. 1d), consistent with the theoretical loss of 54.24 wt%. The Pt1Ag37 nanocluster exhibit good thermal stability, because no decomposition was observed when stored in CH2Cl2 for two days (Fig. S4†).Atomic structure
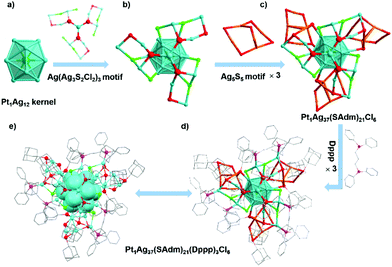
The detailed structure of Pt1Ag37 could be divided into two parts, including the metal kernel and the stabilizing shell. The icosahedral kernel, i.e., Pt1Ag12, was stabilized by a shell including three Ag3(SR)2Cl2 motifs connected via an Ag bridge, three Ag5(SR)5 motifs, and three Dppp motifs (Fig. 2). Besides, the whole Ag@(Ag3(SR)2Cl2-Ag5(SR)5-Dppp)3 shell with a C3 symmetry was bound onto the icosahedral kernel (Fig. S5†). From the other side, the whole trefoil-like shell was constituted of three leave-like units connected by one Ag atom. In Pt1Ag37, the bond lengths between the central Pt and the icosahedron-shell Ag atoms varied from 2.735 to 2.789 Å, with an average distance of 2.752 Å. The Ag–Ag bond lengths ranged from 2.805 to 2.956 Å (averagely, 2.895 Å). Additionally, the remaining six Cl atoms linked to six silver atoms, and the average bond length of Ag–Cl was 2.506 Å. The P atoms of phosphine ligands were also directly attached to Ag atoms with an average bond length of 2.407 Å (Fig. S6†).Transformation from Pt1Ag37 to Pt1Ag28
The reaction between Pt1Ag37 with 40-equivalent of PPh3 gave rise to the generation of a smaller reprted nanocluster, Pt1Ag28(S-Adm)18(PPh3)4,43 fulfilling the ligand exchange induced intercluster transformation. The intercluster transformation was monitored by UV-vis and ESI-MS. According to time-dependent UV-vis spectra (Fig. 3a), the peaks at 408 and 475 nm displayed the characteristic feature of Pt1Ag37 at 0 min. After 5 min, a new peak located at 440 nm corresponding to the Pt1Ag28 nanocluster appeared.43 At t = 10 min to 300 min, the peaks at 408 and 475 attenuated gradually accompanied by the increase of the peak at 440 nm. After 6 hours, the peaks at 408 and 475 nm disappeared, demonstrating the complete intercluster transformation from Pt1Ag37 into Pt1Ag28. The DFT calculation results revealed a larger HOMO–LUMO energy gap for Pt1Ag37 relative to Pt1Ag28 (2.64 eV versus 1.76 eV; Fig. S7†), agreeing with an inverse-proportion trend to size, similar to those of Pt1Ag31 and Pt1Ag28 (1.92 eV versus 1.76 eV).44Concurrently, the time-dependent ESI-MS clearly showed ligand exchange on the Pt1Ag37 nanocluster along with the intercluster transformation (Fig. 3b). At the initial stage, the Pt1Ag37 nanocluster displayed an intense peak at 4574.8 Da. After 5 min, the peak at 4574.8 Da decreased with a new peak emerged at 3637.67 Da, corresponding to the Pt1Ag28 nanocluster (Fig. S8b†). Meanwhile, two new peaks with different intensities centered at 3113.12 Da and 2859.17 Da were observed, which may be identified as intermediates (i.e. [Pt1Ag25(SAdm)15(Dppp)2]2+ and Ag8(SAdm)7(Dppp)2, separately) during the ligand exchange process. As the reaction continued, the intensity of the Pt1Ag28 peak increased, while the intensity of these Pt1Ag37 and intermediates decreased (Fig. 3b, t = 10 and 300 min profiles). After 6 h, the mass peak of Pt1Ag37 disappeared, suggesting a complete intercluster transformation from Pt1Ag37 to Pt1Ag28. It is worth mentioning that with different equivalent of PPh3 ligand, the ligand exchange process proceeded in different ways (Fig. S9†). With the addition of 20 equivalent of PPh3, the reaction proceeded in the same way but with different conversion rate compared with 40 equivalent of PPh3 during the first 60 min. After that, the peak of Pt1Ag37 and Pt1Ag28 decreased simultaneously accompanied by the emergence of new fragment peaks at the range of 3000–3500 Da, indicating the disassociation of both Pt1Ag37 and Pt1Ag28. At t = 360 min, the peaks of Pt1Ag37 and Pt1Ag28 disappeared completely. When the PPh3 ligand was increased to 60 equivalent, the reaction completed within 180 min without the appearance of intermediate peaks at the range of 2500 Da–4000 Da. The 408 nm of peak UV-vis spectra could be used for the kinetic study. After analyzing the fitting coefficients of the functions.59 the half-life periods (T1/2) of reaction with 40 and 60 equivalent of PPh3 were calculated and compared, showing a sequence as 60 eq. (27.43 min) < 40 eq. (38.71 min) (Fig. S10†).
