Open Access Article
Open Access ArticleThermoresponsive polymers in non-aqueous solutions†
Matilde
Concilio
a,
Valentin P.
Beyer
ab and
C. Remzi
Becer
 *ab
*ab
aDepartment of Chemistry, University of Warwick, Coventry, CV4 7AL, UK. E-mail: remzi.becer@warwick.ac.uk
bPolymer Chemistry Laboratory, School of Engineering and Materials Science, Queen Mary University of London, London, E1 4NS, UK
First published on 10th November 2022
Abstract
Thermoresponsive polymers are gaining increasing interest for numerous applications especially in the biomedical and nanotechnology fields. The thermoresponsive behaviour of polymers has been extensively studied in pure water or water/organic solvent systems, however, temperature-induced phase transitions in other organic solvents are less common. Polymers in organic solvents exhibit a broad range of temperature-driven solution behaviours, from LCST and UCST, to sol–gel transitions, to micellization processes, among others, with potential applications as smart materials in electronics, in the lubricant industry, and in the biomedical field. This review article will focus on the thermoresponsive behaviour of polymers in different classes of organic solvents and mixtures thereof to emphasize and demonstrate the versatility and potential of these polymers.
1. Introduction
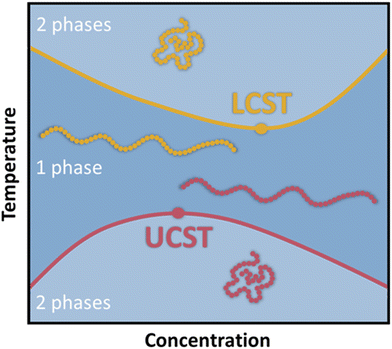
Smart polymers are able to respond to various external triggers such as temperature, light, magnetic fields, mechanical forces, pH and other chemical stimuli, emerging as valuable materials for numerous applications ranging from drug delivery, biosensors to tissue-/surface engineering among many others.1–3 To date, temperature remains the most utilized trigger for the design of stimuli responsive polymers predominantly in aqueous media, which is reflected by a plethora of available reports and review articles discussing and summarizing their synthesis and application.4–13 Thermoresponsive polymers exhibit a miscibility gap in the phase diagram for polymer/solvent mixtures (Fig. 1). Below the lower critical solution temperature (LCST) and above the upper critical solution temperature (UCST), a polymer in a solvents is miscible at any ratio.14It is important to note, that LCST and UCST are exact temperature values describing the minimum or maximum of the binodal curves, respectively. Therefore, constructing the binodal curve of a phase diagram for a given polymer/solvent system is essential for the determination of the exact values for LCST and UCST. Furthermore, the critical solution temperatures are dependent on polymer molecular weight and polydispersity as well as solution components and additives.15–18 Consequently, LCST/UCST values are often inaccurately reported by determining cloud point temperatures (TCP), which refer to the temperature at which phase separation occurs for a specific polymer concentration in solution. Hence, the TCP may be any value on the binodal curve. Additionally, different experimental approaches for the measurement of TCP result in further deviations, impeding the valid comparison of reported data.19,20 As a result, many efforts have been undertaken to facilitate and improve the characterization of thermoresponsive polymers. Predominantly, thermooptical techniques, such as turbidimetry, are utilized for the determination of TCP.21,22 However, the accuracy is strongly correlated to the heating rate and possible sediment formation of the sample.19 Therefore, various analytical approaches have been developed to increase the accuracy of measured TCP, while reducing the sample size and accelerating the analysis in a high-throughput fashion.19,23–28 Furthermore, thermoresponsive polymers have been analyzed by NMR spectroscopy,29 fluorescence microscopy,30 infrared spectroscopy (IR),31,32 dynamic light scattering (DLS),33–35 and calorimetric techniques.15,36–38
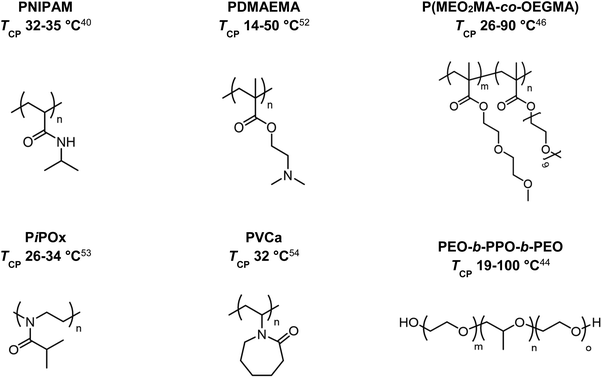
In 1967, the thermal phase transition of poly(N-isopropylacrylamide) (PNIPAM) in aqueous solution was reported for the first time using hydrogen–deuterium exchange experiments.39 Since then, PNIPAM advanced to become one of the most studied polymers in the field of thermoresponsive biomaterials.20,40–43 Similarly, triblock copolymers from poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO), which are summarized under the trademark name Pluronics, are powerful thermoresponsive materials for various applications.44 More recently, copolymers of poly(meth)acrylates with ethylene glycol-derived side chains gained growing attention due to a sharp phase transition and tunable TCP by copolymerizing different monomers.45–48 Similarly, polypeptides and poly(2-oxazolines) are discussed as potential PNIPAM substitutes, showing thermoresponsive behavior in the physiological temperature range (Fig. 2).49–51
The vast number of reports discussing water-soluble polymers with a LCST-type behavior exceeds the scope of this work and the reader is referred to exhaustive literature reviews addressing the topic and summarizing different polymer types.4,6 Instead, we would like to emphasize the current state of thermoresponsive polymers in solvent mixtures and non-aqueous media, while also highlighting polymers with UCST-type phase transitions. Polymers with a UCST behavior are often overlooked in literature reviews or remain merely a side note due to the large quantity of articles discussing materials with LCST-type phase transition in aqueous media for biomedical applications.55,56 For the same reasons, polymers with phase transitions in non-aqueous media are not sufficiently addressed and summarized in literature. In the following, the thermoresponsive behavior of polymers in solvent mixtures and non-aqueous media will be outlined and discussed.
2. Binary mixtures of water and other organic solvents
As explained above, water remains the most used media for the design and study of thermoresponsive polymers. Moreover, its binary mixtures with other organic solvents have also been thoroughly investigated. Among all, the thermoresponsive behaviour of PNIPAM and its copolymers has been widely studied in water/alcohol,57–66 water/DMSO,31,60,67 water/acetone,68 water/THF,58 and water/dioxane binary mixtures.58,69–71 Furthermore, other acrylamide homo and copolymers, such as poly(N,N-dimethylacrylamide) and poly[N-acryloylpiperidine-random-N-acryloylpyrrolidine], have been shown to undergo a phase transition in water/alcohol, water/dioxane, and water/acetone mixtures upon changes in temperature.72–75Another widely-studied class of polymers with thermoresponsiveness especially in water/alcohol mixtures is poly(methyl methacrylate) (PMMA) and its derivatives.76–80 Poly(ethylene glycol) and its derivatives have also been well established as thermoresponsive polymer systems in water/alcohol mixtures, showing LCST,81 UCST,82–84 or both transitions depending on the polymer and mixed solvents composition.85
Additionally, the solution behaviour of poly(2-alkyl-2-oxazoline) homo and copolymers has been studied in detail especially in binary ethanol/water solutions, in which they show both LCST and UCST depending on the length of the alkyl side chains and on the ethanol content.86–90 Polypeptides and glycopeptides have been investigated in water/alcohol mixtures, showing a tuneable UCST transition.91–93
Other classes of polymers showing thermoresponsiveness in water/organic solvent solutions are poly(boronic acid) and its derivatives in water/DMSO, water/DMF, and water/THF solutions,67,94,95 and polysiloxanes in water/acetonitrile and water/ethylene carbonate mixtures.96
Literature offers insight to a plethora of thermoresponsive polymers in water/organic solvents binary mixtures and the reader is referred to an exhaustive review for further information and examples on the UCST behaviour of polymers in alcohol/water mixtures.97 In the following sections, thermoresponsive polymers in pure organic solvents and mixtures thereof will be evaluated and discussed in more detail.
3. Pure alcohols
Apart from water and its binary mixtures, the thermoresponsive behaviour of polymers has been extensively investigated in alcoholic solvents. However, to the best of our knowledge, there are no literature reviews on this topic. For this reason, the thermoresponsive behaviour of polymers in alcohols will be discussed in detail in this section. The examples are grouped by solvents and, therefore, the same polymer classes will recur in the different subsections. In Tables S1–S5,† a complete overview of all examples discussed is provided for more clarity. Representative examples of thermoresponsive polymers in methanol (Table 1), ethanol (Table 2), 1-propanol (Table 3), 2-propanol (Table 4), and other alcoholic solvents (Table 5) are shown below.| Solvent | Type of polymer | Polymer conc. | Phase transition type (Temp. °C) | Ref. |
|---|---|---|---|---|
| MeOH | Poly(γ-4-(propoxycarbonyl)benzyl-L-glutamate)-oligo(ethylene glycol) derived from azido functionalized triethylene glycol monomethylether | 1 mg mL−1 | UCST (∼55) | 93 |
| MeOH | Poly(γ-4-(2-(2-methoxyethoxy) ethoxycarbonyl)benzyl-L-glutamate) | 1 mg mL−1 | UCST(@36.4) | 98 |
| MeOH | Poly(γ-4-(2-(2-(2-methoxyethoxy) ethoxy)ethoxycarbonyl) benzyl-L-glutamate) | 1 mg mL−1 | UCST (@16.0) | 98 |
| MeOH | Poly(γ-propyl-L-glutamate) with p-tolyl pendants and 3-methyl-1,2,3-triazolium iodide linkages | 0.5 mg mL−1 | UCST (∼26.8) | 99 |
| MeOH | Poly(γ-propyl-L-glutamate) tetra-O-acetyl-D-(+)-mannopyranoside conjugate | 5 mg mL−1 | UCST (@27.4) | 100 |
| MeOH | Poly(2-(2-(2-acetoxyethoxy)ethoxy)ethyl methacrylate) | 1 wt% | UCST (@26) | 101 |
| MeOH | Imidazolium ionic liquid-based nanogels | 4.5 wt% | Sol–gel transition (∼7) | 102 |
| MeOH | Poly(N-cyclopropyl-2-cyanohex-4-enamide) | 10 mg mL−1 | UCST (∼63) | 103 |
| MeOH | Poly(methyl 4-(2-(acryloyloxy)propanamido)butanoate) | 1 wt% | UCST (@6–27) | 104 |
| MeOH | Poly(1-(cyclohexylamino)-1-oxopropan-2-yl acrylate) | 1 wt% | UCST (@−37 to −20) | 104 |
| Solvent | Type of polymer | Polymer conc. | Phase transition type (Temp. °C) | Ref. |
|---|---|---|---|---|
| EtOH | Polypeptide bearing azobenzene and triethylene glycol spacers and 1-butylimidazolium side-chain end groups | 8 mg mL−1 | UCST (∼30) | 105 |
| EtOH | Poly(2-(2-(2-(2-((tetrahydrofuran-2-yl)oxy)ethoxy)-ethoxy)ethoxy) ethyl methacrylate) | 1 wt% | UCST (∼25) | 106 |
| EtOH | Poly(2-(dimethylamino) ethyl methacrylate-co-methacrylic acid-co-oligo(ethylene glycol) methyl ether methacrylate) | 1 mg mL−1 | UCST (∼10) | 107 |
| EtOH | Poly(2-(dimethylamino)ethyl methacrylate-b-3-phenylpropyl methacrylate) | 21 wt% | Sol–gel transition (∼70) | 108 |
| EtOH | Poly(N-isopropyl-2-cyanohex-4-enamide) | 10 mg mL−1 | UCST(@40) | 103 |
| EtOH | Poly(2-nonyl-2-oxazoline) | 5 mg mL−1 | UCST (∼48) | 86 |
| EtOH | Poly(octadecyl vinyl ether-random-2-methoxyethyl vinyl ether) | 1 wt% | UCST (∼25) | 109 |
| Solvent | Type of polymer | Polymer conc. | Phase transition type (Temp. °C) | Ref. |
|---|---|---|---|---|
| PrOH | Poly((γ-4-oligo(ethylene glycol) benzyl-L-glutamate)-random-(γ-benzyl-L-glutamate)) | 0.2 mg mL−1 | UCST (@25.6–90) | 110 |
| PrOH | Poly(2-(2-(2-(2-((tetrahydrofuran-2-yl)oxy)ethoxy)-ethoxy)ethoxy) ethyl methacrylate) | 1 wt% | UCST (∼22) | 106 |
| PrOH | Poly(2-hydroxyethy methacrylate) | 1 wt% | UCST (@35.8–45.9) | 111 |
| PrOH | Poly(N-(4-vinylbenzyl)-N,N-dibutylamine) | 2 wt% | UCST (@48.0) | 112 |
| Solvent | Type of polymer | Polymer conc | Phase transition type (Temp °C) | Ref. |
|---|---|---|---|---|
| iPrOH | Poly(ethylene glycol) 6 kDa | 0.050 wt% | UCST (@21) | 113 |
| iPrOH | Poly(poly(ethylene glycol) methacrylate) | 0.0625 wt% | UCST (<5) | 113 |
| iPrOH | Poly(acrylic acid)/poly(poly(ethylene glycol) methacrylate) blend | 0.0625/0.0625 wt% | UCST (@42) | 113 |
| iPrOH | PEG-armed star polymers, poly((ethylene glycol) methyl ether methacrylate-b-methyl methacrylate-b-ethylene glycol dimethyl acrylate) | 3 wt% | UCST (∼30) | 114 |
| iPrOH | PEG-armed Ru(II)-star polymers, poly((ethylene glycol) methyl ether methacrylate-b-methyl methacrylate-b-ethylene glycol dimethylacrylate-b-4-(diphenylphosphino)styrene-Ru(II)) | 3 wt% | UCST (∼31) | 114 |
| iPrOH | Poly(oligo(ethylene glycol) methyl ether methacrylate-b-N-isopropylacrylamide) | 5 mg mL−1 | UCST (<0) | 115 |
| iPrOH | Poly(oligo(ethylene glycol) methyl ether methacrylate-b-N,N-diethylacrylamide) | 5 mg mL−1 | UCST (@0.5) | 115 |
| iPrOH | Poly(2-(dimethylamino) ethyl methacrylate-co-methacrylic acid)-graft-oligo(ethylene glycol) methyl ether methacrylate | 1 mg mL−1 | UCST (@50) | 107 |
| iPrOH | Poly(vinylidene fluoride)-graft-poly(diethylene glycol methyl ether methacrylate) | 0.09%w/v | UCST (@27.5) | 85 |
| iPrOH | Poly(N-(2-methacrylaoyxyethyl)pyrrolidone-b-methyl methacrylate) | 5 wt% | Sol–gel transition (@27–50) | 116 |
| Solvent | Type of polymer | Polymer conc. | Phase transition type (Temp. °C) | Ref. |
|---|---|---|---|---|
| BuOH | Poly(N-phenyl maleimide-co-n-octadecyl vinyl ether) | 0.1 wt% | UCST (@67) | 117 |
| iBuOH | Poly(oligo(ethylene glycol) methyl ether methacrylate) | 16 mg mL−1 | UCST (@30.4) | 82 |
| iBuOH | Poly(2-hydroxyethyl methacrylate) | 1 wt% | UCST (@68.8–71.4) | 111 |
| secBuOH | Poly(oligo(ethylene glycol) methyl ether methacrylate) | 16 mg mL−1 | UCST (@26.4) | 82 |
| secBuOH | Poly(2-hydroxyethyl methacrylate) | 1 wt% | UCST (@13.5–14.0) | 111 |
| 2-Methyl-1-butanol | Poly(oligo(ethylene glycol) methyl ether methacrylate) | 16 mg mL−1 | UCST (@22.2) | 82 |
| Glycerol | Poly(2-hydroxyethyl methacrylate) | 1 wt% | UCST (@71.8–83.6) | 111 |
| PeOH | Poly((γ-4-oligo(ethylene glycol) benzyl-L-glutamate)-random-(γ-benzyl-L-glutamate)) | 0.2 mg mL−1 | UCST (@43.3–86) | 110 |
| PeOH | Poly(oligo(ethylene glycol) methyl ether methacrylate) | 16 mg mL−1 | UCST (@40.9) | 82 |
| iPeOH | Poly(oligo(ethylene glycol) methyl ether methacrylate) | 16 mg mL−1 | UCST (@35.7) | 82 |
| 4-Methyl-2-pentanol | Poly(oligo(ethylene glycol) methyl ether methacrylate) | 16 mg mL−1 | UCST (@34.0) | 82 |
| HexOH | Poly(N-phenyl maleimide-co-n-octadecyl vinyl ether) | 0.1 wt% | UCST (∼47) | 117 |
| HepOH | Poly(oligo(ethylene glycol) methyl ether methacrylate) | 16 mg mL−1 | UCST (@53.3) | 82 |
| 2-Octanol | Poly(oligo(ethylene glycol) methyl ether methacrylate) | 16 mg mL−1 | UCST (@47.7) | 82 |
| OctOH | Poly(N-phenyl maleimide-co-n-octadecyl vinyl ether) | 0.1 wt% | UCST (∼35) | 117 |
| OctOH | Poly(oligo(ethylene glycol) methyl ether methacrylate-b-(N-isopropylacrylamide-co-pentafluorophenyl acrylate)) | 1 mg mL−1 | Self-assembly (@0) | 115 |
| Dodecanol | Poly(oligo(ethylene glycol) methyl ether methacrylate) | 16 mg mL−1 | UCST (@75.4) | 82 |
| CyclohexOH | Poly(styrene-b-methyl methacrylate) | ∼0.15–20 wt% | UCST (∼65–75) | 118 |
| BnOH | Poly(γ-benzyl L-glutamate) | 15–40 wt% | Sol–gel transition (@60–70) | 119 |
3.1. Thermoresponsive behaviour in methanol (MeOH)
Methanol is one of the most studied alcoholic solvents for the evaluation of the thermoresponsive behaviour of polymers in solution. The temperature-induced phase transitions of synthetic polypeptides in alcohols and, in particular, in methanol has been widely studied especially for their potential application in the biomedical field as smart materials. For example, the polypeptide poly((γ-4-oligo(ethylene glycol) benzyl-L-glutamate)-random-(γ-benzyl-L-glutamate)) containing an oligo(ethylene glycol) (OEG) molar content of 0.75 mol% and a degree of polymerization (DP) of 3 prepared via triethylamine-initiated ring-opening polymerization (ROP) exhibited a UCST-type phase transition in methanol.110 The TCP increased from 4 to 34.2 °C with increased concentration from 0.2 to 1 mg mL−1, as demonstrated by visual inspection and UV-vis analyses.In another report, polypeptides containing OEG pendants synthesized via copper-mediated [3 + 2] alkyne–azide 1,3-dipolar cycloaddition (CuAAC) between poly(γ-4-(propargoxycarbonyl)benzyl-L-glutamate) (P1) or poly(γ-4-(4-propargoxyphenoxycarbonyl)benzyl-L-glutamate) (P2) and azido functionalized OEG (N3-OEGx) were also found to have thermoresponsive behaviour in MeOH (Fig. 3A).93
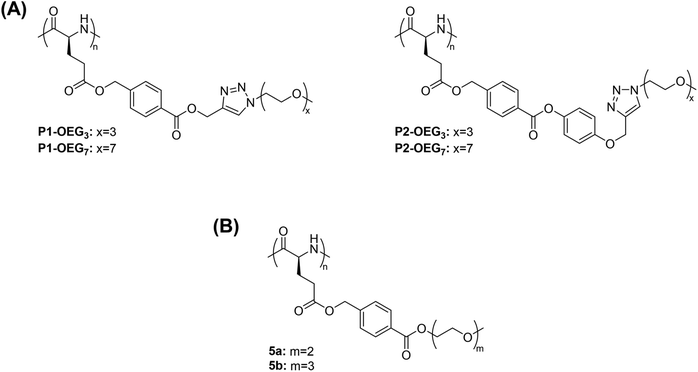 | ||
| Fig. 3 Chemical structure of polypeptides with OEG pendants. (A) Polypeptides obtained by reacting poly(γ-4-(propargoxycarbonyl)benzyl-L-glutamate) (P1) or poly(γ-4-(4-propargoxyphenoxycarbonyl)benzyl-L-glutamate) (P2) with azido functionalized OEG,93 and (B) poly(γ-4-(2-(2-methoxyethoxy) ethoxycarbonyl)benzyl-L-glutamate) (5a) and poly(γ-4-(2-(2-(2-methoxyethoxy)ethoxy)ethoxycarbonyl) benzyl-L-glutamate).98 | ||
The thermoresponsive behaviour was dependent on both the type of linkage (i.e. phenylene or benzoic acid phenyl ester linkages) and the length of the OEG pendants. On the one hand, P1-OEG3 with phenylene linkages and short OEG pendants exhibited a UCST phase transition in MeOH at around 55 °C at a polymer concentration of 1 mg mL−1, as showed by variable-temperature UV-vis spectroscopy measurements. On the other hand, P2-OEG7 with benzoic acid phenyl ester linkages and long OEG pendants underwent a UCST phase transition at 49.5 °C at the same polymer concentration. At low temperatures, the polymers aggregated, but at elevated temperatures the hydrogen bonding interactions between the polar linkages, such as the ester bonds and 1,2,3-triazole groups, the OEG pendants of the polymers and the alcoholic molecules, became more favoured compared to the polymer–polymer ones resulting in the dissolution of the polymer.93
In another example, polypeptides with OEG pendants synthesised from OEGylated N-carboxyanhydride monomers by ROP using triethylamine as initiator, namely poly(γ-4-(2-(2-methoxyethoxy) ethoxycarbonyl)benzyl-L-glutamate) (5a) and poly(γ-4-(2-(2-(2-methoxyethoxy)ethoxy)ethoxycarbonyl) benzyl-L-glutamate) (5b), exhibited a UCST-type phase transition in MeOH (Fig. 3B).98 In the transmittance curves, no hysteresis between the heating a cooling cycles was observed due to a lack of intra- and intermolecular hydrogen bonding interactions between the OEG pendants, which prevented the formation of large aggregates. The thermoresponsive behaviour was attributed to H bonding interactions between the ether groups on the OEG pendants and the OH groups of the alcoholic solvent, which became greater with the increase in temperature, as demonstrated by variable-temperature UV-vis spectroscopy, variable-temperature 1H NMR, and DLS measurements. Furthermore, the UCST phase transition was found to be strongly dependent on the polymer concentration, the length of the OEG pendants, and the length of the main chain.
