Temperature- and solvent-induced reversible single-crystal-to-single-crystal transformations of TbIII-based MOFs with excellent stabilities and fluorescence sensing properties toward drug molecules†
Abstract
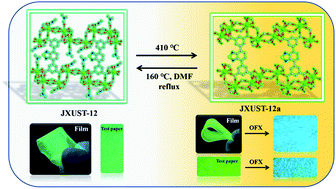
Recently, single-crystal-to-single-crystal conversion has been a hot topic in the field of metal–organic framework (MOF) materials, which could improve the stability and properties due to structural changes. A new TbIII-based MOF ([Tb4(BTDI)3(DMF)4]n, JXUST-12) has been designed and synthesized by the incorporation of TbIII ions and H4BTDI ligands (5,5′-(benzo[c][1,2,5]thiadiazole-4,7-diyl)diisophthalic acid) featuring a fluorescent unit. Very interestingly, the reversible single-crystal-to-single-crystal transformation between JXUST-12 and {[Tb4(BTDI)3(H2O)4]·4H2O·solvents}n (JXUST-12a) occurred, and thus the enhancement of water and pH stabilities has been achieved. Because of the presence of TbIII ions and the fluorescent organic ligand, JXUST-12 and JXUST-12a can be used as luminescent sensors for the detection of drugs (NFT = nitrofurantoin, NZF = nitrofurazone, and DCN = 2,6-dichloro-4-nitroaniline). Furthermore, theoretical caculations and experimental studies suggest that the luminescence quenching of JXUST-12 and JXUST-12a for the detection of NFT, NZF and DCN may be attributed to a competitive energy absorption and photoinduced electron transfer mechanism. Remarkably, fluorescent test papers and composite films based on JXUST-12 and JXUST-12a have been developed to simply detect drugs through the visual change of the luminescence colour.


 Please wait while we load your content...
Please wait while we load your content...