The Pt1Ag37 and Pt1Ag28 displayed significantly different structures. Along with the intercluster transformation, the icosahedral Pt1Ag12 kernel of Pt1Ag37 was converted into a face-centered cubic (FCC) Pt1Ag12 kernel in Pt1Ag28 (Fig. 3c). Besides, the surface shell was changed from three Ag8(SR)7Cl2Dppp trefoil-like motifs to four Ag4(SR)6(PPh3)1 cage-like motifs by sharing the terminal thiol ligands, among which the vertex Dppp ligands on Pt1Ag37 were peeled off. The difference in bond lengths was also observed and compared. The Pt–Ag average bond length (2.752 Å) in the Pt1Ag37 kernel was shorter than that of Pt1Ag28 (2.783 Å), suggesting the more robust Pt–Ag bond in Pt1Ag37 (Table S1†). Besides, the Ag(kernel)–S(motif) and Ag(motif)–S(motif) bonds in Pt1Ag37 were also shorter than those of Pt1Ag28. However, the Ag–Ag bonds of the icosahedral (averagely, 2.895 Å) and Ag(motif)–P(motif) (averagely, 2.407 Å) bonds in Pt1Ag37 were much longer than those in Pt1Ag28. The average distance between the terminal Ag and kernel Ag atom in Pt1Ag12 of Pt1Ag37 was 6.961 Å, much longer than that in Pt1Ag28 (4.290 Å), which was attributed to the bulky peripheral ligands of Pt1Ag37. The transformation of the outermost shell further caused a change towards the core–shell interaction, which altered the innermost Pt1Ag12 kernel from an icosahedral configuration of Pt1Ag37 to a FCC configuration of Pt1Ag28.
Photoluminescent property
The photoluminescence (PL) spectra of Pt1Ag37 and Pt1Ag28 was analyzed and compared (Fig. 4a). The excitation spectrum of Pt1Ag37 nanocluster was shown in Fig. S11.† Upon excitation at 410 nm, the Pt1Ag37 nanocluster (in CH2Cl2 solution) emits at 789 nm, with a PL quantum yield (QY) of 1.49%. By comparison, the Pt1Ag28 nanocluster emits at 747 nm with a higher PL QY of 4.9% upon excitation at 360 nm.‡![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 Moreover, the PL lifetime of Pt1Ag37 was 17.53 ns measured by time-correlated single-photon counting (TCSPC) (Fig. S12†), whereas that for Pt1Ag28 was determined as 3.97 μs.47 Such a PL enhancement may be attributed to the different PL mechanism induced by different electronic structure in Pt1Ag37 and Pt1Ag28, respectively (Fig. S7†). Specifically, the LUMO density of Pt1Ag37 was concentrated mainly on Ag core structures, while the HOMO density distributed over the metal core and SR motif structures. In this context, the PL of Pt1Ag37 might originate from the metal-to-metal-to-ligand charge transfer (MMLCT) process.48 For Pt1Ag28, the PL was ascribed to ligand-to-metal charge transfer (LMCT) based on the excited state behavior analysis.43 The temperature-dependent fluorescence of Pt1Ag37 in CH2Cl2 was also monitored, showing a 37.5-fold enhancement on the PL intensity along with the temperature-lowering process from 290 to 80 K and a bule-shifted emission from 789 to 768 nm (Fig. 4b and c). The increase of the fluorescence intensity may result from the restriction of intramolecular vibration as the temperature decreased.60 Meanwhile, the red-shift of PL peak may be ascribed to the decreased molecular transition energy as the temperature increased. The temperature dependent optical absorption of Pt1Ag37 in CH2Cl2 was further investigated and a 1.2-fold enhancement of the peaks at 408 and 475 nm was shown in the corresponding temperature-lowering process (Fig. 4d). Additionally, two new peaks locating at 327 and 435 nm appeared and enhanced gradually in the cooling process. Accordingly, the PL QY of the Pt1Ag37 nanocluster at 80 K was enhanced from 1.49% to 46.56% (1.49% × 37.5/1.2 = 46.56%) during the temperature-lowering process.