In a recent report, polypeptides bearing p-tolyl pendants and 3-methyl-1,2,3-triazolium linkages synthesized via N-alkylation of poly(γ-propyl-L-glutamate)-graft-(4-methylbenzene) and subsequent ion-exchange reactions, underwent a UCST-type phase transition in MeOH (Fig. 4).99 It was shown by transmittance measurements that poly(γ-propyl-L-glutamate) with p-tolyl pendants and 3-methyl-1,2,3-triazolium iodide linkages (P2) with a DP of 54 or 92 had similar TCP values around 26.8 °C at a polymer concentration of 0.5 mg mL−1. However, P254 with a shorter main chain length exhibited a lower initial aggregation temperature and a sharper solution phase transition compared to P292 (Fig. 4). The thermoresponsive behaviour of P2 and its solubilization upon increase in temperature were attributed to the van der Waals interactions between the hydrophobic groups and the alkyl groups of the alcoholic solvent, and to the H bonding interactions between the polar ester and ether bonds and the hydroxyl groups of the solvent molecules. A similar behaviour was also observed in the case of poly(γ-propyl-L-glutamate) with p-tolyl pendants and 3-methyl-1,2,3-triazolium tetrafluoroborate linkages (P4) at a high concentration (10 mg mL−1), but a value for its TCP was not reported.
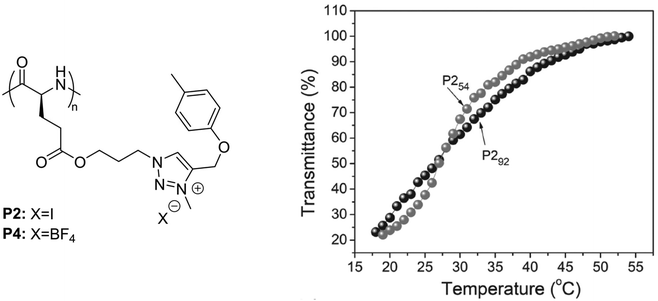 | ||
| Fig. 4 Chemical structure of the polypeptides bearing p-tolyl pendants and 3-methyl-1,2,3-triazolium linkages, and plots of transmittance versus temperature of 0.5 mg mL−1 MeOH solutions of P254 and P292. Adapted from ref. 99 Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
In another example, polypeptides containing 1-butyl, 1-hexyl, or 1-dodecyl side-chains synthesized by CuAAC between poly(γ-4-(propargoxycarbonyl)benzyl-L-glutamate) and 1-azidoalkanes exhibited a UCST phase transition in MeOH, which was dependent on the length of both the alkyl side-chains and the main chain.91 While polypeptides with a long main chain (DP ≥ 62) were insoluble in MeOH, polypeptides with a short main chain (i.e. DP = 41–44) showed UCST behaviour, with TCP of 55, 50 and 54.7 °C when the length of the alkyl pendants was varied from 1-butyl, to 1-hexyl, to 1-dodecyl, respectively. Furthermore, lowering the polymer concentration resulted in a decrease in the TCP values.
Sugar-containing polypeptides have also shown temperature-induced solution behaviours in methanol. This is the case of glycopolypeptides with different contents of tetra-O-acetyl-D-(+)-mannopyranoside and tetra-O-hydroxyl-D-(+)-mannopyranoside pendants synthesised via CuAAC, which showed a UCST-type phase transition in MeOH, that was dependent on both the content and DP of the two monosaccharides (Fig. 5).100 On the one hand, glycopolypeptides containing only tetra-O-acetyl-D-(+)-mannopyranoside pendants (i.e.5a, DP = 36) exhibited a UCST phase transition at 7.4 °C at 1 mg mL−1, which increased to 27.4 °C at 5 mg mL−1, as shown by variable-temperature UV-vis spectroscopy, Fourier Transform Infrared (FTIR) spectroscopy, and dynamic light scattering (DLS). On the other hand, the thermoresponsive behaviour of glycopolypeptides containing both tetra-O-acetyl-D-(+)-mannopyranoside and tetra-O-hydroxyl-D-(+)-mannopyranoside pendants (i.e.5c and 5d) was highly dependent on the ratio (x and y, respectively) of the two monosaccharide units. The TCP of the glycopolypeptides in MeOH (5 mg mL−1) increased from 33.4 to 35 °C, when decreasing the molar content of the acetylated sugar unit from 85 to 44 mol%. A mechanism for the UCST-type phase transition of the obtained glycopolypeptides based on the competition between side-chain/side-chain interactions and side-chain/solvent interactions was proposed. At high temperatures, the hydrogen bonding interactions between the acetyl groups and the alcoholic molecules resulted in the solvation of the polymer, while the hydrophobic interactions led to polymer aggregation at low temperatures. Moreover, the incorporation of proton donating groups, such as tetra-O-hydroxyl-D-(+)-mannopyranoside, led to the formation of hydrogen bonds between the side-chains, resulting in higher TCP values due to the need of higher energy to break the polymer aggregates. The authors stated that these thermoresponsive glycopolypeptides could be potentially applied as smart stationary phases for chromatography.
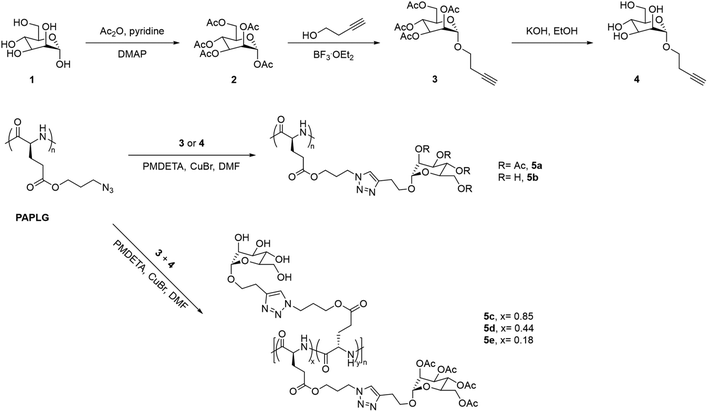 | ||
| Fig. 5 Synthetic route of the glycopolypeptides with mannose pendants.100 | ||
Polypeptoides are another class of polymers showing thermoresponsiveness in alcoholic media. For instance, the ROP of N-allyl glycine (NAG) and N-octyl glycine (NOG) using benzylamine as initiator followed by the thiol–ene/yne coupling reaction with mercaptoacetic acid resulted in the synthesis of carboxylic acid-containing polypeptoides with a UCST-type solution behaviour.120 A random copolymer with a 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 ratio between the average DP of NAG and NOG (PNAG49-r-PNOG14-COOH) underwent a UCST phase transition at 50.8 °C in MeOH at a polymer concentration of 2 mg mL−1, as demonstrated by transmittance measurements. The TCP values were dependent on the polymer concentration, and, at a fixed concentration, it increased upon increasing the DP of NOG due to a reduced solubility of the polymer in the alcoholic solvent caused by the longer alkyl chains.
14 ratio between the average DP of NAG and NOG (PNAG49-r-PNOG14-COOH) underwent a UCST phase transition at 50.8 °C in MeOH at a polymer concentration of 2 mg mL−1, as demonstrated by transmittance measurements. The TCP values were dependent on the polymer concentration, and, at a fixed concentration, it increased upon increasing the DP of NOG due to a reduced solubility of the polymer in the alcoholic solvent caused by the longer alkyl chains.
Other classes of OEG-containing polymers resulted to be thermoresponsive in methanol. For example, a series of poly(oligo(ethylene glycol) (acyloxy)methacrylate)s (PAEEOnMA) containing OEG units and different ester substituents synthesised via RAFT polymerization exhibited a UCST behaviour, which was dependent on the molecular structure, molecular weight, and concentration of the polymer, as shown by turbidity measurements (Fig. 6).101 It was demonstrated that the TCP decreased with increasing number of OEG units and carbon atoms in the alkyl substituents of the ester groups. For example, the increase of the OEG units from 2 to 4 resulted in a decrease of the TCP from 26 to 4.5 °C, for 1 wt% polymer solutions in MeOH. Moreover, for polymers with similar DP and the same number of OEG units, the increase in the number of carbon atoms in the alkyl groups of the ester resulted in the complete solubilization of the polymers bearing longer alkyl chains (i.e. R = CH2CH3 and CH2CH2CH3).
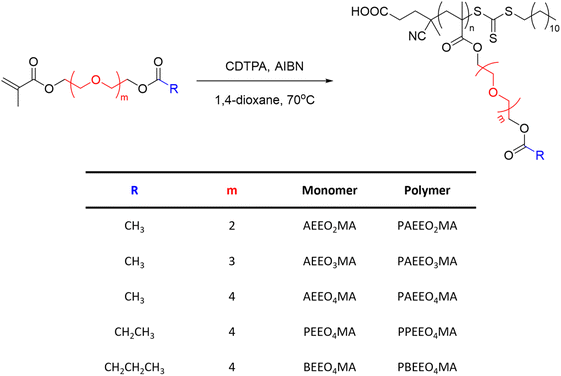 | ||
| Fig. 6 Schematic representation of the synthetic procedure used to synthesize a series of poly(oligo(ethylene glycol) (acyloxy)methacrylate)s.101 | ||
It has also been reported that poly(N-propionyl-aspartic acid/ethylene glycol) (PPAE) synthesized by polycondensation of L-aspartic acid and ethylene glycol exhibited a UCST-type phase transition in MeOH.121 However, no TCP values or analyses on the polymer solution have been stated. The polymers were non-toxic and biocompatible, making them promising candidates for biomedical applications.
A ethylene glycol dimethacrylate (EGDMA) cross-linker was used for the synthesis of imidazolium ionic liquid-based cross-linked polymeric nanogels (ImIL-based CLPNs) obtained via FRP of 1,4-butanediyl-3,3′-bis-1-vinyl imidazolium halides ([BVIm]X = 1·X, X = Cl, Br), which showed both a UCST and a sol–gel transition in MeOH depending on the feed ratio between 1·Br and EGDMA (Fig. 7).102 On the one hand, at a 1·Br/EGDMA feed ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol, the 4.5 wt% CLPN solution was translucent at temperatures above 28 °C and became cloudy when cooled below 25 °C, as shown by turbidity and DLS measurements. On the other hand, when the feed ratio was increased to 10
1 mol/mol, the 4.5 wt% CLPN solution was translucent at temperatures above 28 °C and became cloudy when cooled below 25 °C, as shown by turbidity and DLS measurements. On the other hand, when the feed ratio was increased to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol, the polymer solution turned into a gel at temperatures below −7 °C, as supported by temperature-dependent oscillatory shear rheological measurements (Fig. 7C). The increase in the 1·Br content resulted in the enhancement of the interactions between CLPN and the methanol molecules. In addition, 1H NMR spectroscopy, IR spectroscopy, and X-ray diffraction were used to elucidate the interactions between the ImIL-based CLPN and MeOH. All analyses showed that the formation of a solution of CLPN in MeOH was related to the partial replacement of C–H—Br− bonds with hydrogen bonding interactions between the imidazolium ring and the methanol molecules. Therefore, the formation of a thermoresponsive CLPN gel in MeOH at low temperatures required the enhancement in the hydrogen bonds between the imidazolium ring, Br−, and MeOH, while at high temperatures the H-bond network was destroyed resulting in the formation of a CLPN MeOH solution.
1 mol/mol, the polymer solution turned into a gel at temperatures below −7 °C, as supported by temperature-dependent oscillatory shear rheological measurements (Fig. 7C). The increase in the 1·Br content resulted in the enhancement of the interactions between CLPN and the methanol molecules. In addition, 1H NMR spectroscopy, IR spectroscopy, and X-ray diffraction were used to elucidate the interactions between the ImIL-based CLPN and MeOH. All analyses showed that the formation of a solution of CLPN in MeOH was related to the partial replacement of C–H—Br− bonds with hydrogen bonding interactions between the imidazolium ring and the methanol molecules. Therefore, the formation of a thermoresponsive CLPN gel in MeOH at low temperatures required the enhancement in the hydrogen bonds between the imidazolium ring, Br−, and MeOH, while at high temperatures the H-bond network was destroyed resulting in the formation of a CLPN MeOH solution.
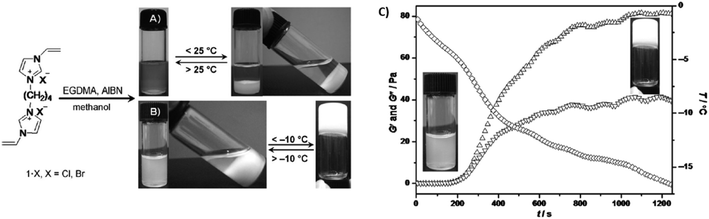 | ||
Fig. 7 One-step synthesis of ImIL-based CLPN, and images of the thermoresponsive behaviour of CLPN solution with a 1·Br/EGDMA feed ratio of (A) 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol, and (B) 10 1 mol/mol, and (B) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol mol−1. (C) Shear storage modulus (G′, ▲) and shear loss modulus (G′′; ▼) as a function of temperature (◆) for a 4.5 wt% CLPN MeOH solution with a 10 1 mol mol−1. (C) Shear storage modulus (G′, ▲) and shear loss modulus (G′′; ▼) as a function of temperature (◆) for a 4.5 wt% CLPN MeOH solution with a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1·Br/EGDMA feed ratio and corresponding appearance of the sample. Adapted from ref. 102 Copyright 2012 WILEY-VCH Verlag GmbH & Co. KGaA. 1 1·Br/EGDMA feed ratio and corresponding appearance of the sample. Adapted from ref. 102 Copyright 2012 WILEY-VCH Verlag GmbH & Co. KGaA. | ||
Other classes of polymers undergo temperature-induced phase transitions in MeOH, as shown by the examples given below.
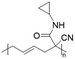
Poly(N-cyclopropyl-2-cyanohex-4-enamide) synthesized via ROP of 1-cyano-1-pentafluorophenoxycarbonyl-2-vinylcyclopropane and post-polymerization modification of the obtained homopolymer with cyclopropylamine exhibited a UCST-type behaviour in MeOH.103 The polymer phase transition was dependent on the polymer concentration, with TCP values decreasing from around 63 to around 54 °C at 10 and 2.5 mg mL−1, respectively, as shown by turbidity measurements.
Turbidity measurements of a 1 mg mL−1 MeOH solution of poly(N-(6-acetamidopyridin-2-yl)acrylamide) obtained by free radical polymerization (FRP) demonstrated the UCST-type thermoresponsive behaviour of the polymer with a TCP of 50 °C.73
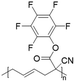
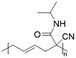
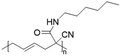
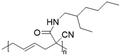
In another report, poly(methyl 4-(2-(acryloyloxy)propanamido)butanoate) and poly(1-(cyclohexylamino)-1-oxopropan-2-yl acrylate) were synthesized by FRP using functionalized acrylate monomers obtained from the Passerini three-component reaction (Passerini-3CR) of acrylic acid and a series of isocyanides and aldehydes (Fig. 8).104 Because of the hydrophilicity/hydrophobicity balance, the resulting polyacrylates showed a UCST behaviour in MeOH at 6–27 and −37 to −20 °C, respectively, as shown by UV-vis analyses of 1 wt% polymer solutions.
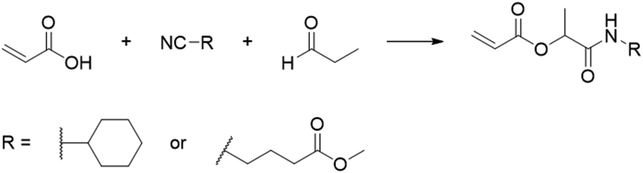 | ||
| Fig. 8 Passerini-3CR of acrylic acid, different isocyanides, and propionaldehyde yielding the acrylates used in the synthesis of thermoresponsive polymers with a UCST-type phase transition in MeOH.104 | ||
Magnetic fluids based on magnetite coated with a covalently bonded polymeric shell of poly(2-methoxyethyl methacrylate) (PMEMA) have been reported to show a UCST-type behaviour in MeOH, forming stable dispersions above UCST and precipitated below it.122 From turbidity measurements, the TCP was found to decrease from around 38 to 19 °C with the increase in the length of the PMEMA arms from to 11 to 35.1 kDa, which was not observed for the free polymers in solution. This behaviour was attributed to the surface attachment of the arms, which resulted in a less beneficial entropic contribution to the solvation process compared to free polymer chains. This effect was less pronounced for long chains since the free volume of chain segments increased with increasing distance from the solid core. These core–shell particles, which combine the properties of both thermoresponsive polymers and magnetic fluids, could be promising materials for easily recoverable polymer supported magnetic separation kits and catalytic systems.
In another example, a random copolymer of octadecyl vinyl ether (ODVE) and 2-methoxyethyl vinyl ether obtained via living cationic polymerization underwent a UCST-type phase transition in methanol at around 20 °C, which was attributed to the crystallization of the long alkyl chains of the octadecyl vinyl ether monomer.109
Finally, poly(N-(4-vinylbenzyl)-N,N-diethylamine) (PVEA) obtained by RAFT polymerization exhibited a LCST phase transition in MeOH, which was strongly dependent on the polymer DP and concentration.112,123 The effect of the addition of a cosolvent on the LCST-type behaviour was also evaluated by analysing PVEA DP = 104 MeOH/EtOH solutions. The TCP increased from 39.5 to 65 °C with increased EtOH content from 0 to 50 wt%, and the polymer became soluble above 50 w% of EtOH.
3.2. Thermoresponsive behaviour in ethanol (EtOH)
The thermoresponsive behaviour of polymers in ethanol has also been widely investigated. Many of the previously mentioned polymers have similar thermoresponsive behaviours in both MeOH and EtOH, therefore, they will appear again in this subsection.As expected, PEO-containing or OEGylated polymers have shown thermoresponsiveness also in ethanol. For example, PEO homopolymer exhibited a UCST phase transition due to the dissolution of the crystalline areas formed upon quenching of a polymer solution at temperatures above the melting temperature.124 The TCP varied from around −5 to 10 °C when the polymer concentration was increased from 0.01 to 0.165 vol%, as extrapolated from small-angle neutron scattering (SANS) measurements.
Various OEGylated polypeptides exhibited a UCST-type behaviour in EtOH.93,110 Polypeptides obtained from CuAAC of poly(γ-4-(propargoxycarbonyl)benzyl-L-glutamate) with azido functionalized triethylene glycol monomethylether (P1-OEG3) or azido functionalized poly(ethylene glycol) methyl ether 350 (P1-OEG7), and poly(γ-4-(4-propargoxyphenoxycarbonyl)benzyl-L-glutamate) with azido functionalized poly(ethylene glycol) methyl ether 350 (P2-OEG7) exhibited UCST phase transition in ethanol (Fig. 3A).93 At a polymer concentration of 1 mg mL−1, the TCP of P1-OEG3 was higher than the one of P1-OEG7 (70.5 °C and 53.1 °C, respectively) due to a decrease in the hydrogen bonding interactions between the OEG side chains and the alcoholic molecules as the length of the OEG pendants decreased. Moreover, the TCP of P2-OEG7 was higher than that of P1-OEG7 (i.e. 64 °C) due to the presence of more hydrophobic linkages.
Poly((γ-4-oligo(ethylene glycol) benzyl-L-glutamate)-random-(γ-benzyl-L-glutamate)) copolymers also exhibited a UCST-type behaviour in EtOH at a maximal concentration above 0.2 mg mL−1.110 For polymers with the same OEG DP, a decrease in the TCP values was observed with increasing OEG molar content due to an enhancement of the H bonding interactions between the oxygen of the OEG pendants and the hydroxyl groups of the alcoholic solvent, which facilitated the solvation of the polymers.
