Modulated luminescence of zero-dimensional bimetallic all-inorganic halide clusters†
Chao
Zhang‡
a,
Xuezhen
Feng‡
b,
Qilin
Song
a,
Chaocheng
Zhou
a,
Lin
Peng
a,
Xiaolin
Liu
*a,
Hong
Chen
 *b and
Jia
Lin
*b and
Jia
Lin
 *a
*a
aDepartment of Physics, Shanghai University of Electric Power, Shanghai 200090, China. E-mail: xlliu@shiep.edu.cn; jlin@shiep.edu.cn
bShenzhen Key Laboratory of Interfacial Science and Engineering of Materials, State Environmental Protection Key Laboratory of Integrated Surface Water-Groundwater Pollution Control, Guangdong Provincial Key Laboratory of Soil and Groundwater Pollution Control, School of Environmental Science and Engineering, Southern University of Science and Technology, Shenzhen 518055, China. E-mail: chenh3@sustech.edu.cn
First published on 13th June 2022
Abstract
Zero-dimensional (0D) metal halide clusters formed as isolated metal halide polyhedral structures show excellent optical properties and have great potential for various applications in optoelectronic devices. In this study, we reported a series of Rb8CuB(III)3Cl18 single crystals (SCs) constructed from Cu(I) and B(III) (B = In, Tb, etc.) containing lead-free bimetallic halide clusters. Density functional theory calculations have been used to explore the thermodynamic stability of the expected bimetallic halide clusters, in which the ionic radius of B(III) trivalent metal cations should be within the range of 0.74 to 0.99 Å in order to form a stable Rb8CuB(III)3Cl18 0D structure in line with the same structure configuration. By analyzing the effects of different B(III) cations on the structural parameters and optical-physical properties, we found that the luminescence of SCs could be modulated by different B(III) cations, while the luminescence originated from the charge transfer from Cu(I) ions to metal-chloride octahedra. This study reported the rational design of novel-structured Cu(I)-based inorganic lead-free metal halide materials, which paves the way for the exploration of new luminescent materials.
Introduction
Halide perovskites, with a chemical formula of ABX3 (where A represents Cs+, MA+ or FA+, etc.; B represents Pb2+ or Sn2+, etc.; and X represents halide ions), have attracted significant attention attributed to their excellent optoelectronic properties, such as high light absorption coefficient, long carrier diffusion length, and tunable bandgap.1–4 Such outstanding properties make them potential candidates for solar cells,5–8 light-emitting diodes,9–12 and detectors.13–16 Currently, large-scale applications of halide perovskites are plagued by the toxicity of lead (Pb2+).17 Replacing Pb2+ with other nontoxic elements while maintaining excellent properties has become a challenge. Lead-free luminescent perovskite materials are of great emergency. In previous reports, Pb2+ has been replaced with divalent Sn2+ or Ge2+, and trivalent Bi3+ or Sb3+, because these cations have an identical electron configuration to Pb2+, but less toxicity. However, the divalent Sn2+ or Ge2+ in the perovskite structure can be easily oxidized to tetravalent Sn4+ or Ge4+, which affects the framework stability and optical performance of the perovskite structure.18–20 Simply employing +3 valence state cations to construct a single B site perovskite limits the possibility of forming a three-dimensional (3D) corner-shared perovskite structure due to the charge balance problem. Low-dimensional crystal structures, such as A2B(III)X5, A3B(III)X6, and A3B(III)2X9, are frequently achieved. Such perovskite-related metal halides usually have larger optical bandgaps and lower carrier mobilities than 3D perovskites, which are unsuitable for photovoltaic devices. Moreover, by replacing two Pb2+ ions with a +1 and +3 cation pair, double perovskite structure A2B(I)B(III)X6 can be formed, in which two kinds of octahedra arrange alternately. However, due to the strong local electric field between the ordered B(I) and B(III) sites in the 3D lattice, the double perovskites show inferior optoelectronic performance than conventional 3D perovskites.21,22To tune the luminescence properties of perovskites, the dimensional engineering of halide materials has been heavily employed. By limiting the coordination number of metal ions at the B(I) position in double perovskites, the low coordination prevents the B(I) metal atoms from forming octahedra with the surrounding halogens. This integration disintegrates the 3D double perovskite structure, leading to the formation of lower-dimensional structures. As a result, the electrical conductivity toward the metal halide bond network is reduced.23,24 The low-dimensional metal halides possess high exciton binding energies and large bandgaps due to quantum and dielectric limitations.25,26 In addition, increasingly isolated octahedral units would enhance exciton localization, which in turn enhances radiative recombination, resulting in high luminescence efficiencies.27–29
In this study, based on the idea of replacing Pb(II) ions with monovalent Cu(I) ions and trivalent In(III) ions, we introduced a new all-inorganic copper–indium halide structure, Rb8CuIn3Cl18. The single crystals (SCs) were synthesized via a vacuum solid-state reaction method, preventing the influence of water and oxygen during the SC growing process. The Cu(I) ion has been chosen because the Cu 3d10 electron is unstable at its high energy level and must be hybridized with 4s and 4p states to reduce the energy. Lower coordination numbers of Cu(I) with halides distorted the d10 orbitals, resulting in stronger d–s and d–p hybridization, thus, debarring the formation of 6-fold coordination.30 Considering the thermodynamic stability and octahedral factors, we further constructed a series of zero-dimensional (0D) clusters based on this crystal structure and investigated the relationship between the structural stability and radius of trivalent-metal cations. We concluded that stable Cu-based cluster structures could be formed only when the ionic radius of trivalent-metal cations was within 0.74–0.99 Å, and the optimum structure appeared to have an ionic radius of 0.86 Å. Combining the optical properties with structural data of the reported isomorphic SCs, we further elucidated the luminescence mechanism in the obtained materials.