43 Moreover, the PL lifetime of Pt1Ag37 was 17.53 ns measured by time-correlated single-photon counting (TCSPC) (Fig. S12†), whereas that for Pt1Ag28 was determined as 3.97 μs.47 Such a PL enhancement may be attributed to the different PL mechanism induced by different electronic structure in Pt1Ag37 and Pt1Ag28, respectively (Fig. S7†). Specifically, the LUMO density of Pt1Ag37 was concentrated mainly on Ag core structures, while the HOMO density distributed over the metal core and SR motif structures. In this context, the PL of Pt1Ag37 might originate from the metal-to-metal-to-ligand charge transfer (MMLCT) process.48 For Pt1Ag28, the PL was ascribed to ligand-to-metal charge transfer (LMCT) based on the excited state behavior analysis.43 The temperature-dependent fluorescence of Pt1Ag37 in CH2Cl2 was also monitored, showing a 37.5-fold enhancement on the PL intensity along with the temperature-lowering process from 290 to 80 K and a bule-shifted emission from 789 to 768 nm (Fig. 4b and c). The increase of the fluorescence intensity may result from the restriction of intramolecular vibration as the temperature decreased.60 Meanwhile, the red-shift of PL peak may be ascribed to the decreased molecular transition energy as the temperature increased. The temperature dependent optical absorption of Pt1Ag37 in CH2Cl2 was further investigated and a 1.2-fold enhancement of the peaks at 408 and 475 nm was shown in the corresponding temperature-lowering process (Fig. 4d). Additionally, two new peaks locating at 327 and 435 nm appeared and enhanced gradually in the cooling process. Accordingly, the PL QY of the Pt1Ag37 nanocluster at 80 K was enhanced from 1.49% to 46.56% (1.49% × 37.5/1.2 = 46.56%) during the temperature-lowering process.