In another example, poly(γ-4-(2-(2-methoxyethoxy) ethoxycarbonyl)benzyl-L-glutamate) and poly(γ-4-(2-(2-(2-methoxyethoxy)ethoxy)ethoxycarbonyl) benzyl-L-glutamate), OEGylated polypeptides obtained from the ROP of N-carboxyanhydrides, showed a UCST phase transition in ethanol, which was dependent on the polymer concentration, and on the DP of both the side and the main chains.98
Furthermore, a homopolypeptide bearing azobenzene and triethylene glycol spacers and 1-butylimidazolium pendants with iodide counter anions (P(Azo-OEG3-ImI)) showed a UCST-type phase transition in EtOH at around 30 °C at a polymer concentration of 8 mg mL−1, which was strongly affected by the polymer concentration in solution (Fig. 9).105
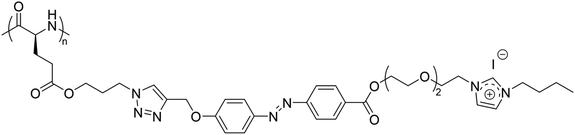 | ||
| Fig. 9 Chemical structure of the homopolypeptide P(Azo-OEG3-ImI).105 | ||
As in the case of methanol, polypeptides containing 1-butyl, 1-hexyl, or 1-dodecyl side-chains showed a UCST-type behaviour in EtOH.91 For polymers having similar main chain length, a decrease in the TCP from 71.1 to 56.9 °C was observed with the increase in the alkyl side-chain length from 4 to 12 carbon atoms. For the same polymer type, higher TCP values were recorded with increasing main chain length or polymer concentration due to an enhancement of hydrophobic interactions and entanglement between polymer chains.
Glycopolymers also displayed thermoresponsive behaviour in EtOH. For example, glycopolypeptides containing different amounts of tetra-O-acetyl-D-(+)-mannopyranoside and tetra-O-hydroxyl-D-(+)-mannopyranoside pendants exhibited UCST-type phase transition with TCP values dependent on the content and the DP of the monosaccharides.100 Glycopolymers containing only tetra-O-acetyl-D-(+)-mannopyranoside pendants showed a TCP of 51.9 °C, but when the tetra-O-acetyl-D-(+)-mannopyranoside molar content was decreased from 100 to 44, the TCP increased from 51.9, to around 60 °C, respectively, due to an enhancement of the H bonding interactions between the proton donating groups in the side-chains.
Another class of OEGylated polymers which have been studied for their thermoresponsive behaviour in EtOH are poly(methacrylate)s. For example, poly(oligo(ethylene glycol) methyl ether methacrylate) (POEGMA) with a molecular weight of 23.2 kDa synthesised via RAFT polymerization exhibited a UCST-type behaviour in EtOH at 21.9 °C at a polymer concentration of 16 mg mL−1, as showed by cloud point measurements.82 Furthermore, it has been shown that poly(oligo(ethylene glycol) (acyloxy)methacrylate)s containing OEG units and various ester substituents obtained via RAFT polymerization exhibited a tunable UCST-type phase transition, depending on the polymer structure, molecular weight, and concentration (Fig. 6).101 For example, a 1 wt% poly(2-(2-(2-acetoxyethoxy)ethoxy)ethyl methacrylate) solution exhibited a TCP at 55 °C, which decreased to 36 °C when the number of OEG units was increased from 2 to 4. Moreover, the alkyl substituents in the ester groups also affected the TCP values. In fact, the increase in the number of carbon atoms resulted in a decreased TCP from 36 °C for poly(2-(2-(2-(2-(2-(acetoxy)ethoxy)ethoxy)ethoxy)ethoxy) ethyl methacrylate), to 8 °C for poly(2-(2-(2-(2-(2-(butyrylacyloxy)ethoxy)ethoxy)ethoxy)ethoxy) ethyl methacrylate).
A UCST-type phase transition was also observed for poly(2-(2-methoxyethoxy)ethyl methacrylate), which was strongly dependent on the polymer molecular weight.123 In fact, at a polymer concentration of 2 wt%, the TCP increased from 13.4 to 20.6 °C when the polymer DP was increased from 29 to 89. At higher DP, the polymer–polymer interactions were more favourable, and higher temperatures were required to disrupt these interactions, resulting in higher TCP values.
Another example is poly(2-(2-(2-(2-((tetrahydrofuran-2-yl)oxy)ethoxy)-ethoxy)ethoxy) ethyl methacrylate) (PTFEO4MA) containing oligo ethylene glycol moieties and acetal groups synthesised via RAFT polymerization, which showed a UCST-type phase transition in EtOH with a TCP at around 25 °C (Fig. 10).106
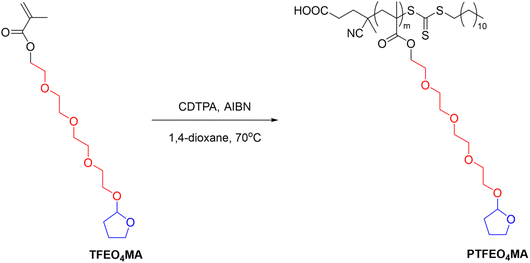 | ||
| Fig. 10 Schematic depiction of the RAFT polymerization of poly(2-(2-(2-(2-((tetrahydrofuran-2-yl)oxy)ethoxy)-ethoxy)ethoxy) ethyl methacrylate) (PTFEO4MA).106 | ||
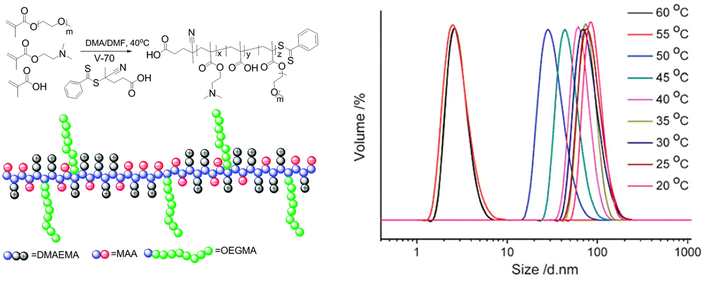
Furthermore, the RAFT copolymerization of a neutral oligo(ethylene glycol)methyl ether methacrylate (Mn = 480 Da) with two charged co-monomers, namely 2-(dimethylamino)ethyl methacrylate and methacrylic acid, yielded a thermoresponsive brush copolymer polyampholyte with a UCST phase transition in EtOH (Fig. 11).107 At temperatures higher than 10 °C the polymer was completely soluble with particle size of 6 nm corresponding to unimers, as shown by DLS analysis. When the temperature was decreased to 5 °C, the particle size increased to about 45 nm, indicating the formation of nanostructures. This thermoresponsive behaviour was attributed to the collapse of the polymer due to strong electrostatic interactions between positive and negative charges, while the ethylene glycol side chains acted as solvophilic corona, stabilizing the nanostructures and preventing their aggregation.
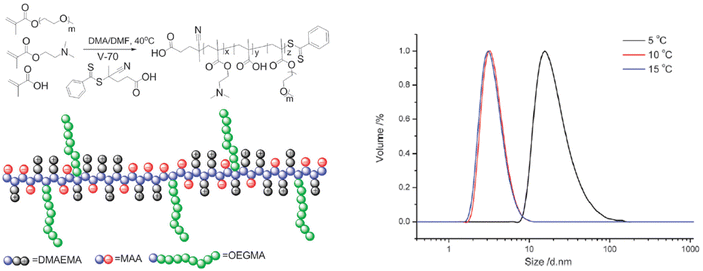 | ||
| Fig. 11 Schematic representation of the synthesis and structure of the brush copolymer polyampholyte (left), and size distribution at various temperatures for a 1 mg mL−1 ethanol solution of the brush copolymer determined by DLS (right). Adapted from ref. 107 Copyright 2015 Royal Society of Chemistry. | ||
Furthermore, block copolymers consisting of a poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA) segment (DP = 20) and a 3-phenylpropyl methacrylate (PPPMA) block (DP = 47) (PDMAEMA20-b-PPPMA47) obtained by RAFT dispersion polymerization formed a pure worm phase at 21 wt% solids in ethanol at room temperature.108 The block copolymer exhibited a gradual macroscopic thermoreversible degelation–gelation behaviour when heated up to 70 °C due to a worm-to-sphere morphological transition, as supported by 1H NMR, DLS, and TEM analyses (Fig. 12).
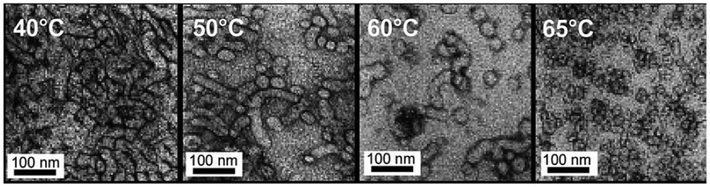 | ||
| Fig. 12 TEM images of the PDMAEMA20-b-PPPMA47 block copolymer at 40, 50, 60 and 65 °C demonstrating the gradual temperature-induced worm-to-sphere morphology transition. Reproduced from ref. 108 Copyright 2014 Royal Society of Chemistry. | ||
Various poly(acrylate)s have also shown to undergo temperature-induced phase transition in EtOH, as in the case of poly(methyl acrylate) and its copolymers. Poly(methyl acrylate) with a DP of 60 synthesised via solution atom transfer radical polymerization (ATRP) exhibited a UCST behaviour at 60 °C at a concentration of 5 mg mL−1.125 The TCP could be tuned by copolymerization of poly(methyl acrylate) with other monomers, such a styrene and diethylene glycol ethyl ether acrylate, into diblock copolymers. In the latter case, the UCST was attributed to the transition from micelles to individually dissolved polymer chains, as demonstrated by turbidity and DLS measurements.84
In another example, poly(1-(benzylamino)-1-oxopropan-2-yl acrylate) and poly(methyl 4-(2-(acryloyloxy)propanamido)butanoate) obtained by FRP using acrylates synthesised via the Passerini-3CR showed UCST-type phase transition in ethanol in a temperature range of 55–74 °C and 6–19 °C, respectively, as demonstrated by visual inspection and UV-vis analyses of 1 wt% polymer solutions.104
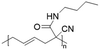
Vinylcyclopropane-derivatives could also undergo UCST phase transition in EtOH. For example, poly(1-cyano-N-propylcarboxyamidovinylcyclopropane) obtained by post-polymerization modification of poly(1-cyano-1-pentafluorophenoxycarbonyl-2-vinylcyclopropane) with n-propylamine showed a UCST-type behaviour in EtOH at around 34 °C at a concentration of 1 mg mL−1.126 As expected, the TCP was found to slightly decrease with the decrease in the polymer concentration. Furthermore, polymers obtained from the ROP of poly(1-cyano-1-pentafuorophenoxycarbonyl-2-vinylcyclopropane) (PCPFPCVP) and its further post-polymerization modification with five different primary amines showed UCST behaviour in ethanol (Table 6).103 At a concentration of 10 mg mL−1, poly(N-isopropyl-2-cyanohex-4-enamide) (VCP2J1) exhibited the highest TCP at 40 °C, while the butylamide-derivative VCP2J3 showed a TCP of 23 °C. The increase in the chain hydrophobicity resulted in a further decrease of the TCP to 15 °C for poly(N-hexyl-2-cyanohex-4-enamide) (VCP2J4). Interestingly, the polymer with 2-(ethyl)hexyl alkyl chains (VCP2J5) exhibited a higher TCP at 25 °C. It was also found that the increase in size and, thus, in hydrophobicity of the amine moiety, led to an increase in the minimal concentration required to observe UCST behaviour due to an enhancement of the polymer solubility in the alcoholic solvent.
Poly(2-alkyl-oxazoline)s are another class of polymers that shows temperature-driven phase transitions not only in water/alcohol mixtures, but also in pure alcohols, such as ethanol. To exemplify, poly(2-nonyl-2-oxazoline) and poly(2-benzyl-2-oxazoline) obtained by cationic ring-opening polymerization underwent a UCST-type phase transition in ethanol at a concentration of 5 mg mL−1 at around 48 and −12 °C, respectively.86 In the case of poly(2-phenyl-2-oxazoline) solutions at the same polymer concentration, two TCP values at around 40 and at 48.1 °C have been reported.88,89 Nevertheless, the copolymerization of 2-phenyl-2-oxaozline with 2-nonyl-2-oxazoline in a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio resulted in a drastic decrease in the TCP to around 10 °C due to an increased solubility of the statistical copolymer in EtOH.89
10 ratio resulted in a drastic decrease in the TCP to around 10 °C due to an increased solubility of the statistical copolymer in EtOH.89
As shown above for solutions in methanol, poly(octadecyl vinyl ether-random-2-methoxyethyl vinyl ether) also exhibited a fully reversible UCST-type phase transition at around 25 °C in ethanol at a concentration of 1 wt% due to the crystallization of the side chains of the octadecyl vinyl ether units.109
3.3. Thermoresponsive behaviour in 1-propanol (PrOH)
Polymers also exhibit thermoresponsive behaviours in 1-propanol (PrOH) As shown above for MeOH and EtOH, various polypeptides show temperature-induced phase transitions in this alcohol. For example, poly((γ-4-oligo(ethylene glycol) benzyl-L-glutamate)-random-(γ-benzyl-L-glutamate)) with different OEG molar content and DP underwent a UCST-type phase transition with a maximal concentration (Cm) dependent on the OEG content and on the side-chain length.110 OEGylated poly(γ-4-(propargoxycarbonyl)benzyl-L-glutamate) and poly(γ-4-(4-propargoxyphenoxycarbonyl)benzyl-L-glutamate) derived from azido functionalized triethylene glycol monomethylether (P1-OEG3), or azido functionalized poly(ethylene glycol) methyl ether 350 (P1-OEG7 and P2-OEG7), exhibited a UCST phase transition in PrOH at a polymer concentration of 1 mg mL−1 (Fig. 3A).93 The TCP decreased with increasing OEG chain length, due to decreased hydrogen-bonding interactions between the OEG pendants and the alcoholic solvent, and increased with the presence of more hydrophobic linkages.The UCST phase transition of the polypeptides poly(γ-4-(2-(2-methoxyethoxy) ethoxycarbonyl)benzyl-L-glutamate) and poly(γ-4-(2-(2-(2-methoxyethoxy)ethoxy)ethoxycarbonyl) benzyl-L-glutamate) in PrOH was also strongly dependent on the polymer concentration and on the length of both the OEG pendants and of the main chain.98
The glycopolypeptide poly(γ-propyl-L-glutamate) tetra-O-acetyl-D-(+)-mannopyranoside containing poly(L-glutamate) main-chain and mannose pendants also underwent a UCST phase transition at 58.3 °C in PrOH at a concentration of 1 mg mL−1 (Fig. 6).100
Furthermore, it has been shown that the non-linear PEG analogue POEGMA exhibited a UCST-type phase transition in PrOH at 29.1 °C at a concentration of 16 mg mL−1, as demonstrated by the measurement of the cloud point values by optical transmission analysis.82 In another example, poly(2-(2-(2-(2-((tetrahydrofuran-2-yl)oxy)ethoxy)-ethoxy)ethoxy) ethyl methacrylate) (PTFEO4MA) exhibited a UCST behaviour in PrOH with a TCP around 22 °C at a polymer concentration of 1 wt%.106
Another poly(methacrylate) exhibiting a UCST behaviour in PrOH is poly(2-hydroxyethyl methacrylate), with TCP shifting to higher values with increased molecular weight and concentration of the polymer in solution.111
Finally, poly[N-(4-vinylbenzyl)-N,N-dibutylamine] with a DP of 72 showed a UCST-type behaviour in 1-propanol with a TCP of 48 °C.112 It has also been demonstrated that the addition of a 10 wt% of cosolvent (i.e. n-butanol) to a 2 wt% polymer solution in PrOH resulted in the decrease of the TCP from 48 to 29 °C, while the addition of 10 wt% of nonsolvents (i.e. methanol, ethanol, or 2-propanol) led to a rise in the TCP value from 48 to 75 °C.
3.4. Thermoresponsive behaviour in 2-propanol (iPrOH)
The thermoresponsive behaviour of polymers has also been studied in the structural isomer of PrOH, 2-propanol (iPrOH). Examples of thermoresponsive (glycol)polypeptides in iPrOH are present in literature, although this solvent has been less studied than the previously mentioned alcoholic solvents. For instance, a glycopolymer consisting of a poly(γ-propyl-L-glutamate) main-chain and tetra-O-acetyl-D-(+)-mannopyranoside side chains underwent a UCST phase transition at 62.2 °C at a concentration of 1 mg mL−1. This thermoresponsive behaviour was attributed to hydrophobic interactions and H-bonding interactions between polymer chains, and the formation of hydrogen bonds between the acetyl groups on the polymer and the OH groups of the solvent, as confirmed by FTIR spectroscopy.100 As expected, OEGylated polypeptides with different hydrophobic linkage groups (i.e. phenyl and benzoic acid phenyl ester linkages) and OEG pendants with different lengths also showed a UCST behaviour in iPrOH at a concentration of 1 mg mL−1, which was strongly influenced by the OEG chain length and the hydrophobicity of the linkage groups.93Apart from OEGylated polypeptides, Pegylated and OEGylated polymers often show thermoresponsive behaviour and, in particular, UCST phase transition in alcoholic media. For instance, the thermoresponsive behaviour of PEG and its analogues has been extensively studied in iPrOH. It has been shown via turbidity measurements that a 5 mg mL−1 iPrOH solution of PEG homopolymer with a molecular weight of 6 kDa exhibited a UCST-type phase transition at 15.8 °C accompanied by a large hysteresis.127 In another example, PEG 6 kDa homopolymer synthesised via ATRP exhibited a UCST behaviour in iPrOH (C = 0.05 wt%) at 21 and 39 °C during the cooling and heating cycles of transmittance measurements, respectively.113
Interestingly, the TCP of PEG could be tuned by blending PEG with poly(acrylic acid) (PEG/PAA), and poly(poly(ethylene glycol) methacrylate) with PAA (PPEGMA/PAA), exhibiting a UCST phase transition in iPrOH, where the PAA alone is insoluble.113 In both cases, the thermoresponsive behaviour was found to be dependent on the ratio between the polymers (Fig. 13), and on the molecular weight of both the proton acceptor (PEG and PPEGMA) and proton donor (PAA) polymers. In the case of the PEG/PAA blends, the thermoresponsive behaviour was attributed to significant interactions between the H-donor COOH groups of PAA and the H-acceptor oxygen atoms of PEG. The solubility of the polymers blend decreased with decreasing temperature until a critical value was reached at the TCP below which a polymer-rich phase appeared due to the presence of interpolymer H bonding interactions. The temperature-induced behaviour of PPEGMA/PAA blends was attributed to the formation of interpolymer complexes by H bonding interactions between the PAA and the comb-like polymacromonomer brushes bearing short PEG side chains. The solution behaviour of the polymers pairs was also analysed in MeOH/iPrOH mixtures. Since MeOH was a good solvent for all polymers, a decrease in the TCP values with increasing content of MeOH was expected. However, changes in the transition temperature were observed only above 20%v/v MeOH content in iPrOH.
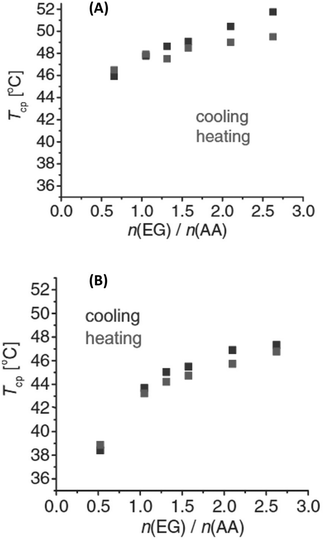 | ||
Fig. 13 Cloud point temperatures of (A) PEG/PAA and (B) PPEGMA/PAA solutions in iPrOH as a function of EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA monomeric unit molar ratios with 0.0625 wt% PAA concentration. Reproduced from ref. 113 Copyright 2017 WILEY-VCH Verlag GmbH & Co KGaA, Weinheim. AA monomeric unit molar ratios with 0.0625 wt% PAA concentration. Reproduced from ref. 113 Copyright 2017 WILEY-VCH Verlag GmbH & Co KGaA, Weinheim. | ||
PEG has also been used for the synthesis of more sophisticated architectures showing thermoresponsiveness in iPrOH. As an example, PEG-armed star polymers with Ru(II)-enclosed microgel cores have been synthesised by cross-linking the living PEGMA arms with a dimethacrylate linking agent in the presence of a phosphine-ligand functionalized styrene and RuCl2(PPh3)3 as catalyst (Fig. 14).114 The solution behaviour of the PEGMA prepolymer, PEGMA-methyl methacrylate diblock copolymer (PEGMA-MMA block), and unreacted PEGMA-MMA diblock copolymer containing the ruthenium catalyst (PEG-Ru block), as well as star polymers consisting of only PEG (PEG star), and PEG-Ru star polymers was analysed in iPrOH. The PEG-Ru star polymers exhibited a UCST behaviour at around 31 °C, which was dependent on both the structure and the composition. The star polymers resulted in a higher TCP compared to the linear counterparts due to the higher hydrophobic content (i.e. MMA, phosphine, linking agent). Moreover, the introduction of the Ru catalyst resulted in an increase of the UCST for both linear and star polymers.