Results and discussion
The novel luminescent all-inorganic lead-free metal halide Rb8CuIn3Cl18 SCs were obtained via a vacuum solid-phase reaction method.31 From the pre-synthesis experiments, we found that the growth temperature of Rb8CuIn3Cl18 SCs was lower than the average melting point of all the involved precursors. Thus, the target product cannot be obtained via a one-pot solid-chemistry synthesis. Under this circumstance, the entire SC growth process needs to be divided into two steps: pre-crystallization and re-crystallization. As displayed in Fig. 1a, RbCl, CuCl, and InCl3, with the mole ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, were ground and sealed in a 9 mm-diameter quartz ampoule in a vacuum. The as-prepared quartz ampoule was annealed in a muffle furnace at 700 °C for 24 h and then cooled to room temperature. The main product obtained at the high temperature was confirmed to be Rb3InCl6 using powder X-ray diffraction (PXRD) (Fig. S1†). After that, the quartz ampoule containing Rb3InCl6 SCs was annealed at 400 °C for 5 days. The block-like Rb8CuIn3Cl18 SCs were obtained after cooling to room temperature.
3, were ground and sealed in a 9 mm-diameter quartz ampoule in a vacuum. The as-prepared quartz ampoule was annealed in a muffle furnace at 700 °C for 24 h and then cooled to room temperature. The main product obtained at the high temperature was confirmed to be Rb3InCl6 using powder X-ray diffraction (PXRD) (Fig. S1†). After that, the quartz ampoule containing Rb3InCl6 SCs was annealed at 400 °C for 5 days. The block-like Rb8CuIn3Cl18 SCs were obtained after cooling to room temperature.
To understand the two-step growth process of Rb8CuIn3Cl18 SCs, thermal stability was analyzed using thermogravimetry-differential scanning calorimetry (TG-DSC) under a nitrogen atmosphere. The white sample powder was heated from 25 to 800 °C at a constant rate of 10 °C min−1. No weight loss was found until the temperature increased to 400 °C (Fig. S2†). The thermal stability was slightly lower than the previously reported low-dimensional all-inorganic Cu(I)-based halides such as Rb2CuCl3, CsCu2Cl3, and Cs3Cu2Cl5.32–34 This may be related to the nature of the 3-fold coordination number of Cu(I) in Rb8CuIn3Cl18. When the temperature increased from 400 to 650 °C, the sample powder lost 15.54% of the weight. From the TG-DSC curves, the thermal decomposition process of Rb8CuIn3Cl18 SCs above 650 °C is consistent with that of Rb3InCl6 SCs, indicating that the weight lost can be ascribed to the destruction of the Cu–Cl bond in Rb8CuIn3Cl18 and the formation of Rb3InCl6 (Fig. S2†). After raising the temperature above 650 °C, the sample powder continued to lose weight, indicating the thermal decomposition of Rb3InCl6. The different formation energies of Rb3InCl6 and Rb8CuIn3Cl18 structures could further shed light on the necessity of multi-step preparation. The formation energy of Rb8CuIn3Cl18 (−5.34 eV) is much lower than that of Rb3InCl6 (−1.0 eV), which means that in order to avoid Rb3InCl6 impurities, the growth of Rb8CuIn3Cl18 SCs needs a lower temperature environment. Therefore, 700 °C and 400 °C were selected as the pre-crystallization and re-crystallization temperatures to prepare Rb8CuIn3Cl18 SCs, respectively.