Optical activity
Compared with the monodentate ligand PPh3 for Pt1Ag28, the bidentate ligand Dppp for Pt1Ag37 shows more opportunities to functionalizing nanoclusters. By controlling the enantioselectivity of the peripheral Dppp ligand, the enantioselective synthesis of optically pure intrinsic chiral Pt1Ag37 nanocluster could be achieved. The chiral ligands (2S,4S)-2,4-bis(diphenylphosphino) pentane (2S,4S-Bdpp) and (2R,4R)-2,4-bis(diphenylphosphino) pentane (2R,4R-Bdpp) were exploited to substitute the achiral Dppp ligand in the nanocluster synthesis. The UV-vis spectra of the racemic Pt1Ag37 and R/S-Pt1Ag37 nanoclusters in CH2Cl2 were measured. As shown in Fig. 5a, the racemic Pt1Ag37 and two enantiomers R/S-Pt1Ag37 nanoclusters exhibited two strong bands at 405 and 482 nm, demonstrating their similar geometric and electronic compositions and structures. ESI-MS results demonstrated that two peaks, corresponding to [Pt1Ag32(SAdm)16(Bdpp)3Cl6]3+ and [Pt1Ag32(SAdm)16(Bdpp)3Cl7]2+ with a loss of an Ag5S5 motif, were detected (Fig. 5b and Fig. S13†). | ||
| Fig. 5 (a) UV-vis spectra of R/S-Pt1Ag37 and Rac-Pt1Ag37. (b) ESI-MS spectra of S-Pt1Ag37 in CH2Cl2. CD (c) and temperature-dependent CPL spectra (d) of the enantiomers. | ||
Significantly, compared with the optically inactive Pt1Ag37, the newly obtained R/S-Pt1Ag37 nanoclusters displayed apparently optical activity. The circular dichroism (CD) spectra of the R/S-Pt1Ag37 solutions exhibited mirror-images signals around 250, 287, 326, 355, 401, 476, and 501 nm (Fig. 5c). The anisotropy factors g = ΔA/A = θ[mdeg]/(32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 980 × A) were calculated, and the maximum anisotropy factor was up to 7.0 × 10−4 at 501 nm (Fig. S14a†). Furthermore, the CD spectra of pure R/S-Bdpp ligands were quite different from that of R/S-Pt1Ag37 (Fig. S15†). These results demonstrated that the CD signals of R/S-Pt1Ag37 originated from the nanoclusters but not the free ligands, confirming the chirality of R/S-Pt1Ag37. The temperature-dependent circularly polarized luminescence (CPL) signals were then collected (Fig. 5d). The R/S-Pt1Ag37 performed obvious CPL responses that corresponded to their emission bands (600–900 nm) (Fig. S16†). Besides, the intensity of CPL signals significantly enhanced with the temperature drop process from 290 to 190 K, consistent with variation of the absorption and emission of such nanoclusters. The calculated glum values were all at the level of 10−3 (Fig. S14b†) with a highest glum value of ±2 × 10−3 at about 887 nm.
980 × A) were calculated, and the maximum anisotropy factor was up to 7.0 × 10−4 at 501 nm (Fig. S14a†). Furthermore, the CD spectra of pure R/S-Bdpp ligands were quite different from that of R/S-Pt1Ag37 (Fig. S15†). These results demonstrated that the CD signals of R/S-Pt1Ag37 originated from the nanoclusters but not the free ligands, confirming the chirality of R/S-Pt1Ag37. The temperature-dependent circularly polarized luminescence (CPL) signals were then collected (Fig. 5d). The R/S-Pt1Ag37 performed obvious CPL responses that corresponded to their emission bands (600–900 nm) (Fig. S16†). Besides, the intensity of CPL signals significantly enhanced with the temperature drop process from 290 to 190 K, consistent with variation of the absorption and emission of such nanoclusters. The calculated glum values were all at the level of 10−3 (Fig. S14b†) with a highest glum value of ±2 × 10−3 at about 887 nm.
4 Conclusions
In summary, we synthesized a Pt1Ag37 nanocluster via a one-pot method, and operated the phosphine ligand exchange-induced intercluster transformation from Pt1Ag37 to Pt1Ag28. The UV-vis and ESI-MS were performed to track the intercluster transformation. Structurally, the Pt1Ag37 nanocluster contained an icosahedral Pt1Ag12 kernel, in contrast to the FCC Pt1Ag12 kernel of the Pt1Ag28 nanocluster. Besides, these two Pt@Ag nanoclusters presented significantly different surface motif structures. A 3.3-fold enhancement on PL QY was accomplished via the ligand exchange from Pt1Ag37 to Pt1Ag28. Furthermore, chiral R/S-Ag37 nanoclusters enantiomers could be obtained by substituting achiral Dppp into chiral bidentate phosphine ligands. The chiral R/S-Ag37 nanoclusters displayed obvious optical activity. Our results suggest that the phosphine ligand exchange can be exploited as an efficient approach to fulfill the size evolution and fluorescence regulation of metal nanoclusters, and hopefully contributes more to the fabrication of noble metal nanoclusters, for potential biological, electronic and catalytic applications.Author contributions
Y. Z. carried out experiments, analyzed the data and wrote the manuscript. X. K. revised the manuscript and C. X. completed DFT calculations. S. J. assisted the X-ray structure analysis. C. F. assisted the measurements of UV-vis and ESI-MS. M. Z. and D. H. designed the project, analyzed the data, and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support by NSFC (21631001, 21871001, and 91961121), the Ministry of Education, and the University Synergy Innovation Program of Anhui Province (GXXT-2020-053).References
- R. Jin, G. Li, S. Sharma, Y. Li and X. Du, Toward Active-Site Tailoring in Heterogeneous Catalysis by Atomically Precise Metal Nanoclusters with Crystallographic Structures, Chem. Rev., 2021, 121, 567–648 CrossRef CAS PubMed.