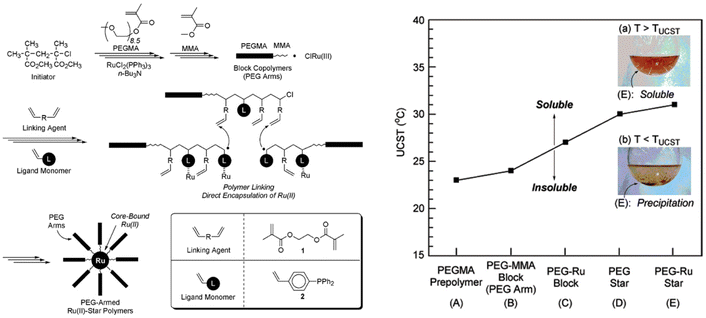 | ||
| Fig. 14 (Right) Schematic depiction of the synthesis of PEG armed star polymers with Ru(II)-enclosed microgel core via Ru(II)-catalysed living radical polymerization. (Left) UCST of 3 wt% solutions of various PEGMA polymers in iPrOH. Inset pictures: (a) PEG-Ru star (E) in iPrOH above UCST, (b) PEG-Ru star (E) in iPrOH below UCST. Adapted from ref. 114 Copyright 2007 American Chemical Society. | ||
Among all PEG analogues, the solution behaviour of POEGMA and its copolymers has been extensively investigated in 2-propanol. POEGMA homopolymers bearing a pentafluorophenyl ester end group and a dithioester end group showed UCST phase transitions in iPrOH, which was strongly dependent on the polymer molecular weight and concentration.82,114,115 The phase transition behaviour was also influenced by the addition of cosolvents and nonsolvents, resulting in an increased polymer solubility and, thus, a decreased TCP when chloroform, a good solvent, was added, and in a lowered solubility and increased TCP when hexane, a non-solvent, was used.82 In another report, the RAFT copolymerization of OEGMA with the commercially available oligo(ethylene glycol)phenyl ether acrylate (OEGPhA) in different ratios, resulted in the synthesis of a series of thermoresponsive copolymers exhibiting UCST-type behaviour in iPrOH at a concentration of 10 mg mL−1.83 A linear relationship between the phenyl ether content and the TCP value was found, with TCP increasing from 35.1 to 75.4 °C with increasing OEGPhA content from 21 to 73 mol% due to a decreased solubility of the polymer in the solvent. Furthermore, block copolymers consisting of a POEGMA segment and a PNIPAM (POEGMA-b-PNIPAM) or N,N-diethylacrylamide (PDEAM) block (POEGMA-b-PDEAM) exhibited a UCST phase transition in iPrOH, forming micellar aggregates upon cooling, as shown by turbidity and DLS measurements.115,128
As previously mentioned for EtOH, a brush copolymer polyampholyte composed of OEGMA, 2-(dimethylamino)ethyl methacrylate and methacrylic acid underwent a UCST temperature induced self-assembly at 50 °C in iPrOH at a concentration of 1 mg mL−1.107 This behaviour was ascribed to the collapse of the copolymer caused by the strong electrostatic interactions between the opposite charges into nanostructures stabilized by the solvophilic ethylene glycol side chains. By decreasing the temperature to 50 °C, particles of around 35 nm were observed via DLS analyses (Fig. 15). However, an increase in the particles size was measured when the temperature was further decreased due to the formation of larger agglomerates caused by a less efficient electrostatic stabilization of the OEG side chains in iPrOH compared to the strong electrostatic attractive interactions between the charges. Additionally, a graft-copolymer consisting of a poly(vinylidene fluoride) backbone and poly(diethylene glycol methyl ether methacrylate) side chains exhibited a UCST-type thermoresponsive behaviour at 27.5 °C in iPrOH at a critical composition of 0.09% (w/v), as shown by DLS measurements.85
 | ||
| Fig. 15 Chemical structure and size distribution measured by DLS at various temperatures of a 1 mg mL−1 iPrOH solution of poly(2-(dimethylamino) ethyl methacrylate-co-methacrylic acid)-graft-oligo(ethylene glycol) methyl ether methacrylate. Adapted from ref. 107 Copyright 2015 Royal Society of Chemistry. | ||
In another example, poly(2-(2-methoxyethoxy)ethyl methacrylate) (PMEO2MA) and poly(N-(4-vinylbenyl)-N,N-diethylamine) (PVEA) homopolymers showed a UCST-type phase transition in iPrOH, greatly influenced by the polymer DP.112,123 When PMEO2MA was polymerised in a diblock manner with PVEA, the resulting diblock copolymer exhibited a UCST phase transition at lower temperatures compared to the one of the PMEO2MA homopolymer with the same DP, and closer to the one of the PVEA homopolymer. This was attributed to the increased solubility of the PMEO2MA segment by the highly soluble PVEA block, resulting in a lower TCP than that of the PMEO2MA homopolymer.123
Another ethylene glycol-based polymer exhibiting a UCST thermoresponsive behaviour in iPrOH is poly(2-(2-(2-(2-((tetrahydrofuran-2-yl)oxy)ethoxy)-ethoxy)ethoxy) ethyl methacrylate) with a TCP around 35 °C at a polymer concentration of 1 wt%.106
Poly(oligo(ethylene glycol) methyl ether acrylate) (POEGMeA) and its copolymers with oligo(ethylene glycol) phenyl ether acrylate (OEGPhA) are another class of intensively studied PEG analogues. In iPrOH, the (co)polymers exhibited a UCST behaviour with TCP linearly increasing with increased OEGPhA content.83 In order to elucidate molecular events, a diblock copolymer consisting of a random poly(OEGMeA-co-OEGPhA) block comprising 45 mol% of OEGPhA units, and a soluble poly(dimethylacrylamide) segment (pDMA) was synthesised and analysed via variable temperature NMR spectroscopy in iPrOD-d8 at a concentration of 20 mg mL−1, in which it exhibited a TCP of 45.2 °C. From the analyses, it has been elucidated that the decrease in solubility in alcohols of polymers containing phenyl groups was caused by a promotion of favourable attractive polymer–polymer interactions and a decreased contribution to mixing entropy due to rigidity and lack of conformational isomers of rigid rings, resulting in the broadening of the insolubility regime of the phase diagrams of the phenyl ether-containing polymers.
Poly(oligo(ethylene glycol) (meth)acrylamide)s are other widely studied biocompatible PEG analogues showing a UCST behaviour in iPrOH, as in the case of poly(oligo(ethylene glycol) acrylamide)s (polyOEGAM) and poly(oligo(ethylene glycol) methacrylamide)s (polyOEGMAM).127 The TCP values increased with increasing length of the OEG side chains due to a decreased solubility of the polymer in the alcohol. Moreover, at comparable lengths of the OEG side chains, polyOEGMAMs exhibited higher TCP values compared to polyOEGAMs counterparts.
In a final interesting example, a series of amphiphilic pyrrolidone diblock copolymers consisting of a N-(2-methacrylaoyxyethyl)pyrrolidone block with a fixed DP of 37 and a methyl methacrylate segment with a DP between 117 and 230 was able to form thermoresponsive organogels composed of 3D micellar networks in iPrOH (Fig. 16).116 The organogels underwent a thermoreversible sol–gel transition, which was dependent on the DP of the PMMA block and on the polymer concentration in solution. Because of their biocompatibility and interesting temperature-induced behaviour, these diblock copolymers might be potentially applied in the development of new nanomaterials, in biotechnology, and in transdermal drug delivery.
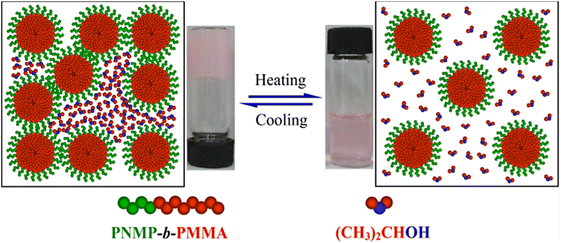 | ||
| Fig. 16 Proposed mechanism of the thermally-induced sol–gel transition of poly(N-(2-methacrylaoyxyethyl)pyrrolidone-block-methyl methacrylate) organogels in iPrOH. Reproduced from ref. 116 Copyright 2017 American Chemical Society. | ||
3.5. Thermoresponsive behaviour in other alcohols
Thermoresponsive polymers have also been investigated in alcohols with slightly longer alkyl chains, such as 1-butanol (BuOH) and its structural isomers, and pentanol (PeOH). As expected, OEGylated polymers have shown thermoresponsive behaviours in these solvents. As an example, POEGMA and its diblock copolymers with PNIPAM or PDEAM exhibited a sharp and reversible UCST phase transition in 1-butanol, which was dependent on both the polymer molecular weight and polymer concentration.82,115 Experiments in different butanol isomers revealed that branching of the alkyl chains had an effect on the solution behaviour of POEGMA polymers. To exemplify, the TCP value of solutions of a 23.2 kg mol−1 POEGMA polymer (C = 16 mg mL−1) decreased from 36.6 °C in BuOH, to 30.4 °C in 2-methyl-1-propanol (iBuOH), to 26.4 °C in 2-butanol (secBuOH), and further to 22.2 °C in 2-methyl-1-butanol. A similar trend was also observed in alcohols with longer chain lengths. Additionally, a linear increase in the TCP with increasing chain length of the alcohol was found, with TCP values ranging from 40.9 °C in 1-pentanol up to 75.4 °C in dodecanol.82(Glyco)polypeptides also show thermoresponsiveness in alcoholic solvents with longer alkyl chains. For example, the TCP values of the glycopolypeptide poly(γ-propyl-L-glutamate) tetra-O-acetyl-D-(+)-mannopyranoside conjugate were found to increase with the number of carbon in the alcoholic solvent due to increased hydrophobic interactions and decreased H bonding interactions between the polymer and the solvent molecules, reaching 69.9 °C and 77.9 °C in BuOH and PeOH, respectively.100 Other polypeptides previously mentioned, such as those obtained by reacting azido functionalized OEG with poly(γ-4-(propargoxycarbonyl)benzyl-L-glutamate) or poly(γ-4-(4-propargoxyphenoxycarbonyl)benzyl-L-glutamate),93 poly((γ-4-oligo(ethylene glycol) benzyl-L-glutamate)-random-(γ-benzyl-L-glutamate)),110 poly(γ-4-(2-(2-methoxyethoxy) ethoxycarbonyl)benzyl-L-glutamate) and poly(γ-4-(2-(2-(2-methoxyethoxy)ethoxy)ethoxycarbonyl) benzyl-L-glutamate),98 also showed a UCST phase transitions in both BuOH and PeOH, with TCP values influenced by the OEG chain length and content.
Another thermoresponsive polymer undergoing a phase transition in a series of aliphatic alcohols at high temperatures is poly(2-hydroxyethyl)methacrylate.111 The solubility of the polymer decreased with increasing molecular weight, which was found to be similar in BuOH and iBuOH (i.e. between 60.8 and 73 °C depending on the polymer molecular weight). On the contrary, the solubility of the polymer in glycerol was very low (TCP between 71.8 and 83.6 °C), while it was highly soluble in secBuOH, resulting in a low TCP at around 14 °C.
Finally, comb-like copolymers obtained from the free radical solution polymerization of n-octadecyl vinyl ether and N-phenyl maleimide underwent a reversible UCST-type behaviour at 67 °C in BuOH at a concentration of 0.1 wt% (Fig. 17).117 This behaviour was dependent on the polymer concentration and it was ascribed to the aggregation of the long alkyl chains of the vinyl ether monomer at low temperatures. Furthermore, the TCP value decreased with increasing chain length of the alcoholic solvent due to an enhanced solubility of the polymer in longer alkyl chain alcohols, such as 1-hexanol (HexOH) and 1-octanol (OctOH). The TCP in BuOH could be tuned by adding proper co-solvents. In fact, the addition of toluene as good solvent resulted in a decrease in the TCP, while the addition of ethylene glycol as nonsolvent increased the TCP.
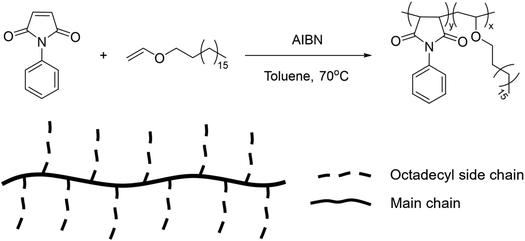 | ||
| Fig. 17 Schematic representation of the synthetic route and chemical structure of the comb-like copolymers.117 | ||
The most studied long-chained alcohol for the solution behaviour of polymers is 1-octanol. Based on the thermoresponsive behaviour of POEGMA, its block copolymers with PNIPAM (POEGMA-b-PNIPAM) and PDEAM (POEGMA-b-PDEAM), and two polymers of POEGMA-b-[PNIPAM-co-poly(pentafluorophenyl acrylate) poly(pentafluorophenyl acrylate) (POEGMA-b-(PNIPAM-co-PPFPA))] containing different amounts of PFP ester, formed well-defined spherical inverted micelles in OctOH upon cooling, with TCP values around room temperature, as demonstrated by DLS and UV-vis analyses.115,128 Interestingly, the PNIPAM and PDEAM blocks did not significantly influence the transition temperature of the final diblock copolymers.
Furthermore, PEG analogues obtained from the post-polymerization of poly(pentafluorophenyl(meth)acrylate) with α-amino, ω-methoxy functionalized di(ethylene glycol), tri(ethylene glycol), and PEG-350, PEG-750 and PEG-5k showed thermoresponsiveness in OctOH.127 Polymers with long ethylene glycol side chains, longer than PEG-750 for polyacrylamides and longer than tri(ethylene glycol) for polymethacrylamides, underwent a UCST phase transition in OctOH, with TCP values increasing with increased EG length. However, the increase in the EG side chain length also resulted in the broadening of the hysteresis between the heating and cooling transition temperatures of the polymers (Fig. 18). This behaviour was attributed to the PEG analogues behaving as pure PEG in terms of redissolution characteristics when the OEG side chain length was increased.
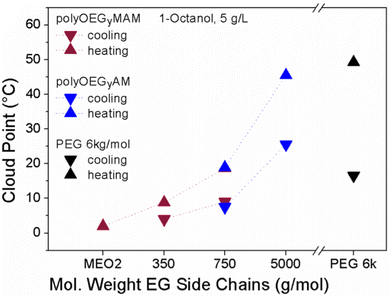 | ||
| Fig. 18 Cloud points of poly(acrylamide)s (blue), poly(methacrylamide)s (purple) and reference PEG 6k (black) measured during cooling (▼) and heating (▲) OctOH at a polymer concentration of 5 mg mL−1, showing an increased hysteresis with an increase of OEG chain length. Adapted from ref. 127 Copyright 2012 American Chemical Society. | ||
Cyclic alcohols are not intensively studied in relation to the thermoresponsive behaviour of polymers, indicated by the few reports present in literature. One example is the thermoresponsive behaviour of polystyrene and poly(methyl methacrylate) homopolymers, which exhibited a UCST phase transition in cyclohexanol, with TCP increasing with increasing molecular weight of the polymer, as demonstrated by thermooptical analyses.118 This behaviour was more accentuated for PS than for PMMA. A diblock copolymer consisting of a PS and a PMMA block exhibited TCP in between those of the two homopolymers with similar molecular weight. In another example, poly(γ-benzyl L-glutamate) have demonstrated to undergo a reversible sol–gel transition in benzyl alcohol (BnOH), forming crystalline fibrillar aggregates at room temperature and having melting temperatures in the range 60–70 °C depending on the sample preparation.119
In this section, we have thoroughly discussed the thermoresponsive behaviour of different classes of polymers in alcoholic solvents with increasing alkyl chain length. Thermoresponsive polymers predominantly exhibit a UCST-type phase transition in pure alcoholic systems, even though a few examples of polymers with a LCST or sol–gel transitions have also been highlighted. As discussed above, the thermoresponsive behaviour of polymers in alcohols is strongly dependent on various parameters, such as polymer composition (e.g. DP, functional groups, etc.), architecture and concentration, as well as on the choice of the solvent and the addition of cosolvents and nonsolvents in the binary system. By carefully selecting these parameters, a precise tuning of the TCP can be achieved. Although many examples of thermoresponsive polymers in alcoholic solvents are present in literature, their potential applications are still limited. For instance, these polymers are often synthesised and their thermoresponsive behaviour in alcohols studied to gain a fundamental understanding about the structure–properties relationship of specific polymer/solvent systems, and their potential applications, especially in the biomedical field, are not generally discussed or investigated.
4. Hydrocarbons
In the previous sections of this review, the solution behaviour of polymers in aqueous media, alcohol water mixtures, and pure alcohols was discussed. It was further pointed out that macromolecules with thermoresponsive properties are omnipresent for water-based applications. However, pure organic systems are less common, with (aliphatic) hydrocarbons catching even less attention. However, polymers showing a thermoresponsive behaviour in hydrocarbons are gaining an increasing interest especially in the lubricant industry for their application as polymeric oil additives, such as viscosity modifiers, oil thickeners, and friction reducers. Therefore, in this section, we would like to discuss polymer solutions with temperature-induced phase transitions in aliphatic and aromatic hydrocarbons, and in oils in more detail (Table S6†). Representative examples are highlighted in Table 7.| Solvent | Type of polymer | Polymer conc. | Phase transition type (Temp. °C) | Ref. |
|---|---|---|---|---|
| Hexane/achiral benzyl amine | Substituted chiral poly(phenylacetylene) | 1 mmol L−1 | Conformational transition (@3) | 129 |
| Hexane/achiral benzyl amine | Substituted chiral/achiral copoly(phenylacetylene) | 1 mmol L−1 | Conformational transition (@16) | 129 |
| Hexane | Poly(octadecyl vinyl ether) | 1 wt% | UCST (∼9) | 109 |
| Methylcyclohexane | Polystyrene | 1–25 wt% | LCST (∼215–250), UCST (∼35–70) | 130 |
| Cyclohexane | Polystyrene | 1–25 wt% | LCST (∼215–240), UCST (∼10–30) | 130 |
| Heptane | Polystyrene-b-polyisoprene | 0.001–10 mg mL−1 | Cylinders-to-spheres (∼35) and vesicles-to-cylinders transition (∼40) | 131 |
| n-Octane | Poly(stearyl methacrylate-b-3-phenylpropyl methacrylate) | 20–30 wt% | Worm-to-sphere transition (∼70) | 132 |
| Decane | Poly(octadecyl vinyl ether)-b-poly(isobutyl vinyl ether)ODVE and IBVE | 20 wt% | Sol–gel transition (∼17) | 109 |
| n-Dodecane | Poly(benzyl methacrylate-b-lauryl methacrylate) | 5–20 wt% | Worm-to-sphere transition (∼50) | 133 |
| n-Dodecane | Graft-copolymers with polyolefin backbones and poly(butyl methacrylate-stat-lauryl methacrylate) side chains | 2 mg mL−1 | UCST (∼80) | 134 |
| n-Dodecane | Poly(lauryl acrylate-b-benzyl acrylate) | 15 wt% | Worm-to-sphere transition (∼15) | 135 |
| n-Tetradecane | Poly(stearyl methacrylate-b-3-phenylpropyl methacrylate) | 20 wt% | Worm-to-sphere transition (∼85) | 136 |
| n-Hexadecane | Poly(tert-octyl acrylamide-b-N,N-dimethylacrylamide) | 1.0 wt% | UCST (@55) | 137 |
| Isohexadecane | Poly(lauryl acrylate-b-benzyl acrylate) | 15 wt% | Worm-to-sphere transition (∼67) | 135 |
| Toluene | Poly(octadecyl vinyl ether) | 1 wt% | UCST (∼4) | 109 |
| Toluene | Poly(3-((4R,5R)-4,5-bis(hydroxydiphenylmethyl)-2-methyl-1,3-dioxolane-2-yl)propyl acrylate) + chiral effectors | 10 mg mL−1 | UCST or sol–gel transition (ND) | 138 |
| Toluene | Poly(3-((4R,5R)-4,5-bis(hydroxydiphenylmethyl)-2-methyl-1,3-dioxolane-2-yl)propyl acrylate) + (S) or (R)-2-methylpiperidine | 25 mg mL−1 | UCST (∼10–30)-LCST (∼20–60)-UCST (∼45–75) | 138 |
| Toluene | Poly(vinyl phenol-alt-N-octadecyl maleimide) | 5 wt% | Sol–gel transition (∼RT) | 139 |
| 1,2,4-Triethylbenzene | Poly(11-(4-((E)-4-butylstyryl)phenoxy)undecyl methacrylate) | 2 wt% | UCST (@49.7) | 140 |
| Dioctyl phthalate, dibutyl phthalate, diethyl phthalate | Poly(styrene-b-dimethylsiloxane) | 1 wt% | Thermotropic transition (@70) | 141 |
| Hydrocarbon oil | Poly(lauryl methacrylate-b-styrene-b-lauryl methacrylate) | 0.1–16 wt% | Swelling of micelles (∼80) | 142 |
| Mineral oil | Poly(stearyl methacrylate-b-benzyl methacrylate) | 10 wt% | Vesicle-to-worm transition (@135) | 143 |
| Polyalphaolefin | Poly(alkyl methacrylate)s with different alkyl side chain lengths | 1 wt% | UCST (0.5–138) | 144 |
| Polyalphaolefin | Poly(n-butyl methacrylate-co-n-hexyl methacrylate)-b-poly(2-ethylhexyl methacrylate-co-lauryl methacrylate)-b-poly(n-butyl methacrylate-co-n-hexyl methacrylate) | 14.3–15 wt% | Sol–gel transition (∼0–63.5) | 144 |
| Decamethylcyclopentasiloxane silicone oil | Poly(dimethylsiloxane-b-2-(dimethylamino) ethyl methacrylate) | 0.25–25 wt% | Worm-to-sphere transition (@32) | 145 |
| Yubase-4 oil | Poly(2-stearyl-2-oxazoline-co-2-ethyl-2-oxazoline) | 5 mg mL−1 | UCST (∼80–49) | 146 |
| Yubase-4 oil | Poly(methacrylic acid-co-2-ethylhexyl methacrylate)-graft-poly(2-stearyl-2-oxazoline-co-2-ethyl-2-oxazoline) | 5 mg mL−1 | UCST (∼40–51) | 146 |
4.1. Thermoresponsive behaviour in aliphatic solvents
Despite the few studies on thermoresponsive polymers in aliphatic solvents, unique solution behaviours and self-assembly of polymers upon changes in temperature have been reported in literature. It has to be noticed that in the case of some solvents, only a few examples have been found in literature. Nevertheless, also in this case, the polymers will be classified based on the solvent, following the order of increasing number of carbons in the aliphatic chain.Hexane is the shortest aliphatic hydrocarbon that has been used in the study of the temperature-responsive behaviour of polymers.