The crystal structure of the novel Rb8CuIn3Cl18 SCs was determined by single-crystal X-ray diffraction (SCXRD). Rb8CuIn3Cl18 SCs displayed a monoclinic structure with a space group of R/3c (Z = 3, a = b = 12.656 Å, c = 75.15 Å). The detailed crystallographic data and experimental refinement parameters are shown in Table S1,† and the bond lengths are listed in Table S2.† The asymmetric unit in the crystal structure of Rb8CuIn3Cl18 consisted of two types of layers (Fig. 1b). Type I layer consisted of spatially well-isolated [InCl6]3− octahedra with surrounding Rb+ cations, similar to that in Rb3InCl6 SCs (Fig. S3†).29 Each [InCl6]3− octahedron was composed of a unique In site in a 3+ oxidation state coordinated by six chlorine anions, with an average In–Cl bond length of 2.505 Å. In the type II layer, two Cu(I) ions combined with three [InCl6]3− octahedra to form a paddle-wheel-like [Cu2(InCl6)3]7− cluster, and the clusters were separated by Rb+. The average In–Cl bond length in the cluster was 2.525 Å, which was almost equal to that in the type I layer. The two types of layers stacked alternatively along the c-axis following the 61 screw axis symmetry, forming a trigonal structure. The PXRD pattern of the as-synthesized Rb8CuIn3Cl18 powder matched well with the simulated one obtained from SCXRD (Fig. 1c), certifying the purity of Rb8CuIn3Cl18 SCs. Energy-dispersive X-ray spectroscopy (EDS) mapping showed a uniform distribution of Rb, Cu, In, and Cl elements (Fig. S4†), in which the atomic ratio of Rb/Cu/In/Cl was determined to be 7.86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.06
3.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.15, which was approximately equal to the stoichiometric proportion of the structure formula as obtained from SCXRD.
17.15, which was approximately equal to the stoichiometric proportion of the structure formula as obtained from SCXRD.
In our previous work, Rb8B(I)Sc3Cl18 SCs were synthesized by using d10 monovalent metal-cationic Cu(I) or Ag(I) occupying the B(I)-site, respectively.22 By analyzing their luminescence properties, it was considered that the metal cation at the B(I)-site was the switch of the luminescence of the 0D structure because the B(I) ion oxidation characteristics in the cluster determined whether the SCs were emissive. Here, we further explored the influence of B(III) ions on the overall structure by considering the thermodynamic stability and structural factors. A series of Rb8CuB(III)3Cl18 structural models were constructed by using different B(III) ions. The Cu–Cl bond lengths in these optimized structural models are shown in Fig. 2a. When the trivalent-metal cation radius was between 0.67 and 0.99 Å, the Cu–Cl bond length was less than 2.6 Å, which was within the typical Cu–Cl bond length range (Table S3†). If the B(III) ion had a radius of 1.01 Å, the distance between Cu ion and the nearest Cl ion was 3.47 Å, which was much larger than the maximum value of the Cu–Cl bond. In addition, the octahedral factor is an important criterion to predict the stability of the perovskite structure,35,36 described by the formula μ = rB/rX (where rB represents the ionic radius of B(III) cations, and rX represents the ionic radius of halogen). When rB was less than 0.74 Å, μ was less than 0.41, and the lattice tended to be distorted to form a layered geometry with edge- or face-sharing polyhedra, thus, failing to form stable cluster structures. As shown in Fig. 2b, the formation energy of structures with different B(III) cations first decreased and then increased with increasing ionic radius. The ionic radius of 0.86 Å referred to the minimum formation energy of −6.2 eV, illustrating that the radius of B(III) cations should be within 0.74 and 0.99 Å to form this type of Cu(I)-connected octahedral clusters. We selected rare-earth ions to build the models because of two reasons. First, rare-earth ions show a stable trivalent state which satisfies the valence requirements. Second and more importantly, rare-earth elements have the same outermost structure of two s electrons, resulting in similar physical and chemical properties. The selection of rare-earth elements could effectively avoid the interference of chemical activity, electronegativity, melting point, and other factors for predicting the influence of the B(III) ionic radius on the structure. To verify the accuracy of the calculation results, we used the same solid-state method to synthesize a new 3-fold coordinated Cu(I)-based SCs with the same structure as Rb8CuIn3Cl18, however, with different B(III) ionic radius, namely Rb8CuTb3Cl18. As confirmed by SCXRD, Rb8CuTb3Cl18 crystallized within the same space group of R/3c. A paddle-wheel-like cluster structure has been involved in the crystal structure (Fig. S5†). The Cu–Cl bond lengths obtained from SCXRD were consistent with the calculated value (Table S4†).
In the following, the photophysical properties of Rb8CuIn3Cl18 SCs were explored. From the UV-visible absorption spectrum (Fig. 3a), the Rb8CuIn3Cl18 SCs had a distinct absorption edge at approximately 376 nm, with an estimated bandgap of 3.19 eV. The steady-state photoluminescence (PL) spectrum of Rb8CuIn3Cl18 displayed a broadband emission with a peak centered at 482 nm (2.57 eV) under 305 nm excitation (Fig. 3b). The large Stokes shift of 177 nm suggested that the PL of Rb8CuIn3Cl18 SCs was not from the band-edge emission. The PL quantum yield (PLQY) of Rb8CuIn3Cl18 SCs was approximately 4%. Although the performance was not exceptional compared with other 0D metal halides, it performed better than all-inorganic 3D CsPbX3 SCs with approximately 1% PLQY.37 From the PL decay curve (Fig. 3c), a carrier lifetime of approximately 257 ns was determined. The CIE coordinate of Rb8CuIn3Cl18 SCs was (0.16, 0.23) (Fig. 3d), corresponding to the sky-blue emission. For Rb8CuTb3Cl18 SCs, the luminescence properties were consistent with the intrinsic luminescence of Tb3+ due to the transition between different energy levels of 4f electrons (Fig. S6†).38,39 In addition, the material stability was evaluated. Rb8CuIn3Cl18 SCs showed good stability under ambient conditions (25 °C, 50% ± 5% relative humidity), and significant emission was observed at 482 nm after 5 h of aging (Fig. S7†). In comparison, Rb8CuB(III)3Cl18 SCs with rare-earth ions at the B(III) site decomposed very quickly and became non-emissive after exposure to air for a few minutes.