- J. Yan, B. K. Teo and N. Zheng, Surface Chemistry of Atomically Precise Coinage–Metal Nanoclusters: From Structural Control to Surface Reactivity and Catalysis, Acc. Chem. Res., 2018, 51, 3084–3093 CrossRef CAS PubMed.
- X. Kang and M. Zhu, Intra-cluster growth meets inter-cluster assembly: The molecular and supramolecular chemistry of atomically precise nanoclusters, Coord. Chem. Rev., 2019, 394, 1–38 CrossRef CAS.
- Q. Tang, G. Hu, V. Fung and D.-E. Jiang, Insights into Interfaces, Stability, Electronic Properties, and Catalytic Activities of Atomically Precise Metal Nanoclusters from First Principles, Acc. Chem. Res., 2018, 51, 2793–2802 CrossRef CAS PubMed.
- Y. Jin, C. Zhang, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Shell engineering to achieve modification and assembly of atomically-precise silver clusters, Chem. Soc. Rev., 2021, 50, 2297–2319 RSC.
- Z. Liu, Z. Wu, Q. Yao, Y. Cao, O. J. H. Chai and J. Xie, Correlations between the fundamentals and applications of ultrasmall metal nanoclusters: Recent advances in catalysis and biomedical applications, Nano Today, 2021, 36, 101053 CrossRef CAS.
- A. Fernando, K. L. D. M. Weerawardene, N. V. Karimova and C. M. Aikens, Quantum Mechanical Studies of Large Metal, Metal Oxide, and Metal Chalcogenide Nanoparticles and Clusters, Chem. Rev., 2015, 115, 6112–6216 CrossRef CAS PubMed.
- Q. Yao, X. Yuan, T. Chen, D. T. Leong and J. Xie, Engineering Functional Metal Materials at the Atomic Level, Adv. Mater., 2018, 30, 1802751 CrossRef PubMed.
- I. Chakraborty and T. Pradeep, Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- P. Chakraborty, A. Nag, A. Chakraborty and T. Pradeep, Approaching Materials with Atomic Precision Using Supramolecular Cluster Assemblies, Acc. Chem. Res., 2019, 52, 2–11 CrossRef CAS PubMed.
- W. Kurashige, Y. Niihori, S. Sharma and Y. Negishi, Precise synthesis, functionalization and application of thiolate-protected gold clusters, Coord. Chem. Rev., 2016, 320–321, 238–250 CrossRef CAS.
- G. Li and R. Jin, Atomically Precise Gold Nanoclusters as New Model Catalysts, Acc. Chem. Res., 2013, 46, 1749–1758 CrossRef CAS PubMed.
- P. Zhang, X-ray Spectroscopy of Gold–Thiolate Nanoclusters, J. Phys. Chem. C, 2014, 118, 25291–25299 CrossRef CAS.
- W. Kurashige, Y. Niihori, S. Sharma and Y. Negishi, Recent Progress in the Functionalization Methods of Thiolate-Protected Gold Clusters, J. Phys. Chem. Lett., 2014, 5, 4134–4142 CrossRef CAS PubMed.
- Y. Yu, Q. Yao, Z. Luo, X. Yuan, J. Y. Lee and J. Xie, Precursor engineering and controlled conversion for the synthesis of monodisperse thiolate-protected metal nanoclusters, Nanoscale, 2013, 5, 4606–4620 RSC.