In a recent example, a phenylacetylene homopolymer having two hydroxyl groups and a chiral pinanyl substituent (Fig. 19) underwent a reversible twist in the conformation from optical inactive cis-transoidal to one handed helical cis-cisoidal at a critical twisting temperature (CTT) of 3 °C upon heating a 1 mmol L−1 hexane solution in the presence of achiral benzyl amine (20 vol%), as shown by absorption and circular dichroism (CD) measurements.129 Furthermore, copolymers consisting of a chiral unit and an achiral unit, both bearing OH groups, showed a thermotropic twisting behaviour in hexane/benzyl amine with a CTT of 16 °C, which was found to be independent of the polymer concentration. However, this thermoresponsive behaviour was strongly dependent on both the amine concentration and chemical structure, as well as on the composition of the copolymer. The thermoresponsive behaviour was attributed to the release of the amine from the polymer upon heating and the presence of chiral moieties in the polymer, which resulted in the twisting of the polymer in a specific direction, as shown by 1H NMR analyses.
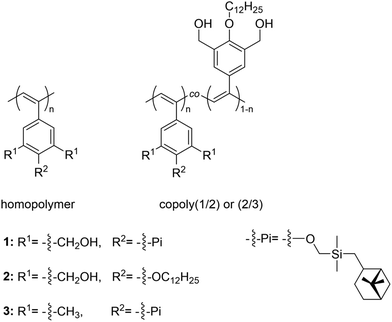 | ||
| Fig. 19 Schematic depiction of the chemical structure of phenylacetylene homo and copolymers.129 | ||
Linear poly(octadecyl vinyl ether) (poly(ODVE)) obtained via living cationic polymerization has also shown a UCST phase transition at around 9 °C in hexane due to the crystallization of the long alkyl side chains, as shown by UV-vis spectrometry measurements of the transmittance of 1 wt% polymer solutions.109,147
The solution behaviour of polystyrene homopolymers with different molecular weight distributions has been studied in various aliphatic solvents, such as cyclohexane and methylcyclohexane, using thermooptical analyses (TOA) and cloud point measurements.21,130 The homopolymers showed both LCST and UCST phase transitions with TCP values strongly influenced by the polymer molecular weight and concentration. The incorporation of polystyrene into more complex architectures, such as star polystyrene polymers, resulted in a temperature-induced phase transition in the same solvent at a concentration of 5 mg mL−1, which was more pronounced for the star polymers compared to the linear analogues, as shown by field-flow fractionation (ThFFF) and DLS analyses.148
The copolymerization of styrene into diblock copolymers composed of a polystyrene block of 21.6 kDa and a polyisoprene block of 4.3 or 6 kDa via sequential anionic polymerization resulted in copolymers showing a temperature dependant self-assembly behaviour in heptane, a good solvent for the isoprene block.131 Their thermoresponsive behaviour was studied and visualized by light scattering measurements and atomic force microscopy (AFM) at a concentration range of 0.001–10 mg mL−1. Depending on the length of the isoprene segment, the block copolymers were able to self-assemble into micelles or vesicles at room temperature. Upon heating the mixture to 40 °C, a reversible transition into spherical micelles for the polymer with the shorter polyisoprene segment, and into cylindrical micelles for the longer polyisoprene segment, was observed due to a change in the quality of the solvent, as demonstrated by the calculation of the temperature variation of the second virial coefficient (A2) for linear polyisoprene in heptane. The initial morphologies were once again obtained upon cooling back to 25 °C. However, it has been noticed that the growth rates of both the cylindrical micelles and the vesicles were slower compared to the rate of dissolution at high temperatures, which was attributed to slow kinetics near the glass transition temperature (Tg).
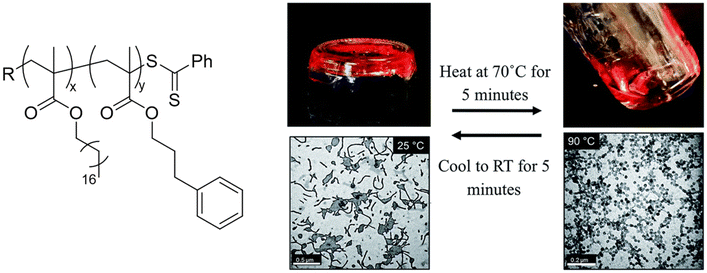
Macro-chain transfer agents (macro-CTAs) consisting of poly(stearyl methacrylate) homopolymers (PSMA) have been used for the non-polar RAFT dispersion polymerization of 3-phenylpropyl methacrylate (PPPMA) in n-octane.132 At 20 wt%, a series of diblock copolymers showing the full range of morphologies (i.e. spheres, cylinders and vesicles) was obtained via polymerization induced self-assembly (PISA) by using low DP macro-CTAs (i.e. 18 and 19 units of SMA) and by varying the DP of the PPPMA block. The complexity of the nanoparticles increased from spheres to worms to vesicles by increasing the DP of the PPPMA block from 31 to 130, as confirmed by transmission electron microscopy (TEM) studies. By heating the polymer solutions from 25 to 70 °C, block copolymers forming only pure worm phases (i.e. PSMA18-b-PPPMA71) could undergo a fast reversible thermally-induced shift from gels to free-flowing solutions due to a worm-to-sphere transition (Fig. 20). This fast morphological transition was facilitated by the change in the relative solvation of the PPPMA core-forming block and by its low Tg.
 | ||
| Fig. 20 Chemical structure and digital images showing the reversible, temperature-induced degelation–gelation for the PSMA18-b-PPPMA71 copolymer at 30 wt% in n-octane (top), and TEM images of the PSMA18-b-PPPMA71 block copolymer nanoparticles prepared at 30 wt% in n-octane (bottom). Reproduced from ref. 132 Copyright 2015 Royal Society of Chemistry. | ||
Block copolymers consisting of a thermoresponsive poly(ODVE) segment with a DP of 200 and a decane-soluble poly(isobutyl vinyl ether) block (DP = 200) were shown to exhibit a thermoreversible sol–gel transition in decane.109 The polymer solution was a transparent sol at room temperature, and it turned into a stiff opaque gel upon cooling, as demonstrated by dynamic viscoelasticity measurements. Moreover, thermally induced reversible physical gelation was achieved in various organic solvents for different block copolymers by varying the thermosensitive segment corresponding to a solvent.
In another report, sterically-stabilized nanoparticles consisting of a poly(tert-octyl acrylamide) segment (DP = 82), and a poly(dimethylacrylamide) block (DP = 150) (POAAPDMAC150) directly synthesised in decane via PISA underwent a UCST phase transition at 2 °C at a concentration of 1 wt%, as demonstrated by turbidity measurements and DLS analyses.137
Interestingly, alternating copolymers of 2-chloroethyl vinyl ether and maleic anhydride showed a LCST behaviour in propyl acetate/n-alkanes mixed solvents at a fixed polymer concentration, with TCP values shifting to higher temperatures by increasing the length and the dielectric constant of the n-alkane (i.e. hexane < octane < decane < dodecane).149,150 Additionally, a linear relationship between the content of n-octane in the mixed solvents and the change in the TCP was observed. The LCST of the alternated copolymer could be tuned from 76.3 to 30.9 °C by increasing the n-octane content in the mixed solvents from 6 to 16 wt%, with the TCP decreasing with increasing polymer concentration in the dilute region, and becoming almost constant in the high concentration region.150
Among all aliphatic hydrocarbons, n-dodecane is probably one of the most studied ones, usually used as model alkane for the evaluation of the properties of potential polymeric oil additives. Diblock copolymers of lauryl methacrylate and benzyl methacrylate (PLMA-b-PBzMA) obtained by RAFT polymerization were demonstrated to form spheres, worms or vesicles via PISA at 20 wt% solid content in n-dodecane at 70 °C when the DP of the PLMA stabilizer block was relatively low.133 The worm state (i.e. PLMA16-PBzMA37) formed a free-standing gel at 20 °C, which underwent degelation upon heating above 50 °C due to a worm-to-sphere transition. This morphological transition was irreversible for dilute solutions (0.10%w/w), but it became reversible when the polymer concentration was increased up to 20%w/w, as confirmed by TEM images (Fig. 21). The reversible worm-to-sphere transition was attributed to a change in the relative volume fractions occupied by the PBzMA core-forming and stabilizer blocks caused by surface plasticization of the PBzMA block, as confirmed by variable temperature 1H NMR analyses and small-angle X-ray scattering (SAXS) studies. Two possible mechanisms have been proposed for the observed morphological transition upon heating: (A) the sequential budding of spheres, and (B) a series of random worm cleavages (Fig. 22). However, the appearance of a second minor population of isolated spheres in the TEM image (Fig. 21B, red arrows) suggested that the former mechanism was more likely to occur.133
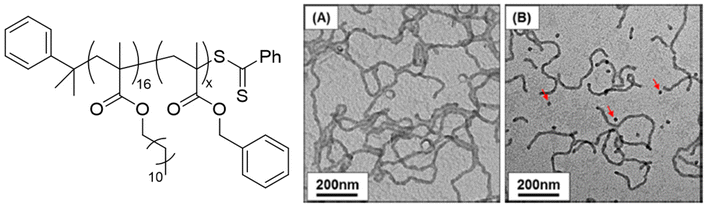 | ||
| Fig. 21 Chemical structure and TEM images showing the thermoreversible morphological transition of a 20%w/w PLMA16-PBzMA37 worm gel diluted to 0.01% w/w solid prior to prepare the TEM grids, at (A) 20 °C, and (B) after heating to 90 °C and cooling back to 20 °C. Reproduced from ref. 133 Copyright 2014 American Chemical Society. | ||
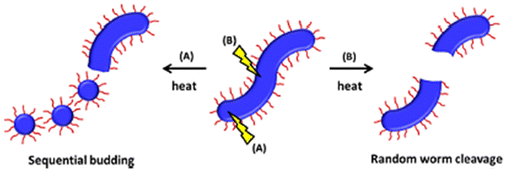 | ||
| Fig. 22 Schematic representation of the two possible mechanisms for the worm-to-sphere transition of a PLMA16–PBzMA37 diblock copolymer dispersion in n-dodecane upon heating: (A) sequential budding of spheres, and (B) random worm cleavage. Reproduced from ref. 133 Copyright 2014 American Chemical Society. | ||
Similarly, poly(alkyl methacrylate)s have been employed to obtain thermoresponsive copolymers with more complex architectures. For example, well-defined graft-copolymers consisting of polyolefin backbones and poly(alkyl methacrylate) side chains have been synthesised via a combination of ring-opening metathesis polymerization of cyclic monomers (i.e. cyclooctene COE, 3-ethyl cyclooctene EtCOE, and α-bromoisobutyrate functionalized cis-cyclooctene BrICOE), ATRP of the alkyl methacrylates (i.e. butyl methacrylate BuMA, and lauryl methacrylate LMA), and subsequent hydrogenation of the unsaturated backbone.134 The graft-copolymers having a COE backbone containing 8 mol% of BrICOE and side chains with a [LMA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BuMA] molar ratio of 39
[BuMA] molar ratio of 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61 (L40B60-C50) showed a UCST-type phase transition in n-dodecane. As suggested by SANS measurements, clusters containing methacrylate-rich domains were observed at low temperatures due to the low solubility of the side chains in the solvent, while the high temperature favoured the “deaggregation” into individual polymer chains due to an increase in the solubility of the methacrylate side chains.
61 (L40B60-C50) showed a UCST-type phase transition in n-dodecane. As suggested by SANS measurements, clusters containing methacrylate-rich domains were observed at low temperatures due to the low solubility of the side chains in the solvent, while the high temperature favoured the “deaggregation” into individual polymer chains due to an increase in the solubility of the methacrylate side chains.
By changing the methacrylate backbone into the acrylate counterpart, a series of all-acrylic poly(lauryl acrylate)-block-poly(benzyl acrylate) (PLA-b-PBzA) diblock copolymers was synthesised via RAFT polymerization in aliphatic hydrocarbon solvents.135 The whole range of morphologies (i.e. spheres, worms, and vesicles), confirmed by conventional TEM, DLS and cryo-TEM, was obtained by PISA depending on the polymer concentration, DP of the core and stabilizer blocks, as well as on the choice of the solvent (Fig. 23). For 15%w/w PLA14-PBzA60 dispersions in both n-dodecane and isohexadecane, the formation of a free-standing gel at 20 °C was observed. The gel underwent a worm-to-sphere transition when the temperature was increased up to 80 °C. Additionally, as demonstrated by rheology measurements, the copolymer presented a second critical gelation temperature upon cooling below 15 °C, which was attributed to the stiffening of the worms on approaching the Tg of the benzyl acrylate block.
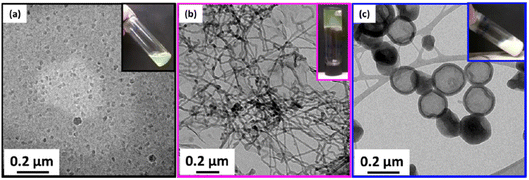 | ||
| Fig. 23 Cryo-TEM images of poly(lauryl acrylate)-block-poly(benzyl acrylate) copolymers in n-heptane, forming (a) spheres, (b) worms, and (c) vesicles depending on the polymer concentration and DP of the blocks. Digital photographs of the concentrated dispersions are also shown. Reproduced from ref. 135 Copyright 2015 American Chemical Society. | ||
In another report, sterically-stabilized nanoparticles consisting of a poly(tert-octyl acrylamide) segment (DP = 85), and a poly(dimethylacrylamide) block (DP = 150) directly synthetized in dodecane via PISA exhibited a UCST-type phase transition with a TCP of 27 °C. This behaviour was attributed to the poor solvation of the poly(tert-octyl acrylamide) stabilizer block in the hydrocarbon at lower temperatures.137
Poly(ε-allyl-ε-caprolactone) (PCL) synthesised via ROP using Mg(BHT)2(THF)2 and post-polymerized via photoinitiated thiol–ene addition using a series of commercially available alkyl thiols (i.e. 1-hexanethiol, 1-octanethiol, 1-ethyl hexanethiol, 1-decanethiol, 1-dodecanethiol, and 1-hexadecanethiol) was also found to be thermoresponsive in n-dodecane.151 The alkyl-functionalized polymers exhibited a UCST phase transition, which was dependent on the length of the alkyl thiol and the polymer concentration. The increase in the alkyl chain length on the PCL backbone from C6 to C12 resulted in an enhancement of its solubility in the hydrocarbon, and the polymers became semi-crystalline when the alkyl chain length reached 10 carbons, as demonstrated by DLS and UV-vis analyses.
Interestingly, the possibility of using temperature as stimulus to control the foamability of an emulsion system in n-dodecane/water has also been investigated.152 In this example, a system composed of four components, n-dodecane and aqueous 0.3 M NaNO3 solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), tetraethylene glycol monododecyl ether (surfactant), and azobenzene randomly modified poly(acrylic acid sodium salt) (1 mol% modification) as the amphiphilic photoresponsive polymer (PRP), was studied as a “proof-of-principle” for the design of stimuli-responsive foams. The surfactant could stabilize both direct and inverse emulsions below and above the phase inversion temperature (PIT). Above the PIT, a decreased foamability was observed, which was attributed to an increased ability of emulsion drops to enter the air–water interface due to the different interfacial organization. The foamability was recovered by decreasing the temperature below the PIT.
1), tetraethylene glycol monododecyl ether (surfactant), and azobenzene randomly modified poly(acrylic acid sodium salt) (1 mol% modification) as the amphiphilic photoresponsive polymer (PRP), was studied as a “proof-of-principle” for the design of stimuli-responsive foams. The surfactant could stabilize both direct and inverse emulsions below and above the phase inversion temperature (PIT). Above the PIT, a decreased foamability was observed, which was attributed to an increased ability of emulsion drops to enter the air–water interface due to the different interfacial organization. The foamability was recovered by decreasing the temperature below the PIT.
The temperature-responsive solution behaviour of polymers in longer aliphatic hydrocarbon has also been investigated, even though only a few studies are present in literature. For example, the thermoresponsive behaviour of diblock copolymers of 3-phenylpropyl methacrylate (PPMA) and stearyl methacrylate (SMA) obtained via RAFT dispersion polymerization in n-tetradecane using SMA homopolymers as macro-CTAs has been evaluated.136 At 20 wt% solids, nanoparticles with spherical, worm and vesicular morphologies were obtained in situ by employing a PSMA macro-CTA with a fixed DP of 19 (PSMA19), which was chain extended with PPMA (PPPMAx). To exemplify, the diblock copolymer consisting of a PSMA19 segment and a PPPMA85 block was able to form a soft physical gel at 20 wt% solids at ambient temperature due to worm nanoparticle entanglements. Also in this case, the gel underwent degelation upon heating up to 95 °C. This process was completely reversible, and it was attributed to a worm-to-sphere transition caused by an increase in the solvation of the core-forming PPPMA block, as confirmed by DLS and TEM analyses.
Finally, nanoparticles consisting of block copolymers of tert-octyl acrylamide (DP = 85) and dimethylacrylamide (DP = 150) obtained via PISA showed a UCST-type phase transition in n-tetradecane and n-hexadecane, which was attributed to the poor solubility of the tert-octyl acrylamide block in the hydrocarbons at lower temperatures.137
4.2. Thermoresponsive behaviour in aromatic solvents
Aromatic hydrocarbons have also been employed for the evaluation of the thermoresponsive behaviour of polymers. Among all, toluene is the most studied one. Linear poly(octadecyl vinyl ether) and linear poly(2-(4-biphenyloxy)ethyl vinyl ether) (poly(BPOVE)) underwent a UCST phase transition in toluene around 4 °C and 12 °C, respectively.109 As explained above, the UCST phase transition of poly(ODVE) was due to the crystallization of the long alkyl chains (Fig. 24), while the solution behaviour of poly(BPOVE) was caused by the strong interactions between the liquid crystalline mesogenic structures.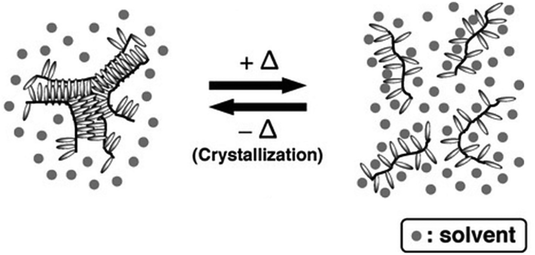 | ||
| Fig. 24 Schematic depiction of the UCST phase transition of poly(ODVE) caused by the crystallization of the long alkyl chains. Adapted from ref. 109 Copyright 2008 Wiley Periodicals, Inc. | ||
Furthermore, it has been shown that a partially fluorinated poly(vinyl ether), namely poly[2-(4,4,5,5,6,6,7,7,7-nonafluoroheptyloxy)ethyl vinyl ether] (poly(9FVE)), synthesised by living cationic polymerization exhibited a UCST-type phase transition in toluene, with the polymer solution being completely transparent at 70 °C, and becoming heterogeneous at 69 °C, as demonstrated by a sharp drop in transmittance in the transmittance vs. temperature plot.153 The polymer thermoresponsiveness was completely reversible with little hysteresis, and it was strongly dependent on the balance between polymer-solvent and polymer–polymer interactions based on their affinity towards each other.
In another report, poly(3-((4R,5R)-4,5-bis(hydroxydiphenylmethyl)-2-methyl-1,3-dioxolane-2-yl)propyl acrylate) (poly(T5)) synthesised via ATRP containing α,α,α′,α′-tetraaryl-2,2-disubstituted 1,3-dioxolane-4,5-dimethanol (TADDOL) moiety in the side chains exhibited single or triple thermoresponsive behaviour in toluene in the presence of chiral guests as effectors (Fig. 25).138 The addition of hydrogen bond acceptors or donors, such as camphor, methanol and borneol, resulted in single thermoresponsiveness, while the addition of quinine, containing four hydrogen bonding sites, led to a sol–gel transition due to the bonding of multiple polymer chains, inducing gelation of the polymer.