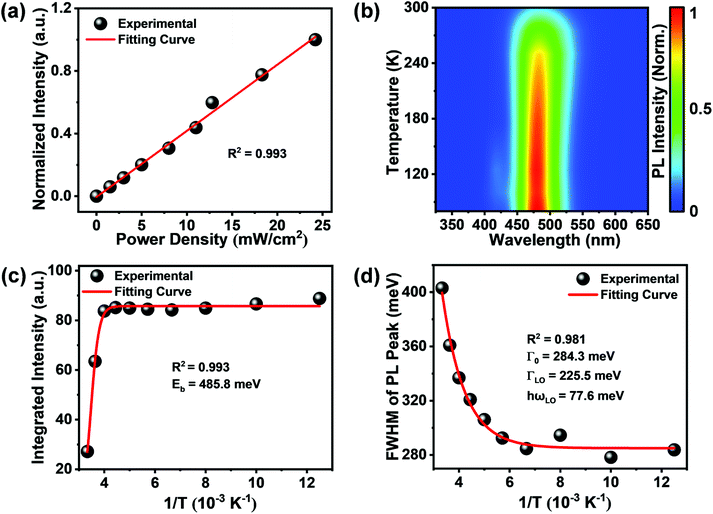
The variation of PL intensity with the excitation power density of Rb8CuIn3Cl18 SCs was investigated. The PL intensity increased linearly with the excitation power density (Fig. 4a), suggesting that the emission was not from permanent defects.40 The temperature-dependent PL spectra of Rb8CuIn3Cl18 SCs in the temperature range of 80–300 K are shown in Fig. 4b. As the temperature decreased, the PL intensity increased. The variation of the integrated PL intensity with temperature can be fitted using an Arrhenius equation (Fig. 4c):41
 | (1) |
The exploration of temperature-dependent PL broadening helps in the understanding of the electron–phonon coupling mechanism. The full width at half-maximum (FWHM) of the PL peak declined with decreasing temperature (Fig. 4d), which can be fitted using the following formula:
 | (2) |
The luminescence mechanism of 0D metal halide Rb8Cu(I)B(III)3Cl18 was further discussed. In our earlier work, under 375 nm light excitation, Rb8CuSc3Cl18 and Rb8CuY3Cl18 SCs showed strong and broad PL emissions. Rare-earth ions of Sc3+ and Y3+, unlike Tb3+ and Eu3+, had empty 4f-orbitals which did not exhibit emission caused by f–f transition. It was hypothesized that the luminescence of the SCs might come from the [B(III)Cl6]3− octahedra. Rb8CuIn3Cl18 SCs showed a single emission peak at 482 nm under 305 nm light excitation, in which In3+, similar to Sc3+ and Y3+ ions, had no intrinsic luminescence properties. Therefore, we deduced that the luminescence of Rb8CuIn3Cl18, similar to Rb8CuSc3Cl18 and Rb8CuY3Cl18, was relevant to the metal halide octahedra due to the unique isolated building units.46,47
To explore the effect of different B(III) ions on the luminescence properties of the Rb8Cu(I)B(III)3Cl18 structure, we analyzed the relationship between the length of B(III)–Cl bond in the [B(III)Cl6]3− octahedra and the emission peak position. As illustrated in Fig. 5a–c, the average B(III)–Cl (B = In, Sc, Y) bond lengths in the three compounds were 2.525 Å, 2.492 Å, and 2.588 Å, respectively. The In–Cl bond length was in between the Sc–Cl and Y–Cl bond lengths. Furthermore, the emission peak position of Rb8CuIn3Cl18 was just located between that of Rb8CuSc3Cl18 and Rb8CuY3Cl18. The results indicated that the origins of luminescence of Rb8CuB(III)3Cl18 (B(III) = In, Sc, Y) were consistent and related to the [B(III)Cl6]3− octahedra. The emitting wavelength of this cluster structure could be tuned by selecting B(III) ions with different ionic radii, endowing this kind of material with a broader range of applications.48 Rb8CuIn3Cl18 had a similar structure to Rb3InCl6. However, their optical properties were very different. Rb3InCl6 had an emission center at 436 nm, and its PL lifetime reached the microsecond level.29,49,50 This indicated that Cu(I) ions in the cluster structure played a crucial role in the luminescence process of the SCs. Furthermore, we used density functional theory (DFT) calculations to explore the electron interactions in Rb8CuIn3Cl18 and elucidate the role of Cu(I) in the luminescence process. The [Cu2(InCl6)3]7− octahedral clusters in Rb8CuIn3Cl18 were isolated by Rb+ and their coupling was negligible, resulting in flat bands similar to other 0D structures (Fig. 5d).51 From the projected density of states (PDOS) in Fig. 5e, the top of the valence band mainly consisted of the Cu d orbital, and the bottom of the conduction band was composed of In s and Cl p orbitals. This was consistent with the role of Cu(I) in Rb8CuSc3Cl18 and Rb8CuY3Cl18. Similar to the