- M. S. Bootharaju, C. P. Joshi, M. R. Parida, O. F. Mohammed and O. M. Bakr, Templated Atom-Precise Galvanic Synthesis and Structure Elucidation of a [Ag24Au(SR)18]− Nanocluster, Angew. Chem., Int. Ed., 2016, 55, 922–926 CrossRef CAS PubMed.
- H. Wu, Y.-G. Fang, R. Anumula, G. N. Andrew, G. Cui, W. Fang, Z. Luo and J. Yao, A mono-copper doped undeca-gold cluster with up-converted and anti-stokes emissions of fluorescence and phosphorescence, Nanoscale, 2021, 13, 5300–5306 RSC.
- L. He, J. Yuan, N. Xia, L. Liao, X. Liu, Z. Gan, C. Wang, J. Yang and Z. Wu, Kernel Tuning and Nonuniform Influence on Optical and Electrochemical Gaps of Bimetal Nanoclusters, J. Am. Chem. Soc., 2018, 140, 3487–3490 CrossRef CAS PubMed.
- J. Lin, W. Li, C. Liu, P. Huang, M. Zhu, Q. Ge and G. Li, One-phase controlled synthesis of Au25 nanospheres and nanorods from 1.3 nm Au : PPh3 nanoparticles: the ligand effects, Nanoscale, 2015, 7, 13663–13670 RSC.
- C. A. Hosier and C. J. Ackerson, Regiochemistry of Thiolate for Selenolate Ligand Exchange on Gold Clusters., J. Am. Chem. Soc., 2019, 141, 309–314 CrossRef CAS PubMed.
- Z. Wu, Q. Yao, S. Zang and J. Xie, Directed Self-Assembly of Ultrasmall Metal Nanoclusters, ACS Mater. Lett., 2019, 1, 237–248 CrossRef CAS.
- M.-M. Xu, Q. Chen, L.-H. Xie and J.-R. Li, Exchange reactions in metal-organic frameworks: New advances, Coord. Chem. Rev., 2020, 421, 213421 CrossRef CAS.
- Y. Niihori, S. Hossain, B. Kumar, L. V. Nair, W. Kurashige and Y. Negishi, Perspective: Exchange reactions in thiolate-protected metal clusters, APL Mater., 2017, 5, 053201 CrossRef.
- X. Kang and M. Zhu, Transformation of Atomically Precise Nanoclusters by Ligand-Exchange, Chem. Mater., 2019, 31, 9939–9969 CrossRef CAS.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, A DNA-based method for rationally assembling nanoparticles into macroscopic materials, Nature, 1996, 382, 607–609 CrossRef CAS PubMed.
- C. K. Kim, P. Ghosh, C. Pagliuca, Z.-J. Zhu, S. Menichetti and V. M. Rotello, Entrapment of Hydrophobic Drugs in Nanoparticle Monolayers with Efficient Release into Cancer Cells, J. Am. Chem. Soc., 2009, 131, 1360–1361 CrossRef CAS PubMed.
- Y. Negishi, H. Horihata, A. Ebina, S. Miyajima, M. Nakamoto, A. Ikeda, T. Kawawaki and S. Hossain, Selective formation of [Au23(SPhtBu)17]0, [Au26Pd(SPhtBu)20]0 and [Au24Pt(SC2H4Ph)7(SPhtBu)11]0 by controlling ligand-exchange reaction, Chem. Sci., 2022, 13, 5546–5556 RSC.
- S. Gratious, A. S. Nair, S. Mukherjee, N. Kachappilly, B. Pathak and S. Mandal, Gold Deassembly: From Au44(SPh-(t)Bu)28 to Au36(SPh-(t)Bu)24 Nanocluster through Dynamic Surface Structure Reconstruction, J. Phys. Chem. Lett., 2021, 12, 10987–10993 CrossRef CAS PubMed.
- C. Zhu, T. Duan, H. Li, X. Wei, X. Kang, Y. Pei and M. Zhu, Structural determination of a metastable Ag27 nanocluster and its transformations into Ag8 and Ag29 nanoclusters, Inorg. Chem. Front., 2021, 8, 4407 RSC.
- M. Lucarini and L. Pasquato, ESR spectroscopy as a tool to investigate the properties of self-assembled monolayers protecting gold nanoparticles, Nanoscale, 2010, 2, 668–676 RSC.