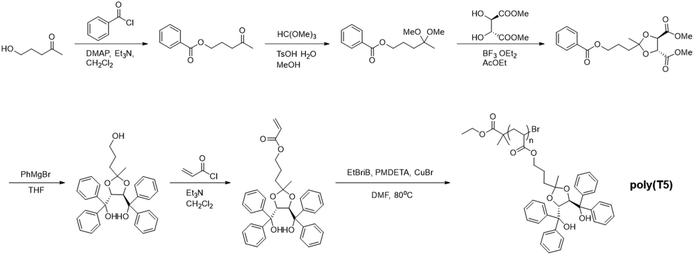 | ||
| Fig. 25 Schematic depiction of the synthesis of poly(3-((4R,5R)-4,5-bis(hydroxydiphenylmethyl)-2-methyl-1,3-dioxolane-2-yl)propyl acrylate (poly(T5)).138 | ||
Interestingly, a triple thermoresponsive behaviour was observed in the presence of (S) or (R)-2-methylpiperidine.138 Upon heating, the polymer solution underwent a UCST phase transition, followed by a LCST transition and again by a UCST transition. This unique triple thermoresponsiveness was attributed to the formation of multiple supramolecular complexes between the host polymer and the guest molecules, and it was dependent on the stoichiometry change between the host and the guest, and the concentration of both the polymer and the effector, as demonstrated by transmittance, DLS, and NMR studies (Fig. 26). This supramolecular strategy is believed to be of importance for the design of highly ordered responsiveness of functional polymer materials.
 | ||
| Fig. 26 Schematic depiction of the process of the schizophrenic thermoresponsive behaviour of poly(T5) in presence of (S) or (R)-2-methylpiperidine in toluene. Reproduced from ref. 138 Copyright 2020 American Chemical Society. | ||
Alternating copolymers of vinyl phenol and alkyl-substituted maleimide with an appropriate alkyl chain length also exhibited interesting thermoresponsive behaviours in toluene (Fig. 27).139 A 1 wt% poly(vinyl phenol-alt-N-dodecyl maleimide) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) solution showed a UCST-type phase transition at around 60 °C in toluene, and a sol–gel transition around room temperature when the polymer concentration was increased to 5 wt%. Comparatively, a 50
50) solution showed a UCST-type phase transition at around 60 °C in toluene, and a sol–gel transition around room temperature when the polymer concentration was increased to 5 wt%. Comparatively, a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 poly(vinyl phenol-alt-N-octadecyl maleimide) exhibited a reversible sol–gel transition in toluene around room temperature at both analysed polymer concentrations. The two copolymers underwent a sol–gel transition also in other aromatic solvents, such as anisole, p-cymene, cumene, 1,2,4-trichlorobenzene, and nitrobenzene at a concentration of 5 wt%. This thermoresponsive behaviour was attributed to hydrogen bonding interactions between phenol units, which became weaker on heating, resulting in the temperature-induced phase transition, as shown by variable temperature 1H NMR measurements. Moreover, it was demonstrated that the transition temperature was dependent on the molecular weight of the alternating copolymer as well as on the amount of phenol groups in the polymer chains.
50 poly(vinyl phenol-alt-N-octadecyl maleimide) exhibited a reversible sol–gel transition in toluene around room temperature at both analysed polymer concentrations. The two copolymers underwent a sol–gel transition also in other aromatic solvents, such as anisole, p-cymene, cumene, 1,2,4-trichlorobenzene, and nitrobenzene at a concentration of 5 wt%. This thermoresponsive behaviour was attributed to hydrogen bonding interactions between phenol units, which became weaker on heating, resulting in the temperature-induced phase transition, as shown by variable temperature 1H NMR measurements. Moreover, it was demonstrated that the transition temperature was dependent on the molecular weight of the alternating copolymer as well as on the amount of phenol groups in the polymer chains.
 | ||
| Fig. 27 Schematic depiction of the synthesis of alternating copolymers of vinyl phenol and alkyl substituted maleimide.139 | ||
In another report, the solution behaviour of the liquid crystalline polymer poly(11-(4-((E)-4-butylstyryl)phenoxy)undecyl methacrylate) (PMAS) synthesised by RAFT polymerization was evaluated in both toluene and 1,2,4-triethylbenzene.140 The polymer exhibited a UCST phase transition at 26.3 and 49.7 °C in 2 wt% polymer solutions in toluene and 1,2,4-triethylbenzene, respectively, characterized by a large hysteresis. The thermoresponsive behaviour was dependent on both the polymer concentration and molecular weight. Scanning electron microscopy (SEM) and TEM imaging of the PMAS at temperatures above and below the UCST, and the investigation of the polymer after dimerization with stilbene using UV light irradiation confirmed that the thermoresponsive behaviour was driven by the crystallization of PMAS in the evaluated solvents.
Poly(γ-benzyl-L-glutamate) homopolymers synthesised via nickel-mediated ROP formed thermoreversible gels in toluene with gel–sol transition temperature between 48 and 53 °C depending on the polymer molecular weight and concentration.154 From TEM and AFM imaging, it has been demonstrated that gels formed at a very low concentration (0.05 wt%) consisted of spherical aggregates sticking together, with thin nanofibers radiating from them. The increase in the polymer concentration resulted in the formation of a self-supportive gel with a nanofiber 3D network.
The effect of the addition of a second solvent on the thermoresponsive behaviour of polymer solutions in toluene has also been studied. For example, poly[2-(vinyloxy)-ethyl-1-butylimidazolium chloride] (poly([BuIm][Cl])) bearing imidazolium pendants underwent a LCST-type phase transition at around 60 °C in a mixture of toluene/1-butanol (90/10% w/w), which was induced by the addition of the good solvent, 1-butanol, in the nonpolar solvent, toluene.155 It was demonstrated by 1H NMR and differential scanning calorimetry (DSC) measurements that, at low temperatures, the interactions between the polymer pendants and the alcohol resulted in the polymer solubilization, while the increase in temperature led to the weakening of the aforementioned interactions resulting in favoured counter anion-induced pendant–pendant interactions and, eventually, in the phase separation.
Furthermore, a reversible twist in the conformation from optical inactive cis-transoidal to cis-cisoidal has been reported for a phenylacetylene homopolymer having two hydroxyl groups and a chiral pinanyl substituent in toluene/2-amino-1-butanol and toluene/DMSO mixtures (4/1 v/v) upon a change in temperature.129
The thermoresponsive behaviour of polymers in aromatic solvents has been further investigated with polystyrene block copolymers. A poly(styrene-b-dimethylsiloxane) diblock copolymer with block molecular weights of 4 and 12 kDa, respectively, was synthesised via anionic polymerization in a series of styrene-selective dialkyl phthalates: dioctyl phthalate (DOP), dibutyl phthalate (DBP), and diethyl phthalate (DEP).141 The increase in the selectivity of the mixed solvent at room temperature resulted in a change in the morphology of the equilibrium micelles from spheres (DOP) to cylinders (DBP) to vesicles (DEP). By increasing the temperature above 70 °C, the selectivity of the solvent decreased, leading to a reverse transition from cylinders to spheres in DBP, and from vesicles to cylinders to spheres in DEP. These thermotropic transitions were dependent only on the thermodynamic interactions between the solvent and the blocks in the copolymer, which could be varied by changing the nature of the solvent or the temperature.
4.3. Thermoresponsive behaviour in oils
The thermoresponsive behaviour of polymers has been investigated also in more complex alkane mixtures. For example, poly(lauryl methacrylate-block-styrene-block-lauryl methacrylate) (PLMA-b-PS-b-PLMA) gradient copolymers synthesised via nitroxide-mediated radical polymerization (NMP) were able to form spherical colloidal micelles in a commercially available hydrocarbon oil, as shown by SAXS measurements.142 These micelles, consisting of a pure PS core, an interfacial layer of mixed PS and PLMA, and a pure PLMA corona, swelled upon heating due to the gradual solubilization of the domains in which the two monomers were mixed. On the one hand, the high incompatibility of the PS core with the oil resulted in no swelling and hindered plasticization. On the other hand, the increase in temperature led to the extension of the PLMA corona and in the solubilization of the mixed layer, resulting in the micelles swelling.Another example is the thermally induced morphological transition of diblock copolymer vesicles consisting of stearyl and benzyl methacrylates formed via PISA in mineral oil.143 At appropriate block length, the diblock copolymers underwent a vesicle-to-worm phase transition upon heating from 20 to 150 °C, as assessed by TEM, SAXS, rheology and variable temperature 1H NMR studies. The latter technique showed that at elevated temperatures, the BMA core-forming block was better solvated, resulting in a change in the preferred morphology of the diblock copolymer via surface plasticization. SAXS studies also indicated that on average, three worms were formed per vesicle.
Similarly, a UCST-type behaviour in polyalphaolefin (PAO) was observed for homopolymers and random copolymers of alkyl methacrylates with an appropriate alkyl pendant length (Fig. 28).144 It has been shown that the TCP was strongly dependent on the number of carbon atoms in the alkyl pendant groups for polymers with a similar DP: the longer the alkyl side chain, the lower the UCST in PAO. For instance, the TCP could be tuned from 0.5 °C to 138 °C by decreasing the number of carbons in the alkyl side chains from 8 to 4. Additionally, a series of ABA triblock copolymers containing a PAO-philic middle block (i.e. 2-ethylhexyl methacrylate and lauryl methacrylate copolymers) and temperature-responsive outer blocks (i.e. n-butyl methacrylate and n-hexyl methacrylate copolymers) synthesised by RAFT polymerization using a difunctional chain transfer agent, showed a reversible thermally-induced sol–gel transition in PAO (Fig. 28). This transition could be efficiently tuned from around 0 to 63.5 °C by varying the composition and length of the blocks, as well as the polymer concentration in solution.144
 | ||
| Fig. 28 Schematic depiction of the chemical structure of homopolymers and random copolymers of alkyl methacrylates with linear and branched alkyl side chains (top), and synthetic route of ABA triblock copolymers composed of a PAO-philic middle block and two thermoresponsive outer blocks (bottom).144 | ||
Furthermore, linear polydimethylsiloxane-poly(2-(dimethylamino)ethyl methacrylate diblock copolymers formed a well-defined worm phase at 25% w/w in decamethylcyclopentasiloxane (D5) silicone oil.145 The worms underwent a worm-to-sphere transition upon heating up to 100 °C. This change in morphology was driven by a reversible solvent plasticization of the poly(2-(dimethylamino)ethyl methacrylate cores, as shown by TEM, variable temperature 1H NMR, DLS, and SAXS experiments. It has been observed by both oscillatory rheology studies and shear-induced polarized light imaging measurements that the degelation occurred at around 32 °C, while the full worm-to-sphere conversion required higher temperatures (∼110 °C). The thermoresponsive behaviour of the worms upon the addition of a cross-linker was also evaluated. TEM analyses showed that the covalently-stabilized worms failed to undergo the worm-to-sphere transition when heated to 100 °C.
In another report, linear random copolymers of 2-ethyl-2-oxazoline (EtOx) and 2-stearyl-2-oxazoline (SteOx) obtained via cationic ring opening polymerization (CROP) characterized by different ratios between the two monomers exhibited a UCST-type phase transition in Yubase-4 oil.146 The solution behaviour was attributed to the packing of the long alkyl chains of the SteOx units, and the TCP values of the copolymers could be tuned by varying the ratios between the two monomers. Moreover, DSC analyses demonstrated the presence of exothermic crystallization peaks for all copolymers, and the resulting crystallization temperatures (Tc) could be correlated with the TCP values obtained by transmittance measurements. It has been shown that by increasing the overall order of the copolymer structure (i.e. copolymer composition), an increase in the Tc and TCP values in oil was observed due to a better alignment of the polymer chains, which could pack and form crystalline areas. Furthermore, graft copolymers consisting of a methacrylic acid-co-2-ethylhexyl methacrylate backbone and SteOx-co-EtOx side chains, obtained via the grafting-onto method (Fig. 29), also exhibited a UCST solution behaviour, which was found to be dependent on various parameters, such as the conversion, DP, and composition of the side chains, as well as on the grafting density. Also in this case, it was possible to correlate the Tc values obtained via DSC measurements with the TCP values determined by transmittance measurements.
 | ||
| Fig. 29 Schematic representation of the synthetic route used to synthesize graft copolymers consisting of a methacrylic acid-co-2-ethyhexyl methacrylate backbone and SteOx-co-EtOx side chains.146 | ||
In this section, the temperature-induced phase transitions of polymers in aliphatic and aromatic hydrocarbons as well as complex alkane mixtures have been meticulously described. In these solvents, polymers mainly exhibit UCST-type solution behaviours and sol–gel transitions caused by their self-assembly in solution, but a few examples of LCST and triple thermoresponsiveness are also present in literature. Furthermore, other interesting phenomena include conformational transitions and controlled foamability upon changes in temperature. The thermoresponsive behaviour could be tuned not only by the structural and chemical characteristics of the polymers, but also by the addition of solvents, effectors, and other small molecules, which could trigger and vary the temperature-induced behaviour. This class of polymers have gained an increase interest especially for their possible application in the lubricant industry for the production of oil additives.
5. Halogenated solvents
In this section, the thermoresponsive behaviour of polymers in halogenated solvents will be described in detail. Although halogenated solvents have not been intensively investigated in relation to thermoresponsive polymers, in the few examples presented in the literature, it has been demonstrated that polymers have interesting temperature-responsive solution behaviours in these solvents (Table 8 and Table S7†).| Solvent | Type of polymer | Polymer conc. | Phase transition type (Temp. °C) | Ref. |
|---|---|---|---|---|
| CHCl3 | Poly[2-(vinyloxy)-ethyl-1-butylimidazolium chloride] | 2 wt% | LCST (@30) | 155 |
| CHCl3 | Poly[2-(4,4,5,5,6,6,7,7,7-nonafluoroheptyloxy)ethyl vinyl ether] | 1 wt% | UCST (∼20) | 153 |
| CHCl3 | Poly(isobutyl vinyl ether)-b-(2-(4,4,5,5,6,6,7,7,7-nonafluoroheptyloxy)ethyl vinyl ether) | 0.5 wt% | Self-assembly (∼25) | 153 |
| CHCl3 | Poly(isobutyl vinyl ether)-b-(2-(4,4,5,5,6,6,7,7,7-nonafluoroheptyloxy)ethyl vinyl ether) | 20 wt% | Sol–gel transition (∼25) | 153 |
| CHCl3 (+2%EtOH) | Chiral-polymeric ionic liquids (CPILs) with benzyl N-substituent and an imidazole derived from (L)-valine | 0.5 mg mL−1 | LCST (@48.5) | 156 |
| CHCl3 | CPIL-stabilized AuNPs | 1 mg mL−1 | LCST (@31) | 156 |
| CHCl3 | Poly(7-methacryloyloxycoumarin) | 0.1 wt% | LCST (@28) | 157 |
| CHCl3 | Poly((−)-3-methoxycarbonyl-5-(N-methyl-N-(S)-(1-phenylethyl)carbamoyl)phenylacetylene) | 0.0001 mol L−1 | cis–trans isomerization (∼15–20) | 158 |
| DCM | Poly(7-methacryloyloxycoumarin) | 0.3wt% | LCST (∼39) | 157 |
| DCM | Poly(3-((4R,5R)-4,5-bis(hydroxydiphenylmethyl)-2-methyl-1,3-dioxolane-2-yl)propyl acrylate) | 10 mg mL−1 | UCST (∼43) | 138 |
| DCE | Poly(2-(3-butylureido)propyl acrylate) + effectors | 25 mg mL−1 | LCST (@43–57) and UCST (@32–71) | 159 |
| DCE | Poly(3-(3-butylureido)propyl acrylate) (PUA) + effector | 25 mg mL−1 | LCST (∼33) and UCST (∼38) | 160 |
| DCE | Poly(3-(3-butylureido)propyl methacrylate (PUMA) + effector | 25 mg mL−1 | LCST (∼30) and UCST (∼24) | 160 |
| DCE | Poly(3-(3-butylureido)propyl vinyl ether) (PUVE) + effector | 25 mg mL−1 | LCST (∼47) and UCST (∼20) | 160 |
| DCE | Poly((1-pyrene)methyl acrylate) + effectors | 10 mg mL−1 | LCST (@43) | 161 |
| 1,1,2-Trichloroethane | Poly(7-methacryloyloxycoumarin) | 0.3 wt% | LCST (ND) | 157 |
| o-Dichlorobenzene | Poly[(5,6-difluoro-2,1,3-benzothiadiazol-4,7-diyl)-alt-(3,3′′′-di(2-octyldodecyl)-2,2′,5′,2′′,5′′,2′′′-quaterthiophen-5,5′′′-diyl)] | 5 mg mL−1 | Disaggregation (∼75) | 162 |
| Perfluoro(methylcyclohexane) | Poly[2-(4,4,5,5,6,6,7,7,7-nonafluoroheptyloxy)ethyl vinyl ether] | 1 wt% | UCST (∼10) | 153 |
| Perfluorodecalin | Poly[2-(4,4,5,5,6,6,7,7,7-nonafluoroheptyloxy)ethyl vinyl ether] | 1 wt% | UCST (∼20) | 153 |
Among all halogenated solvents, chlorinated solvents and, especially, chloroform (CHCl3), are the most studied in relation to the temperature-induced solution behaviour of polymers. As discussed above, poly(ODVE) showed a UCST-type phase transition in hexane due to the crystallization of the long alkyl side chains. In a similar fashion, the miscibility gap of a 1 wt% solution of poly(ODVE) in chloroform was demonstrated, exhibiting a UCST behaviour with a TCP of −2 °C.109,147 Interestingly, vinyl ether polymers functionalized with imidazolium or pyridinium salt pendants underwent a LCST-type phase separation in CHCl3.155 In fact, a solution of poly[2-(vinyloxy)-ethyl-1-butylimidazolium chloride] in CHCl3 displayed a LCST-type behaviour at 30 °C with no hysteresis, which was found to be dependent on the polymer DP, polymer concentration, the type of pendant counter anions, and the length of the alkyl side chains. Moreover, poly[2-(vinyloxy)ethyl-4-methylpyridinium chloride] (poly([MePy][Cl])) exhibited a fully reversible LCST phase transition at around 35 °C in CHCl3/MeOH mixtures (97/3%w/w), which was strongly affected by the content of MeOH in the mixed solvents. The authors believe that these thermoresponsive polymers might have a potential application in sensor systems and other interactive materials.
Furthermore, poly[2-(4,4,5,5,6,6,7,7,7-nonafluoroheptyloxy)ethyl vinyl ether] (poly9FVE), a partially fluorinated vinyl ether polymer, underwent a UCST phase transition at around 20 °C in CHCl3, which was dependent on the DP of the polymer (Fig. 30).153 Additionally, when 9FVE was copolymerized with isobutyl vinyl ether (IBVE), the resulting block copolymer self-assembled in CHCl3. It was demonstrated by DLS measurements that the diblock copolymer adopted either individual polymer chains or small aggregates at 30 °C at a concentration of 0.5 wt%, whereas the aggregates size sharply increased upon cooling. Moreover, when the diblock copolymer concentration was increased to 20 wt%, the polymer solution underwent a thermally induced sol–gel transition, forming a transparent gel upon cooling from 50 °C to ambient temperature. It was also shown that poly9FVE underwent a UCST-type transition in fluorinated solvents, namely perfluoro(methylcyclohexane) and perfluorodecalin, at around 10 °C and 20 °C, respectively.
 | ||
| Fig. 30 Chemical structure of (A) poly[2-(vinyloxy)-ethyl-1-butylimidazolium chloride] and poly[2-(vinyloxy)ethyl-4-methylpyridinium chloride],155 and (B) the 2-(4,4,5,5,6,6,7,7,7-nonafluoroheptyloxy)ethyl vinyl ether (9FVE) partially fluorinated monomer.153 | ||
Similarly, when dissolving chiral-polymeric ionic liquids (CPILs) in CHCl3, thermoresponsive properties were observed.156 CPILs were synthesised from the corresponding linear poly(vinylbenzyl chloride) polymers via substitution of the chloride groups with chiral imidazoles obtained from several amino acids (Fig. 31). CPILs bearing a benzyl N-substituent and an imidazole derived from (L)-valine (CPIL-4a) exhibited a LCST-type thermoresponsive behaviour at 48.5 °C in CHCl3 (stabilized by 2% EtOH), as shown by transmittance measurements. This thermoresponsive behaviour was attributed to the self-assembly of the IL-like moieties, which resulted in a high-order supramolecular structure with a hierarchical architecture that provided the thermoresponsiveness. The LCST could be tuned over a wide range of temperatures, from 58.8 to 12.5 °C, by varying the substitution at the chiral stereocentres and the amide substitution, the nature of the counter ion and the CPILs concentration. Poly(vinylbenzyl chloride) homopolymer was then used to stabilize gold nanoparticles (AuNPs) by direct synthesis from AuCl4− in the presence of the polymer prepared via RAFT polymerization, followed by the modification of the chloromethyl groups into chiral imidazolium units. Interestingly, this nanocomposite system also showed a LCST phase transition at 31.0 °C in CHCl3 at a concentration of 1 mg mL−1. These systems may open a new avenue for the preparation of chiral polymeric IL-stabilized AuNPs for various applications.
 | ||
| Fig. 31 Schematic depiction of the synthesis of chiral polymeric ionic liquids (CPILs).156 | ||
Similar to ionic charges, interactions with π-electron systems have also shown to induce thermoresponsive behaviour. For example, poly(7-methacryloyloxycoumarin) (P1a) and its derivatives as homopolymers containing photoreactive coumarin units synthesised via FRP exhibited a temperature-induced phase transition depending on the type of substituents (Fig. 32).157 For example, P1a showed a LCST thermoresponsive behaviour in CHCl3 around room temperature (i.e. 28 °C), attributed to the collapse of the CH/π interactions between the solvent molecules and the coumarin ring, and the formation of π/π interactions between the coumarin units. Additionally, for this polymer, a lower TCP (21 °C) was measured for a 0.1 wt% polymer solution in deuterated CDCl3 compared to CHCl3, which was attributed to the deuterium isotope effect. It was further demonstrated that in distilled CHCl3, the TCP could be varied by photo-irradiation due to the reversible [2 + 2] cycloaddition of the coumarin units (dual-stimuli responsive units). The TCP could be tuned from 28 to 57 °C by changing the polymer structure (i.e. by introducing ethyleneoxy spacer units and substituting the α-methyl group with hydrogen), and by introducing different substituents on the coumarin units. A similar LCST-type behaviour was also observed for P1a in 1,1,2-trichloroethane. As indicated by the authors, these polymers might have a potential application in the synthesis of dual-stimuli responsive functional materials.
 | ||
| Fig. 32 Chemical structure of the coumarin-containing polymers.157 | ||
In a similar fashion, a series of well-defined poly(arylene ether sulfone)s (PESs) with different molecular weight distributions obtained by chain-growth condensation polymerization showed a LCST-type phase transition in chloroform characterized by high TCP values due to the high solubility of the polymer in the selected solvent.163 As shown by UV-vis spectrophotometry, the TCP was dependent on the length of the polymer chains, increasing with decreasing molecular weight.