previously reported luminescence process of Cu(I)-based structures, the luminescence came from the intersystem crossing process.52,53 Upon excitation, Cu(I) ions could be oxidized,54 and the electrons were transferred to the connecting [InCl6]3− octahedra in the cluster, making the octahedra the source of the broadband emission (Fig. 5f). To sum up, the luminescence properties of the Rb8CuB(III)3Cl18 structure could be modulated by the Cu(I)-connected [B(III)Cl6]3− octahedra. With the increase of the ionic radius of B(III), the [B(III)Cl6]3− octahedra gradually expanded, accompanied by a continuous red shift of the luminescence.
Conclusion
This study has reported a series of novel all-inorganic Cu(I) based metal halide Rb8CuB(III)3Cl18 (B = In, Tb, etc.) SCs. This kind of structure was composed of alternating [B(III)Cl6]3− octahedral and [Cu2(B(III)Cl6)3]7− cluster layers. In each cluster, three [B(III)Cl6]3− octahedra were connected to two Cu(I) ions to form a paddle-wheel cluster. By DFT calculations, we found that the paddle-wheel-like cluster structure could be created only when the ionic radius of B(III) trivalent metal cations was in the range of 0.74 to 0.99 Å. After studying the relationship between the structures and luminescence properties of Rb8CuB(III)3Cl18 SCs with different B(III) ions, we found that all the luminescence of these SCs was determined by both B(I) connecting ions and B(III) ions in the octahedral centers. Furthermore, the luminescence peak position could be tuned by changing the radius of the central ions of the octahedra. The comprehensive study of the structural factors and luminescence properties of this Rb8CuB(III)3Cl18 structure will guide the future exploration of new metal halides for various light-emitting applications.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 12174246 and 61875119) and Science and Technology Commission of Shanghai Municipality (Grant No. 21010501300). The authors thank BL17B1 beamline of National Facility for Protein Science in Shanghai (NFPS) at Shanghai Synchrotron Radiation Facility and 2D-SMC (proposal No. 2020-1st-2D-024) of the Pohang Accelerator Laboratory for providing the beam time.References
- D. Han, M. Imran, M. Zhang, S. Chang, X.-G. Wu, X. Zhang, J. Tang, M. Wang, S. Ali and X. Li, et al., Efficient light-emitting diodes based on in situ fabricated FAPbBr3 nanocrystals: the enhancing role of the ligand-assisted reprecipitation process, ACS Nano, 2018, 12(8), 8808–8816 CrossRef CAS PubMed.
- H. Huang, M. I. Bodnarchuk, S. V. Kershaw, M. V. Kovalenko and A. L. Rogach, Lead halide perovskite nanocrystals in the research spotlight: stability and defect tolerance, ACS Energy Lett., 2017, 2(9), 2071–2083 CrossRef CAS PubMed.
- L. Chouhan, S. Ghimire, C. Subrahmanyam, T. Miyasaka and V. Biju, Synthesis, optoelectronic properties and applications of halide perovskites, Chem. Soc. Rev., 2020, 49(10), 2869–2885 RSC.
- Z. Xiao, Z. Song and Y. Yan, From lead halide perovskites to lead-free metal halide perovskites and perovskite derivatives, Adv. Mater., 2019, 31(47), 1803792 CrossRef CAS PubMed.
- Y.-H. Lin, N. Sakai, P. Da, J. Wu, H. C. Sansom, A. J. Ramadan, S. Mahesh, J. Liu, R. D. J. Oliver and J. Lim, et al., A piperidinium salt stabilizes efficient metal-halide perovskite solar cells, Science, 2020, 369(6499), 96–102 CrossRef CAS PubMed.
- K. Nishimura, M. A. Kamarudin, D. Hirotani, K. Hamada, Q. Shen, S. Iikubo, T. Minemoto, K. Yoshino and S. Hayase, Lead-free tin-halide perovskite solar cells with 13% efficiency, Nano Energy, 2020, 74, 104858 CrossRef CAS.