- E. S. Shibu, M. A. H. Muhammed, T. Tsukuda and T. Pradeep, Ligand Exchange of Au25SG18 Leading to Functionalized Gold Clusters: Spectroscopy, Kinetics, and Luminescence, J. Phys. Chem. C, 2008, 112, 12168–12176 CrossRef CAS.
- R. Guo, Y. Song, G. Wang and R. W. Murray, Does Core Size Matter in the Kinetics of Ligand Exchanges of Monolayer-Protected Au Clusters, J. Am. Chem. Soc., 2005, 127, 2752–2757 CrossRef CAS PubMed.
- A. C. Templeton, W. P. Wuelfing and R. W. Murray, Monolayer-Protected Cluster Molecules, Acc. Chem. Res., 2000, 33, 27–36 CrossRef CAS PubMed.
- C. Zeng, C. Liu, Y. Pei and R. Jin, Thiol Ligand-Induced Transformation of Au38(SC2H4Ph)24 to Au36(SPh-t-Bu)24, ACS Nano, 2013, 7, 6138–6145 CrossRef CAS PubMed.
- M. P. Maman, A. S. Nair, H. Cheraparambil, B. Pathak and S. Mandal, Size Evolution Dynamics of Gold Nanoclusters at an Atom-Precision Level: Ligand Exchange, Growth Mechanism, Electrochemical, and Photophysical Properties, J. Phys. Chem. Lett., 2020, 11, 1781–1788 CrossRef CAS PubMed.
- J. Wang, Z.-Y. Wang, S.-J. Li, S.-Q. Zang and T. C. W. Mak, Carboranealkynyl-Protected Gold Nanoclusters: Size Conversion and UV/Vis–NIR Optical Properties, Angew. Chem., Int. Ed., 2021, 60, 5959–5964 CrossRef CAS PubMed.
- M. G. Taylor and G. Mpourmpakis, Rethinking Heterometal Doping in Ligand-Protected Metal Nanoclusters, J. Phys. Chem. Lett., 2018, 9, 6773–6778 CrossRef CAS PubMed.
- G. Soldan, M. A. Aljuhani, M. S. Bootharaju, L. G. AbdulHalim, M. R. Parida, A.-H. Emwas, O. F. Mohammed and O. M. Bakr, Gold Doping of Silver Nanoclusters: A 26-Fold Enhancement in the Luminescence Quantum Yield, Angew. Chem., Int. Ed., 2016, 55, 5749–5753 CrossRef CAS PubMed.
- K. Kwak and D. Lee, Electrochemistry of Atomically Precise Metal Nanoclusters, Acc. Chem. Res., 2019, 52, 12–22 CrossRef CAS PubMed.
- J. Yan, H. Su, H. Yang, S. Malola, S. Lin, H. Häkkinen and N. Zheng, Total Structure and Electronic Structure Analysis of Doped Thiolated Silver [MAg24(SR)18]2− (M = Pd, Pt) Clusters, J. Am. Chem. Soc., 2015, 137, 11880–11883 CrossRef CAS PubMed.
- X. Lin, K. Sun, X. Fu, X. Ren, Y. Yang, C. Liu and J. Huang, Correlating Kernel–Shell Structures with Optical Properties of Pt1Ag24 and Pt1Ag14 Nanoclusters, J. Phys. Chem. C, 2021, 125, 2194–2201 CrossRef CAS.
- X. Lin, X. Fu, Y. Yang, X. Ren, J. Tang, C. Liu and J. Huang, Synthesis and Optical Properties of Unique Pt1Ag24 Nanoclusters with Mixed Exterior Motif Structures, Inorg. Chem., 2021, 60, 10167–10172 CrossRef CAS PubMed.
- X. Kang, M. Zhou, S. Wang, S. Jin, G. Sun, M. Zhu and R. Jin, The tetrahedral structure and luminescence properties of Bi-metallic Pt1Ag28(SR)18(PPh3)4 nanocluster, Chem. Sci., 2017, 8, 2581–2587 RSC.