Furthermore, it has been shown that poly(3-((4R,5R)-4,5-bis(hydroxydiphenylmethyl)-2-methyl-1,3-dioxolane-2-yl)propyl acrylate) exhibited a UCST-type thermoresponsive behaviour in CHCl3 around 50 °C at a concentration of 10 mg mL−1 due to the cleavage of the self-associative interactions between TADDOL groups, which could be easily broken by heating, and the solvation of the polymer chains by the solvent molecules.138 A similar thermoresponsive behaviour was also observed in dichloromethane and 1,2-dichlorethane. These polymers might find application for the design of stimuli-responsive polymers with highly ordered responsiveness.
Recently, it has been shown that temperature could also induce an helical inversion of poly((−)-3-methoxycarbonyl-5-(N-methyl-N-(S)-(1-phenylethyl)carbamoyl)phenylacetylene) in CHCl3, which was related to the thermo-driven amide cis–trans isomerization, as shown by UV–vis spectroscopy, electronic circular dichroism (ECD), and vibrational circular dichroism (VCD).158 For instance, at low temperatures the trans conformation was dominant, while the amide bond shifted toward the cis isomer when the temperature increased, causing a higher steric hindrance and, thus, the inversion of the helical sense (Fig. 33). As stated by the authors, this unique thermoresponsive behaviour brings a deeper understanding of the dynamic origin of biological one-handed helix, and it might also have potential as molecular thermoswitch in the field of smart materials.
 | ||
| Fig. 33 Schematic depiction of the possible 3D helical models and the conformations of pendants for poly((−)-3-methoxycarbonyl-5-(N-methyl-N-(S)-(1-phenylethyl)carbamoyl)phenylacetylene) in CHCl3. Adapted from ref. 158 Copyright 2021 American Chemical Society. | ||
Similar to CHCl3, vinyl ether polymers show thermoresponsiveness also in dichloromethane (DCM). For example, poly(ODVE) exhibited a UCST phase transition at 14 °C in DCM, due to the crystallization of the long alkyl chains, as confirmed by the appearance of an exothermic peak in the DSC curve at the phase separation of a poly(ODVE) DCM solution.109,147 The UCST properties remained unchanged when reducing the side chain length to poly(hexadecyl vinyl ether) (poly(HDVE).109
In another report, poly(7-methacryloyloxycoumarin) also showed a temperature-induced solution behaviour in DCM, revealing a LCST-type phase separation near the boiling point of the solvent (∼39 °C) at a concentration of 0.3 wt%, while being completely soluble at a polymer concentration of 0.1 wt%.157
1,2-Dichloroethane (DCE) has also been used for the investigation of polymers thermoresponsiveness in solution. As an example, the solubility and thermoresponsive behaviour of a series of well-defined urea-modified homopolymers obtained from the RAFT polymerization of 2-(3-butylureido)propyl acrylate in DCE was found to be dependent on the presence in solution of effectors, such as alcohols, amides, ureas, and carboxylic acids.159 Upon the addition of the small molecules, solubility was achieved and a thermoresponsive behaviour was observed, as shown by transmittance measurements. It has been demonstrated that the thermosensitivity was strongly dependent on the strength of the hydrogen bonding between the urea groups and the effectors (Fig. 34). Effectors that strongly interacted with the urea groups, such as aliphatic carboxylic acids and dialkylureas, tended to induce UCST-type phase separation, while weakly interacting effectors, such as alcohols, induced an LCST-type behaviour. On the one hand, weak polymer–effector interactions were readily interrupted upon heating, leading to the insolubility of the polymer by aggregation (LCST-type). On the other hand, strong polymer–effector interactions resulted in the cleavage of the polymer–polymer interactions upon heating, leading to a UCST-type behaviour. Both the UCST and LCST phase transitions could be easily tuned by varying the type and the concentration of the effector in solution. Moreover, a quaternary system using two effectors, which were able to induce different thermoresponsive behaviours, namely 1-dodecanol (LCST) and N,N′-butyloctylurea (UCST), was also investigated. At appropriate amounts of the two effectors, both LCST and UCST were achieved, and the LCST + UCST regime could be easily adjusted by slight changes of the amount of the two effectors in the system. These polymer/effector systems might be used in the design of smart thermosensitive systems.
 | ||
| Fig. 34 Thermoresponsive behaviour of poly(2-(3-butylureido)propyl acrylate) induced by the addition of strongly interacting and weakly interacting effectors. Adapted from ref. 159 Copyright 2012 American Chemical Society. | ||
3-(3-Butylureido)propyl acrylate has also been copolymerized with N-[3-(acryloyloxy)propyl]-N,N,N-trihexylammonium tetrakis(3,5-bis(trifluoromethyl)phenyl)borate (TFPB) via RAFT polymerization, resulting in copolymers with self-attractive urea units via hydrogen bonding, and self-repulsive lipophilic ion units via electrostatic interaction.164 The copolymers exhibited a UCST-type thermoresponsive behaviour in DCE, which was dependent on the content of lipophilic units in the polymer. The TFPB units were able to induce the dissolution of the polymer due to ionic dissociation, decreasing the TCP from 65 to 48 °C by increasing the TFPB content in the final copolymer from 4 to 5 mol%. Moreover, the self-association of the urea units was found to be strongly dependent on the temperature, while the ionic dissociation of the ion pairs was only slightly affected. Thus, a considerable decrease of the attractive interactions and a slight reduction of the repulsive forces occurred upon heating, resulting in the UCST-type phase transition (i.e. thermoresponsiveness achieved when the repulsive forces among TFPB units overcame the attractive interactions among urea units upon heating). Furthermore, the addition of salt decreased the concentration of dissociated species in the polymer, weakening the repulsive forces and, consequently, leading to increased TCP values.
The attractive interactions between urea units have also been used to induce thermoresponsiveness to a series of polymers containing N-butylurea groups in the side chains and characterized by different backbones, namely poly(3-(3-butylureido)propyl acrylate) (PUA), poly(3-(3-butylureido)propyl methacrylate (PUMA), and poly(3-(3-butylureido)propyl vinyl ether) (PUVE), synthesised by RAFT polymerization (Fig. 35).160 Their thermoresponsive behaviour was investigated in DCE in the presence of 1-hexanol. All polymers exhibited a LCST-type phase transition, which was attributed to the thermal cleavage of hydrogen bonds between urea groups in the polymer and hydroxyl groups in the effector, resulting in the self-association of the urea units and the precipitation of the polymer chains. By changing 1-hexanol with lauric acid, a UCST behaviour was observed for all polymers, with decreasing TCP values when increasing the effector concentration. Additionally, thermoresponsive behaviour, either LCST or UCST, for these polymers was observed in other organic solvents (e.g. ethyl acetate, chloroform, toluene, cyclohexane, etc.) when 1-hexanol was added as an effector. This behaviour was not observed in more polar solvents, such as acetone. The temperature-induced phase transition could be controlled by varying the solvent polarity and the effector concentration.
 | ||
| Fig. 35 Chemical structure of poly(3-(3-butylureido)propyl acrylate) (PUA), poly(3-(3-butylureido)propyl methacrylate (PUMA), and poly(3-(3-butylureido)propyl vinyl ether) (PUVE) having urea units in the side chains.160 | ||
The dependence of the thermoresponsive behaviour on the presence of effectors has also been investigated for a poly((1-pyrene)methyl acrylate) homopolymer (PPMA) synthesised via RAFT polymerization.161 The solubilization of the polymer in DCE was hindered by the presence of the pyrene units, which were able to aggregate through π–π interactions. For this reason, the addition of electron-accepting molecules as effectors was required to form charge-transfer (CT) complexes with the polymer. Additionally, the low binding constant between the pyrene units and the effectors gave rise to the dissociation of the charge transfer complexes with increasing temperature. Upon heating, the dissociation of the charge transfer complexes between the PPMA and the electron-donating molecules resulted in a decrease in the solubility of the polymer leading to its aggregation and precipitation (Fig. 36). The TCP increased with increasing effector concentration, and decreased at higher PPMA concentrations, indicating that the solubility of the polymer was strongly dependent on the degree of association between the PPMA and the effectors. It was also demonstrated by adding a competitive donor in solution that the solubility of the polymer could be tuned by controlling the degree of association between the pyrene units in the polymer chains and the effectors. Also in this case, these unique interactions and thermoresponsive behaviour might be use for the design of smart thermoresponsive systems.
 | ||
| Fig. 36 Thermoresponsive behaviour of supramolecular complexes caused by CT interactions, and chemical structures of the PPMA polymer and used acceptors. Reproduced from ref. 161 Copyright 2013 WILEY-VCH Verlag GmbH&Co. KGaA, Weinheim. | ||
In another report, poly[(5,6-difluoro-2,1,3-benzothiadiazol-4,7-diyl)-alt-(3,3′′′-dialkyl-2,2′;5′,2′′;5′′,2′′′-quaterthiophen-5,5′′′-diyl)] (PffBT4T) polymers with varying side-chain lengths, namely 2-octyldodecyl (C8C12) and 2-nonyltridecyl (C9C13), exhibited an aggregation-to-dissolved chain transition in o-dichlorobenzene (o-DCB).162 At room temperature, the polymers (5 mg mL−1) aggregated into fabric structures, as shown by AFM, TEM and DLS analyses. Above 75 °C, the polymers were fully dissolved in o-DCB and adopted a semi-flexible coil conformation, as demonstrated by temperature-dependent SANS measurements. Interestingly, PffBT4T-C8C12 formed bigger aggregates compared to PffBT4T-C9C13 at the same temperature, indicating that the longer side chain hindered the further growth of aggregates.
In this section, the thermoresponsive behaviour of polymers in halogenated solvents, especially chlorinated, has been reviewed. In these solvents, polymers exhibit mostly LCST and UCST-type phase transitions, which are mainly caused by the interactions (repulsive or attractive) between specific functional groups present on the polymeric chains, the presence and the nature of effectors in solution, the nature of the counter ions, as well as by the DP and the concentration of the polymer. These polymers have shown interesting temperature-induced solution behaviours which might have a crucial impact on the design and production of polymers for the smart materials field.
6. Ionic liquids
In this section, the thermoresponsive behaviour of polymers in ionic liquids (ILs) will be thoroughly discussed (Table S8†). In Table 9, representative examples are divided depending on the type of IL used, as in the previous sections. However, for a better understanding of the polymer structure/IL structure relationship and its effect on the final temperature-induced behaviour, the examples will be discussed depending on the polymer type.| Solvent | Type of polymer | Polymer conc. | Phase transition type (Temp. °C) | Ref. |
|---|---|---|---|---|
| [C2mim][NTf2] | Poly(benzyl methacrylate) | 3 wt% | LCST (@105) | 165 |
| [C2mim][NTf2] | Poly(benzyl methacrylate-co-styrene) | 3 wt% | LCST (∼70–100) | 166 |
| [C2mim][NTf2] | Poly(benzyl methacrylate-co-methyl metacryalte) | 3 wt% | LCST (∼120–140) | 166 |
| [C2mim][NTf2] | Poly(methyl methacrylate)-b-poly(benzyl methacrylate) | 2 wt% | LCST and LCMT (∼120) | 167 |
| [C2mim][NTf2] | Poly(styrene-co-methyl methacrylate) | 3 wt% | LCST (∼85–170), UCST (∼−5–10), LCST + UCST | 168 |
| [C2mim][NTf2] | Poly(cis-4-phenylazophenyl methacrylate-co-benzyl methacrylate) | 3 wt% | LCST (∼102–103) | 169 |
| [C2mim][NTf2] | Poly(trans-4-phenylazophenyl methacrylate-co-benzyl methacrylate) | 3 wt% | LCST (∼80–83) | 169 |
| [C2mim][NTf2] | Poly(N-isopropylacrylamide) | 1 wt% | UCST (@34) | 170 |
| [C2mim][NTf2] | Poly(benzyl methacrylate)-b-poly(N-isopropylacrylamide) | 1 wt% | LCMT (∼140) | 171 |
| [C2mim][NTf2] | Poly(N-isopropylacrylamide)-b-poly(ethylene oxide)-b-poly(N-isopropylacrylamide) | 10 wt% | Sol–gel (∼20) | 172 |
| [C2mim][NTf2] | Poly(ethyl glycidyl ether) | 10 wt% | LCST (@84.4) | 173 |
| [C2mim][NTf2]/[C4mim]PF6 | Poly(benzyl methacrylate)-b-poly(N-isopropylacrylamide) | 1 wt% | UCMT (∼30) LCMT (∼130) | 171 |
| [C4mim][NTf2] | Poly(n-butyl methacrylate) 48 kDa | 0.25 wt% | LCST (@119) | 174 |
| [C8mim][NTf2] | Poly(3-methylbenzyl methacrylate) | 3 wt% | LCST (@162) | 175 |
| [C4dmim][NTf2] | Poly(2-phenylethyl methacrylate) | 3 wt% | LCST (@136) | 175 |
| [C1mim][NTf2] | Poly(2-phenylethyl methacrylate)-b-poly(methyl methacrylate)-b-poly(2-phenylethyl methacrylate) | 30 wt% | Sol–gel (@23) | 176 |
| [Azo][NTf2]/[C1mim][NTf2] | Poly(2-phenylethyl methacrylate)-b-poly(methyl methacrylate)-b-poly(2-phenylethyl methacrylate) | 30 wt% | Sol–gel (@50.7–61.3) | 176 |
| [C4mim][BF4] | Poly(ethylene oxide)-b-poly(N-isopropylacrylamide) | 1 wt% | UCMT (∼60) LCMT (∼207) | 177 |
| [C2dmim][BF4] | Poly(ethylene oxide) 2 kDa | 60 wt% | LCST (∼110) | 178 |
| [C4mim][PF6] | Poly(trans-4-phenylazophenyl methacrylate-r-N-isopropylacrylamide)-b-poly(ethylene oxide)-b-poly(trans-4-phenylazophenyl methacrylate-r-N-isopropylacrylamide) | 1 wt% | UCMT (@60) | 179 |
| [C4mim][PF6] | Poly(trans-4-phenylazophenyl methacrylate-r-N-isopropylacrylamide)-b-poly(ethylene oxide)-b-poly(trans-4-phenylazophenyl methacrylate-r-N-isopropylacrylamide) | 10 wt% | Sol–gel (@56) | 179 |
| [C4mim][PF6] | Poly(ethyl glycidyl ether)-b-poly(ethylene oxide) | 1 wt% | LCMT (@15) | 180 |
| [C8mim][PF6] | Poly(ethyl glycidyl ether) | 10 wt% | LCST (@67.0) | 173 |
Poly(benzyl methacrylate) and its derivatives are the most studied polymers in relation with their thermoresponsive phase transitions in ionic liquids. For example, a 3 wt% PBzMA solution showed a LCST-type phase transition in 1-ethyl-3-methylimidazolium bis(trifluoromethane sulfone)imide ([C2mim][NTf2]) with a TCP of 105 °C.165,166 The thermoresponsive behaviour was attributed to structure-forming solvation between the aromatic ring of PBzMA and the imidazolium cation of the IL, which led to a negative entropic mixing term and, thus, to the LCST-type behaviour.181,182
The effect of the structure of the IL on the thermoresponsive behaviour of PBzMA and its copolymers has been extensively studied by changing the alkyl chain length of the imidazolium substituent and the anion. In general, it has been observed that for ILs with [Cnmim] cations and [NTf2] anion, the TCP values of PBzMA solutions increased with the length of the alkyl chain of the imidazolium cation.165,166 A similar trend was also observed for ILs having PF6 as anion (Fig. 37).
 | ||
| Fig. 37 T CP values of PBnMA and chemical structure of IL cations with [NTf2] (●) and PF6 anions (○). Reproduced from ref. 165 Copyright 2009 IUPAC. | ||
Furthermore, the TCP value of BzMA could be easily tuned by copolymerization with other solvophilic and solvophobic monomers (Fig. 38). For example, the copolymerization of benzyl methacrylate with the solvophobic styrene monomer resulted in a decrease of the TCP in [C2mim][NTf2] with increasing styrene content.166 When BzMA was copolymerized with the solvophilic methyl methacrylate (MMA), higher TCP values were recorded for polymers with higher MMA contents. Furthermore, diblock copolymers consisting of a MMA block and a BzMA segment showed a LCST-type behaviour in [C2mim][NTf2] (2 wt%), resulting in the formation of micelles at a lower critical micellization temperature (LCMT) of around 120 °C, as demonstrated by DLS measurements.167 Interestingly, copolymers of St and MMA also exhibited thermoresponsive phase transitions in [C2mim][NTf2], which were strongly dependent on the St content.168 To exemplify, depending on the St content, the copolymers were insoluble or showed LCST or UCST phase transitions in the selected IL. Interestingly, those containing 42.2–45.6 mol% of St exhibited both LCST (∼165–170 °C) and UCST (∼−5 °C) behaviours.
 | ||
| Fig. 38 Example of chemical structures of random and block copolymers of BzMA and (A) styrene, (B) methyl methacrylate, (C) 4-phenylazophenyl methacrylate, and (D) 9-(4-vinylbenzyl)-9H-carbazole. | ||
In another report, BzMA was copolymerized with 4-phenylazophenyl methacrylate (AzoMA) and the resulting polymers exhibited a LCST-type phase transition in [C2mim][NTf2], which was found to be strongly dependent on the content and on the cis/trans isomerization of the AzoMA units.169 Turbidity measurements were performed either in the dark, to obtain copolymers with trans-AzoMa units, or under UV irradiation for cis-AzoMA-containing copolymers. The TCP values of 3 wt% [C2mim][NTf2] solutions containing copolymers with 1.9 mol% of cis-AzoMA or trans-AzoMA were around 103 and 83 °C, respectively. When the AzoMA content was increased to 4.1 mol%, the TCP decreased to 102 and 80 °C, for the cis- and trans-AzoMA copolymers, respectively. The difference in TCP values between the two copolymers was attributed to the different strength of the cation–π interaction between the azo moieties of the copolymers in the cis- or trans-form and the IL, with the trans-form AzoMA units forming stronger interactions due to a higher planarity of the structure.
Furthermore, the NMP of BzMA with a small fraction of fluorescent controlling comonomer 9-(4-vinylbenzyl)-9H-carbazole (VBK, 2–11 mol% in the feed) yielded polymers exhibiting LCST-type behaviour in [C2mim][NTf2].183 Since PVBK was insoluble in the selected IL, a decrease in the copolymers solubility with increasing VBK content was expected. For instance, the TCP values of 3 wt% copolymer solutions decreased steeply from 86 to 68 °C when the amount of VBK was increased from 4 to 9 mol%. However, the thermoresponsive behaviour of these two copolymers was not reversible. The authors argued that the incorporation of VBK units resulted in a higher stiffness and lower solubility of the final copolymers which, combined with the π–π interactions between the aromatic structure of VBK and the benzyl groups of BzMA, hindered the diffusion of the IL into the polymer aggregates, resulting in an irreversible phase separation. However, it was also shown that chain extending macroinitiators of selected statistical copolymers with a solvophilic MMA-rich block containing 10 mol% of styrene resulted in diblock copolymers with a fully reversible LCST behaviour in the same IL at higher TCP values.