- J. Lin, M. Lai, L. Dou, C. S. Kley, H. Chen, F. Peng, J. Sun, D. Lu, S. A. Hawks and C. Xie, et al., Thermochromic halide perovskite solar cells, Nat. Mater., 2018, 17(3), 261–267 CrossRef CAS PubMed.
- Z. Ni, C. Bao, Y. Liu, Q. Jiang, W.-Q. Wu, S. Chen, X. Dai, B. Chen, B. Hartweg and Z. Yu, et al., Resolving spatial and energetic distributions of trap states in metal halide perovskite solar cells, Science, 2020, 367(6484), 1352–1358 CrossRef CAS PubMed.
- W. Xu, Q. Hu, S. Bai, C. Bao, Y. Miao, Z. Yuan, T. Borzda, A. J. Barker, E. Tyukalova and Z. Hu, et al., Rational molecular passivation for high-performance perovskite light-emitting diodes, Nat. Photonics, 2019, 13(6), 418–424 CrossRef CAS.
- Q. Shan, J. Song, Y. Zou, J. Li, L. Xu, J. Xue, Y. Dong, B. Han, J. Chen and H. Zeng, High performance metal halide perovskite light-emitting diode: from material design to device optimization, Small, 2017, 13(45), 1701770 CrossRef PubMed.
- M. Lu, Y. Zhang, S. Wang, J. Guo, W. W. Yu and A. L. Rogach, Metal halide perovskite light-emitting devices: promising technology for next-generation displays, Adv. Funct. Mater., 2019, 29(30), 1902008 CrossRef.
- Q. Ge, R. Zheng, J. Lin and H. Chen, Cs2InCl5(H2O): A moisture-stable defective double halide perovskite analogue with broadband emission, Mater. Lett., 2020, 277, 128280 CrossRef CAS.
- L. Sun, W. Li, W. Zhu and Z. Chen, Single-crystal perovskite detectors: development and perspectives, J. Mater. Chem. C, 2020, 8(34), 11664–11674 RSC.
- W. Zhu, W. Ma, Y. Su, Z. Chen, X. Chen, Y. Ma, L. Bai, W. Xiao, T. Liu and H. Zhu, et al., Low-dose real-time X-ray imaging with nontoxic double perovskite scintillators, Light: Sci. Appl., 2020, 9, 112 CrossRef CAS PubMed.
- H. P. Wang, S. Li, X. Liu, Z. Shi, X. Fang and J. H. He, Low-dimensional metal halide perovskite photodetectors, Adv. Mater., 2021, 33(7), 2003309 CrossRef CAS PubMed.
- J. Ma, L. Zhang, J. Wang, R. Zheng, X. Feng, W. Wei, W. Zou, Z. Yang, Y. Zheng and H. Chen, A novel optical sensor for Pb2+ detection based on in situ formation of Ruddlesden-Popper phase (C4H9NH3)2PbBr4 perovskite, Mater. Lett., 2021, 131280 Search PubMed.
- X. Li, F. Zhang, H. He, J. J. Berry, K. Zhu and T. Xu, On-device lead sequestration for perovskite solar cells, Nature, 2020, 578(7796), 555–558 CrossRef CAS PubMed.
- M. H. Kumar, S. Dharani, W. L. Leong, P. P. Boix, R. R. Prabhakar, T. Baikie, C. Shi, H. Ding, R. Ramesh and M. Asta, et al., Lead-free halide perovskite solar cells with high photocurrents realized through vacancy modulation, Adv. Mater., 2014, 26(41), 7122–7127 CrossRef CAS PubMed.
- W. Ning and F. Gao, Structural and functional diversity in lead-free halide perovskite materials, Adv. Mater., 2019, 31(22), 1900326 CrossRef PubMed.
- H. Chen, S. Xiang, W. Li, H. Liu, L. Zhu and S. Yang, Inorganic perovskite solar cells: a rapidly growing field, Sol. RRL, 2018, 2(2), 1700188 CrossRef.
- A. H. Slavney, T. Hu, A. M. Lindenberg and H. I. Karunadasa, A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications, J. Am. Chem. Soc., 2016, 138(7), 2138–2141 CrossRef CAS PubMed.
- J. Lin, H. Chen, J. Kang, L. N. Quan, Z. Lin, Q. Kong, M. Lai, S. Yu, L. Wang and L.-W. Wang, et al., Copper(I)-based highly emissive all-inorganic rare-earth halide clusters, Matter, 2019, 1(1), 180–191 CrossRef.
- J.-P. Correa-Baena, L. Nienhaus, R. C. Kurchin, S. S. Shin, S. Wieghold, N. T. Putri Hartono, M. Layurova, N. D. Klein, J. R. Poindexter and A. Polizzotti, et al., A-site cation in inorganic A3Sb2I9 perovskite influences structural dimensionality, exciton binding energy, and solar cell performance, Chem. Mater., 2018, 30(11), 3734–3742 CrossRef CAS.