- X. Kang, S. Jin, L. Xiong, X. Wei, M. Zhou, C. Qin, Y. Pei, S. Wang and M. Zhu, Nanocluster growth via “graft-onto”: effects on geometric structures and optical properties, Chem. Sci., 2020, 11, 1691–1697 RSC.
- H. Shen and T. Mizuta, An Alkynyl-Stabilized Pt5Ag22 Cluster Featuring a Two-Dimensional Alkynyl–Platinum “Crucifix Motif”, Chem. – Eur. J., 2017, 23, 17885–17888 CrossRef CAS PubMed.
- H. Shen and T. Mizuta, An Atomically Precise Alkynyl-Protected PtAg42 Superatom Nanocluster and Its Structural Implications, Chem. – Asian J., 2017, 12, 2904–2907 CrossRef CAS PubMed.
- X. Kang, X. Wei, S. Wang and M. Zhu, Controlling the Phosphine Ligands of Pt1Ag28(S-Adm)18(PR3)4 Nanoclusters, Inorg. Chem., 2020, 59, 8736–8743 CrossRef CAS PubMed.
- X. Kang and M. Zhu, Tailoring the photoluminescence of atomically precise nanoclusters, Chem. Soc. Rev., 2019, 48, 2422–2457 RSC.
- C. Adamo and V. Barone, Toward reliable density functional methods without adjustable parameters: The PBE0 model, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- E. Runge and E. K. U. Gross, Density-Functional Theory for Time-Dependent Systems, Phys. Rev. Lett., 1984, 52, 997–1000 CrossRef CAS.
- R. van Leeuwen, Causality and Symmetry in Time-Dependent Density-Functional Theory, Phys. Rev. Lett., 1998, 80, 1280–1283 CrossRef CAS.
- M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, G. Petersson, H. Nakatsuji, et al., Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford, CT, 2016 Search PubMed.
- T. Lu and F. Chen, Multiwfn: A multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- Chemcraft-graphical software for visualization of quantum chemistry computations. https://www.chemcraftprog.com.
- W. Sun, S. Jin, W. Du, X. Kang, A. Chen, S. Wang, H. Sheng and M. Zhu, Total Structure Determination of the Pt1Ag9[P(Ph-F)3]7Cl3 Nanocluster, Eur. J. Inorg. Chem., 2020, 6, 590–594 CrossRef.
- Y. Zhou, Y. Shen, J. Xi and X. Luo, Selective Electro-Oxidation of Glycerol to Dihydroxyacetone by PtAg Skeletons, ACS Appl. Mater. Interfaces, 2019, 11, 28953–28959 CrossRef CAS PubMed.
- Q. Li, S. Yang, T. Chen, S. Jin, J. Chai, H. Zhang and M. Zhu, Structure determination of a metastable Au22(SAdm)16 nanocluster and its spontaneous transformation into Au21(SAdm)15, Nanoscale, 2020, 12, 23694–23699 RSC.
- Q. Yuan, X. Kang, D. Hu, C. Qin, S. Wang and M. Zhu, Metal synergistic effect on cluster optical properties: based on Ag25 series nanoclusters, Dalton Trans., 2019, 48, 13190–13196 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1–S16 and Tables S1–S2. CCDC 2166689 for Pt1Ag37(SAdm)21(Dppp)3Cl6. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi01082h |
| ‡ In our previous works, the photoluminescence of Pt1Ag28(SR)18(PR′)4 was characterized using an instrument of HITACHI (F-7000) with a photoluminescence detection limit at 750 nm, which resulted in an inaccurate emission wavelength (i.e., 672 nm). In this work, we tested the PL of nanoclusters by an instrument of HORIBA Scientific (FluoroMax+) with a higher photoluminescence detection limit at 870 nm. And here, we updated the emission wavelength data of Pt1Ag28(S-Adm)18(PPh3)4 to 747 nm. The photoluminescence quantum yields and emission lifetimes were correct in previous works since they were detected by an instrument of HORIBA Scientific (FluoroMax-4P) with a photoluminescence detection limit at 870 nm. |
| This journal is © the Partner Organisations 2022 |