- Z. Xiao, W. Meng, J. Wang, D. B. Mitzi and Y. Yan, Searching for promising new perovskite-based photovoltaic absorbers: the importance of electronic dimensionality, Mater. Horiz., 2017, 4(2), 206–216 RSC.
- C. Katan, N. Mercier and J. Even, Quantum and dielectric confinement effects in lower-dimensional hybrid perovskite semiconductors, Chem. Rev., 2019, 119(5), 3140–3192 CrossRef CAS PubMed.
- B. Traore, L. Pedesseau, L. Assam, X. Che, J.-C. Blancon, H. Tsai, W. Nie, C. C. Stoumpos, M. G. Kanatzidis and S. Tretiak, et al., Composite nature of layered hybrid perovskites: assessment on quantum and dielectric confinements and band alignment, ACS Nano, 2018, 12(4), 3321–3332 CrossRef CAS PubMed.
- C. Zhou, H. Lin, J. Neu, Y. Zhou, M. Chaaban, S. Lee, M. Worku, B. Chen, R. Clark and W. Cheng, et al., Green emitting single-crystalline bulk assembly of metal halide clusters with near-unity photoluminescence quantum efficiency, ACS Energy Lett., 2019, 4(7), 1579–1583 CrossRef CAS.
- C. Zhou, H. Lin, Q. He, L. Xu, M. Worku, M. Chaaban, S. Lee, X. Shi, M.-H. Du and B. Ma, Low dimensional metal halide perovskites and hybrids, Mater. Sci. Eng., R, 2019, 137, 38–65 CrossRef.
- C. Zhang, X. Feng, Q. Song, C. Zhou, L. Peng, J. Chen, X. Liu, H. Chen, J. Lin and X. Chen, Blue-violet emission with near-unity photoluminescence quantum yield from Cu(I)-doped Rb3InCl6 single crystals, J. Phys. Chem. Lett., 2021, 12(33), 7928–7934 CrossRef CAS PubMed.
- Z. Xiao, K. Z. Du, W. Meng, D. B. Mitzi and Y. Yan, Chemical origin of the stability difference between copper(I)-and silver(I)-Based halide double perovskites, Angew. Chem., Int. Ed., 2017, 56(40), 12107–12111 CrossRef CAS.
- C. Zhang, X. Liu, J. Chen and J. Lin, Solution and solid-phase growth of bulk halide perovskite single crystals, Chin. J. Chem., 2021, 39(5), 1353–1363 CrossRef CAS.
- R. Zhang, X. Mao, D. Zheng, Y. Yang, S. Yang and K. Han, A lead-free all-inorganic metal halide with near-unity green luminescence, Laser Photonics Rev., 2020, 14(5), 2000027 CrossRef CAS.
- R. Roccanova, A. Yangui, G. Seo, T. D. Creason, Y. Wu, D. Y. Kim, M.-H. Du and B. Saparov, Bright Luminescence from Nontoxic CsCu2X3 (X = Cl, Br, I), ACS Mater. Lett., 2019, 1(4), 459–465 CrossRef CAS.
- T. D. Creason, A. Yangui, R. Roccanova, A. Strom, M. H. Du and B. Saparov, Rb2CuX3 (X = Cl, Br): 1D all-inorganic copper halides with ultrabright blue emission and up-conversion photoluminescence, Adv. Opt. Mater., 2020, 8(2), 1901338 CrossRef CAS.
- M. R. Filip and F. Giustino, The geometric blueprint of perovskites, Proc. Natl. Acad. Sci. U. S. A., 2018, 115(21), 5397–5402 CrossRef CAS PubMed.
- C. Li, X. Lu, W. Ding, L. Feng, Y. Gao and Z. Guo, Formability of ABX3 (X = F, Cl, Br, I) halide perovskites, Acta Crystallogr., Sect. B: Struct. Sci., 2008, 64(6), 702–707 CrossRef CAS.
- L. N. Quan, R. Quintero-Bermudez, O. Voznyy, G. Walters, A. Jain, J. Z. Fan, X. Zheng, Z. Yang and E. H. Sargent, Highly emissive green perovskite nanocrystals in a solid state crystalline matrix, Adv. Mater., 2017, 29(21), 1605945 CrossRef PubMed.
- H. Pan, S. Wang, X. Dao and Y. Ni, Fluorescent Zn-PDC/Tb3+ coordination polymer nanostructure: A candidate for highly selective detections of cefixime antibiotic and acetone in aqueous system, Inorg. Chem., 2018, 57(3), 1417–1425 CrossRef CAS PubMed.
- V. X. Quang, P. Van Do, N. X. Ca, L. D. Thanh, V. P. Tuyen, P. M. Tan, V. X. Hoa and N. T. Hien, Role of modifier ion radius in luminescence enhancement from 5D4 level of Tb3+ ion doped alkali-alumino-telluroborate glasses, J. Lumin., 2020, 221, 117039 CrossRef CAS.
- Z. Yuan, C. Zhou, Y. Tian, Y. Shu, J. Messier, J. C. Wang, L. J. Van De Burgt, K. Kountouriotis, Y. Xin and E. Holt, et al., One-dimensional organic lead halide perovskites with efficient bluish white-light emission, Nat. Commun., 2017, 8(1), 14051 CrossRef CAS.
- J. Chen, C. Zhang, X. Liu, L. Peng, J. Lin and X. Chen, Carrier dynamic process in all-inorganic halide perovskites explored by photoluminescence spectra, Photonics Res., 2021, 9(2), 151–170 CrossRef.
- Y. Zhang, B. Fan, Y. Liu, H. Li, K. Deng and J. Fan, Quasi-self-trapped Frenkel-exciton near-UV luminescence with large Stokes shift in wide-bandgap Cs4PbCl6 nanocrystals, Appl. Phys. Lett., 2018, 112(18), 183101 CrossRef.
- A. Miyata, A. Mitioglu, P. Plochocka, O. Portugall, J. T.-W. Wang, S. D. Stranks, H. J. Snaith and R. J. Nicholas, Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites, Nat. Phys., 2015, 11(7), 582–587 Search PubMed.
- A. D. Wright, C. Verdi, R. L. Milot, G. E. Eperon, M. A. Pérez-Osorio, H. J. Snaith, F. Giustino, M. B. Johnston and L. M. Herz, Electron–phonon coupling in hybrid lead halide perovskites, Nat. Commun., 2016, 7(1), 11755 Search PubMed.
- J. A. Steele, P. Puech, M. Keshavarz, R. Yang, S. Banerjee, E. Debroye, C. W. Kim, H. Yuan, N. H. Heo and J. Vanacken, et al., Giant electron–phonon coupling and deep conduction band resonance in metal halide double perovskite, ACS Nano, 2018, 12(8), 8081–8090 CrossRef CAS PubMed.
- J. Almutlaq, J. Yin, O. F. Mohammed and O. M. Bakr, The benefit and challenges of zero-dimensional perovskites, J. Phys. Chem. Lett., 2018, 9(14), 4131–4138 CrossRef CAS PubMed.
- M. I. Saidaminov, O. F. Mohammed and O. M. Bakr, Low-dimensional-networked metal halide perovskites: the next big thing, ACS Energy Lett., 2017, 2(4), 889–896 CrossRef CAS.
- S. Adjokatse, H.-H. Fang and M. A. Loi, Broadly tunable metal halide perovskites for solid-state light-emission applications, Mater. Today, 2017, 20(8), 413–424 CrossRef CAS.
- P. Han, C. Luo, S. Yang, Y. Yang, W. Deng and K. Han, All-Inorganic Lead-Free 0D Perovskites by a Doping Strategy to Achieve a PLQY Boost from <2% to 90%, Angew. Chem., Int. Ed., 2020, 59(31), 12709–12713 CrossRef CAS PubMed.
- J. Huang, T. Chang, R. Zeng, J. Yan, Q. Wei, W. Zhou, S. Cao and B. Zou, Controlled Structural Transformation in Sb-Doped Indium Halides A3InCl6 and A2InCl5·H2O Yields Reversible Green-to-Yellow Emission Switch, Adv. Opt. Mater., 2021, 2002267 CrossRef CAS.
- J. Yin, H. Yang, K. Song, A. M. El-Zohry, Y. Han, O. M. Bakr, J.-L. Brédas and O. F. Mohammed, Point defects and green emission in zero-dimensional perovskites, J. Phys. Chem. Lett., 2018, 9(18), 5490–5495 CrossRef CAS PubMed.
- R. Hamze, J. L. Peltier, D. Sylvinson, M. Jung, J. Cardenas, R. Haiges, M. Soleilhavoup, R. Jazzar, P. I. Djurovich and G. Bertrand, et al., Eliminating nonradiative decay in Cu(I) emitters: >99% quantum efficiency and microsecond lifetime, Science, 2019, 363(6427), 601–606 CrossRef CAS PubMed.
- N. M. Khatri, M. H. Pablico-Lansigan, W. L. Boncher, J. E. Mertzman, A. C. Labatete, L. M. Grande, D. Wunder, M. J. Prushan, W. Zhang and P. S. Halasyamani, et al., Luminescence and nonlinear optical properties in copper(I) halide extended networks, Inorg. Chem., 2016, 55(21), 11408–11417 CrossRef CAS PubMed.
- I. L. Malaestean, V. C. Kravtsov, J. Lipkowski, E. Cariati, S. Righetto, D. Marinotto, A. Forni and M. S. Fonari, Partial in situ reduction of copper(II) resulting in one-pot formation of 2D neutral and 3D cationic copper(I) iodide–pyrazine coordination polymers: structure and emissive properties, Inorg. Chem., 2017, 56(9), 5141–5151 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2128946 (Rb8CuIn3Cl18) and 2128947 (Rb8CuTb3Cl18). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2qi00620k |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2022 |