In order to investigate the effect of the structures of polymers and ILs on the temperature-induced phase transition, a series of poly(benzyl methacrylate)-derivatives with different functional groups on their side chains was synthesised and their solution behaviour was studied in six different ILs (Fig. 39).175 It has been observed from transmittance measurements that polymers with solvophobic substituents on the m-position of the phenyl group on the side chains (1b–d) were insoluble in [C2mim][NTf2]. Polymers containing methoxy (1e) and fluoro groups (1f) exhibited a LCST-type phase transition at 54 and 87 °C, respectively. The polymers with a 2-carbon spacer between the phenyl and the ester groups (1g) had a TCP at 42 °C, while when the length of the alkyl spacer was increased to 4 carbon atoms (1h), the resulting polymer was insoluble. In contrast, poly(2-pyridylmethyl methacrylate) (1i), a polar polymethacrylate, was completely soluble over the whole examined temperature range. Also for these polymers, the TCP values increased with increasing length of the alkyl chain on the imidazolium cation of the ILs with a fixed [NTf2] cation. Furthermore, the polymers 1d and 1h, which were insoluble in [C2mim][NTf2], exhibited a LCST phase transition in 1-octyl-3-methylimidazolium bis(trifluoromethane sulfone)imide [C8mim][NTf2] at 162 and 242 °C, respectively. The solution behaviour of PBzMA and the 1g derivative were also evaluated in 1-butyl-2,3-dimethylimidazolium bis(trifluoromethane sulfonyl)imide ([C4dmim][NTf2]), exhibiting TCP values of 176 and 136 °C, respectively. Finally, the temperature-induced phase transition of 1g was studied in binary mixtures of different ILs. The TCP of 1g in [C2mim][NTf2]/[C4mim][NTf2] mixture increased linearly from 42 to 118 °C as the weight fraction of [C2mim][NTf2] increased from 0 to 1. This linear relationship between the TCP and the ratio of the two ILs was also observed for other ILs mixtures and different BzMA-derivatives, making it a useful tool for the prediction and estimation of TCP values that are too low or too high to be directly measured.
 | ||
| Fig. 39 Chemical structures of the PBzMA-derivatives.175 | ||
In another report, the solution behaviour (poly(benzyl methacrylate) and poly(2-phenylethyl methacrylate) (PPhEtMA)), a PBzMA derivative, was evaluated in mixed ILs (1,3-dimethylimidazolium bis(trifluoromethanesulfonyl)amide [C1mim][NTf2] and (1-butyl-3-(4-phenylazobenzyl)imidazolium bis(trifluoromethanesulfonyl)amide [Azo][NTf2], as a small molecular photo trigger.184 The [Azo][NTf2] IL was mainly in its trans form in the absence of light (100%) or under visible light irradiation (85%), while the cis form was the predominant one under UV light irradiation (65%). Interestingly, PPhEtMA was completely soluble in [Azo][NTf2], but insoluble in [C1mim][NTf2]. However, the addition of a small amount of [Azo][NTf2] to [C1mim][NTf2] resulted in the dissolution of the polymer. It has been shown that PPhEtMA underwent a LCST-type phase transition in the solvent mixture, with TCP increasing with increasing [Azo][NTf2] content for both measurements performed in absence of light or under UV irradiation. Furthermore, at all contents, the TCP values measured in the absence of light were higher than those obtained under UV irradiation. Interestingly, the mixture containing 30 wt% [Azo][NTf2] exhibited a reversible phase separation and dissolution at 112 °C under alternating UV and visible light irradiation, due to the cis–trans isomerization of the [Azo][NTf2] IL. A similar behaviour was also observed for PBzMA, with TCP values increasing with increasing [Azo][NTf2] content in the absence of light. However, the TCP values of mixtures containing cis-[Azo][NTf2] were higher than those containing trans-[Azo][NTf2], an opposite behaviour compared to PPhEtMA, which was suggested to be caused by specific interactions of the two polymers with [Azo][NTf2], as demonstrated by 1H NMR measurements.
Furthermore, two thermoresponsive ABA triblock copolymers, PBnMA-b-PMMA-b-PBnMA (BMB) and PPhEtMA-b-PMMA-b-PPhEt, (PMP), exhibited a thermo- and photo-responsive micellization/demicellization in [Azo][NTf2]/[C1mim][NTf2] mixtures.185 As shown by DLS measurements performed in the dark, the size of a 0.1 wt% PMP solution in [Azo][NTf2]/[C1mim][NTf2] (5/95) sharply increased at around 65 °C, a higher temperature compared to the TCP of PPhEtMA homopolymer (49 °C) due to the presence of the solvophilic PMMA block. The increase in the scattering intensity was caused by the formation of micelles consisting of a PPhEtMA core and PMMA corona. As in the case of the PPhEtMA homopolymer, the PMP triblock copolymer exhibited a lower TCP (∼55 °C) when the solution was heated under UV irradiation (i.e. prevalence of cis-IL). The BPB solution in the same ILs mixture in the absence of light showed a lower critical micellization temperature at around 110 °C. As in the case of the PBzMA homopolymer, the LCMT measured under UV irradiation was higher (∼120 °C) compared to the one in trans-IL. Furthermore, at a temperature between the two LCMTs, UV irradiation induced a “unimer-to-micelle” transition for PMP, while a “micelle-to-unimer” transition was observed for BMB (Fig. 40). NMR analyses showed a shift in opposite directions of the chemical shifts for the two polymer/IL solutions, suggesting a strong influence of the photoisomerization of [Azo][NTf2] and the structure of the polymers on the interaction between polymers and [C1mim][NTf2].
 | ||
| Fig. 40 Schematic representation of the micellization/demicellization process of PMP and BMB under UV and visible light irradiation. Reproduced from ref. 185 Copyright 2017 American Chemical Society. | ||
The thermoresponsive behaviour of crosslinked gels of PBzMA and its derivatives in ILs has also been extensively studied. For example, a gel obtained by crosslinking BzMA with ethylene glycol dimethacrylate exhibited a low-temperature swollen and a high-temperature shrunken phase in [C2mim][NTf2] with a phase transition temperature at around 100 °C. The authors believe that these gels could enable the development of environmentally stable smart gel materials for various applications.166
Furthermore, ABA triblock copolymers consisting of two outer PBzMA blocks and a middle PMMA segment (PBzMA-b-PMMA-b-PBzMA) were shown to undergo a low-temperature sol-high-temperature gel transition in [C2mim][NTf2] due to the formation of physical PBnMA cross-linking points.186 At a polymer concentration of 20 wt%, viscoelastic measurements showed that the ABA triblock copolymer formed a viscous solution below 100 °C, and a gel at higher temperatures, due to the high concentration resulting in the aggregation of micelles. The evaluation of the sol–gel reversibility was demonstrated by temperature scanning (0–140 °C) over several days. These polymers may help the development of materials chemistry of soft materials using ILs.
In another example, the ABC triblock copolymer consisting of a PBzMA block, a middle PMMA segment, and a PPhEtMA block (PBzMA-b-PMMA-b-PPhEtMA) underwent a sol–gel transition in [C2mim][NTf2].187 Also in this case, DLS analyses of a 0.1 wt% polymer solution showed the increase in the particle size above 70 °C, indicating the formation of micelles. However, above 120 °C a decrease in size was recorded. The authors suggested that this temperature responsive behaviour was caused by a unimer-to hairy micelle-to flower-like micelle transition due to the double thermosensitivity of the PPhEtMA and PBnMA blocks. Measurements of the frequency dependence of the dynamic moduli at selected temperatures of a 20 wt% PBzMA-b-PMMA-b-PPhEtMA solution showed that the ABC triblock copolymer formed a gel at temperatures above the TCP of PBzMA (105 °C). However, between 40 and 100 °C, an intermediate state was observed, which was attributed to a sol-to-jamming micelles transition due to the LCST phase transition of the PPhEtMA block (∼42 °C), as also confirmed by SAXS analyses. At high temperatures the PPhEtMA and PBnMA blocks connected by physical cross-linking, resulting in the gelation behaviour (Fig. 41).
 | ||
| Fig. 41 Schematic representation of the sol-jamming micelles-gel transition of the PBzMA-b-PMMA-b-PPhEtMA ABC triblock copolymer. Reproduced from ref. 187 Copyright 2016 American Chemical Society. | ||
Triblock copolymers of BzMA-derivatives have also shown sol–gel transitions in ILs. For example, an ABA triblock copolymer consisting of two outer PhEtMA blocks and a middle MMA segment (PMP) synthetized by ATRP of PhEtMA from a bifunctional PMMA macroinitiator exhibited a thermoresponsive behaviour in [C2mim][NTf2]/[C4mim][NTf2] mixtures.188 DLS measurements of 0.1 wt% PMP dissolved in three [C2mim][NTf2]/[C4mim][NTf2] mixtures (100/0, 75/25, 50/50) showed the formation of micelles at higher temperatures. The LCMT of the triblock copolymer increased from 60 to 100 °C with increasing [C4mim][NTf2] fraction from 0 to 50 mol%. Furthermore, rheological measurements of the three 20 wt% PMP ILs solutions showed a low-temperature sol-high-temperature gel transition, with sol–gel transition temperatures increasing from 25 to 65 °C as the [C4mim][NTf2] fraction increased from 0 to 50 mol%. The sol–gel transition temperatures were found to be concentration and solvent dependent.
The thermoresponsive behaviour of PMP has also been evaluated in [C1mim][NTf2] and in a [Azo][NTf2]/[C1mim][NTf2] (10/90 w/w) mixture.176 A 30 wt% PMP solution in [C1mim][NTf2] exhibited a sol–gel transition at 23 °C due to the LCST-type behaviour of the PPhEtMA blocks, which resulted in percolated micellar networks consisting of aggregated PPhEtMA end blocks bridged by solvophilic PMMA middle blocks. To induce photo/thermoresponsive sol–gel transitions, [C1mim][NTf2] was mixed with the photoresponsive [Azo][NTf2] IL. Temperature sweep measurements of viscoelastic moduli and dynamic frequency sweep measurements of a 30 wt% PMP solution in a 10/90 w/w [Azo][NTf2]/[C1mim][NTf2] mixture performed in the dark (i.e. [Azo][NTf2] in trans-state) showed that the PMP behaved as a viscous polymer solution below 40 °C. Above 40 °C, the solution still behaved as a viscoelastic fluid, but at higher temperatures it formed a gel. Under UV irradiation (i.e. photoisomerization of [Azo][NTf2] from trans- to cis-state), the measured gelation temperature was lower than the one measured in the dark, indicating a lower affinity of the PPhEtMA blocks towards the cis-[Azo][NTf2] compared to the trans-[Azo][NTf2].
Another widely studied thermoresponsive polymer in ILs is PNIPAM. Interestingly and contrarily to its thermoresponsive behaviour in aqueous solutions, PNIPAM with a molecular weight of 53 kDa showed a UCST-type phase transition in [C2mim][NTf2] at around 34 °C at a polymer concentration on 1 wt%.170 The TCP values were strongly dependent on the polymer molecular weight and concentration, with TCP increasing with increasing molecular weight and concentration, indicating a strong entropic contribution to the phase transition. Furthermore, PNIPAM gels obtained by using N,N′-methylenebisacrylamide as crosslinker exhibited a reversible swelling/deswelling behaviour in the IL, with a maximum swollen state at around 200 °C. The difference in the transition temperatures between the gels and the polymer in solution was attributed to the reduced conformational entropic effect for the gel due to the high molecular weight of the polymer. PNIPAM (40 kDa) also exhibited a UCST-type phase transition in other ILs, such as 1-ethyl-3-methylimidazolium tetrafluoroborate ([C2mim] [BF4]) and 1-butyl-3-methylimidazolium tetrafluoroborate ([C4mim] [BF4].177
In order to tune its thermoresponsive behaviour in ILs, PNIPAM has been copolymerized with various comonomers (Fig. 42). For example, two block copolymers consisting of a BzMA block (11 kDa) and a PNIPAM segment (PBzMA(11)-b-PNIPAM), or a BzMA segment (22 kDa) and a block of NIPAM/acrylamide random copolymer (PBzMA(22)-b-P(NIPAM-r-AAM)) synthetized via RAFT polymerization showed a thermoresponsive behaviour in [C2mim][NTf2], 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim]PF6), and in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the two ILs.171 In a 1
1 mixture of the two ILs.171 In a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ILs mixture (wt%), the PBzMA(11)-b-PNIPAM diblock copolymer (C = 1 wt%) formed micelles below 30 °C (upper critical micellization temperature, UCMT), near the UCST of PNIPAM, and also above 130 °C, close to the LCST of PBzMA, with a regime of unimers at intermediate temperatures, as shown by DLS measurements. In pure [C2mim][NTf2], the formation of micelles was observed only above 140 °C, however, no micellization at lower temperatures was detected. It has been reported that a 3 wt% PAAM homopolymer solution in [C2mim][NTf2] exhibited a UCST-type phase transition above 150 °C, therefore, the copolymerization of NIPAM with AAM, should induce a micellization of the diblock copolymer at lower temperatures. In fact, the diblock copolymer PBzMA(22)-b-P(NIPAM-r-AAM) underwent micellization at both low and high temperatures, with unimers at intermediate temperatures. This indicated that the self-assembly temperature of the diblock copolymers could be easily tuned, opening the route for the development of various smart materials.
1 ILs mixture (wt%), the PBzMA(11)-b-PNIPAM diblock copolymer (C = 1 wt%) formed micelles below 30 °C (upper critical micellization temperature, UCMT), near the UCST of PNIPAM, and also above 130 °C, close to the LCST of PBzMA, with a regime of unimers at intermediate temperatures, as shown by DLS measurements. In pure [C2mim][NTf2], the formation of micelles was observed only above 140 °C, however, no micellization at lower temperatures was detected. It has been reported that a 3 wt% PAAM homopolymer solution in [C2mim][NTf2] exhibited a UCST-type phase transition above 150 °C, therefore, the copolymerization of NIPAM with AAM, should induce a micellization of the diblock copolymer at lower temperatures. In fact, the diblock copolymer PBzMA(22)-b-P(NIPAM-r-AAM) underwent micellization at both low and high temperatures, with unimers at intermediate temperatures. This indicated that the self-assembly temperature of the diblock copolymers could be easily tuned, opening the route for the development of various smart materials.
 | ||
| Fig. 42 Example of chemical structures of random and block copolymers of PNIPAM and (A) BzMA, (B) P(NIPAM-r-AAM), (C) AzoMA, (D) PEO, (E) PEO in ABA triblock copolymer. | ||
In another example, the thermoresponsive behaviour of well-defined random copolymers of NIPAM and 4-phenylazophenyl methacrylate (AzoMA) synthesised via RAFT polymerization was evaluated in [C2mim][NTf2] in absence or under UV light irradiation.189 The copolymers exhibited a UCST-type phase transition, which was strongly dependent on the AzoMA content and the photochromic state (Fig. 43). For example, the TCP of trans-AzoMA-containing copolymers increased strongly with the increase in the AzoMA content, whereas that of cis-AzoMA-containing copolymers changed only slightly. This was attributed to the nature of the AzoMA monomer, which behaved as solvophobic monomer in its trans-form, and as solvophilic monomer in its cis-state, due to a change in polarity depending on its photoisomerization.
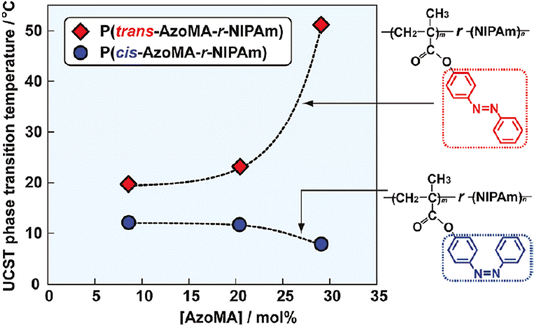 | ||
| Fig. 43 Effect of AzoMA content and photochromic state on the TCP values of random copolymers of NIPAM and AzoMA. Reproduced from ref. 189 Copyright 2011 American Chemical Society. | ||
ABC triblock copolymers consisting of an IL-phobic poly(styrene) block, an IL-philic middle poly(N,N-dimethylacrylamide) segment and a photo/thermoresponsive final poly(4-phenylazophenyl methacrylate-r-N-isopropylacrylamide) block (PS-b-PDMAm-b-P(AzoBnAm-r-NIPAm) synthetized via RAFT polymerization exhibited temperature-induced phase transitions in [C2mim][NTf2].190 As shown by DLS measurements performed in the dark, at a low polymer concentration (1 wt%), the triblock copolymer formed micelles consisting of a PS core and PDMAm-b-P(AzoBnAm-r-NIPAm) corona at 80 °C. By decreasing the temperature to 40 °C and further to 10 °C, the appearance of a bimodal distribution was observed, which was attributed to the formation of micellar aggregates due to the UCST behaviour of P(AzoBnAm-r-NIPAm). Rheological analysis of a 15 wt% triblock copolymer solution showed that the polymer could form percolated micellar networks at low temperatures due to the aggregation of the PS and P(AzoBnAm-r-NIPAm) blocks as physical crosslinks, bridged by the IL-philic PDMAm blocks. With the increase in temperature, a transition from micellar network to jammed micelles was observed, attributed to the disassembly of the thermoresponsive P(AzoBnAm-r-NIPAm) block, resulting in the softening of the gel. Also in this case, the temperature-induced phase transitions were strongly dependent on the photochromic state of the AzoBnAm units. The authors believe that this study might provide a promising strategy for controlling the rheological properties of nonvolatile soft materials via contactless light irradiation that could be used in various applications, such as photoresponsive and photo-healable actuators and soft materials.
The polymerization of PNIPAM with poly(ethylene oxide) into block copolymers showing temperature-induced phase transitions in ILs has also been thoroughly investigated. In contrast to the UCST behaviour of PNIPAM, PEO exhibited a LCST-type transition in ILs. For instance, PEO with a molecular weight of 20 kDa (PEO-20) underwent a reversible LCST behaviour in 1-ethyl-3-methylimidazolium tetrafluoroborate ([C2mim] [BF4]) with TCP values decreasing with increasing polymer concentration.191 Phase diagrams of two PEO homopolymers of 20 kDa and 5 kDa showed convex downward curves, with critical temperatures at 130 and 136 °C, respectively, showing that the TCP values were only weakly dependent on the molecular weight of the polymer. The effect of the alkyl chain on the imidazolium cation of the IL was also evaluated by measuring the transmittance of a 2 wt% PEO-20 solution in [C4mim] [BF4]. The solution showed a LCST-type phase transition at 209 °C, approximately 45 °C higher than in [C2mim] [BF4]. Additionally, in a mixture of the two ILs, a 2 wt% PEO-20 solution exhibited TCP values which increased almost linearly with increasing weight fraction of [C4mim] [BF4].
Interestingly, a noticeable difference in the phase diagrams of 2 kDa PEO solutions in [C2mim] [BF4]and 1-ethyl-2,3-dimethylimidazolium tetrafluoroborate [C2dmim] [BF4], where the H atom in the C2 position of the imidazolium cation was replaced by a methyl group, was observed.178 To exemplify, no changes were recorded at low PEO concentrations, however, a sharp decrease in the TCP values was observed when the concentration was above 30 wt%. The substitution of the most acidic proton of the IL cation with a methyl group lowered the H bonding interactions with the PEO chains, decreasing the PEO miscibility in the IL and, consequently, shifting the TCP to lower values.
By combining PNIPAM and PEO in a diblock copolymer, micelles and inverted micelles could be obtained in [C4mim] [BF4] with a UCMT around 60 °C and a LCMT around 207 °C, close to the TCP of PNIPAM and PEO, respectively.177 The authors concluded that the diblock formed PNIPAM-core micelles at low temperatures, and PEO-core micelles at high temperatures. Similarly to the behaviour of the corresponding homopolymers, in [C4mim] [BF4]/[C2mim] [BF4] mixtures, the UCMT and LCMT values of the diblock copolymer increased and decreased, respectively, with increasing [C2mim] [BF4] weight fraction.
In another report, triblock copolymers consisting of two PNIPAM outer blocks and a PEO middle segment obtained by RAFT polymerization of a PEO homopolymer end-capped with a chain transfer agent, showed a reversible sol–gel transition in [C2mim][NTf2] at a 10 wt% polymer concentration.172 As demonstrated by dynamic shear measurements, the solution behaved as a liquid at high temperatures (∼50 °C), and as a gel at −5 °C, with an intermediate behaviour at intermediate temperatures, and a gelation point around 20 °C. The gelation process was attributed to the noncovalent association of PNIPAM outer blocks undergoing the UCST, bridged by the IL-compatible PEO-middle block.
By incorporating a solvophobic poly(styrene) segment into a pentablock copolymer obtained by chain extension of a poly(styrene)-b-poly(ethylene oxide)-b-poly(styrene) triblock copolymers with NIPAM (PNIPAM-b-PS-b-PEO-b-PS-b-PNIPAM), the sol–gel transition temperatures of the resulting gels in [C2mim][NTf2] could be tuned over a wide temperature range (17–48 °C), as demonstrated by oscillatory shear and temperature-dependent dynamic shear moduli measurements, and DLS analyses.192
ABA triblock copolymers consisting of a middle IL-soluble PEO block and two outer segments of random copolymers of AzoMA and NIPAM exhibited temperature-induced reversible upper critical micellization and gelation in [C4mim]PF6 depending on the polymer concentration.179,193,194 As already explained above, the AzoMA was in its cis-state under UV light irradiation, while it was in its trans-form under visible light irradiation or in the absence of light. As expected, the UCMT of the ABA copolymers (1 wt%) was found to be strongly dependent on the photochromic state of the AzoMA, with values of 60 °C and 44 °C for trans-ABA and cis-ABA copolymers, respectively.179 In addition, a temperature-induced sol–gel transition was observed for 20 wt% ABA triblock copolymer solutions in both cis- and trans-states in [C4mim]PF6 under both UV or visible light. Also in this case, the gelation temperatures were strongly dependent on the cis- or trans-state of the azobenzene units.
PEO has also been copolymerized with poly(n-butyl methacrylate (PnBMA) to form diblock copolymers undergoing temperature-induced micellization with tunable LCMT in ILs mixtures. PnBMA exhibited a LCST-type phase transition in [C4mim][NTf2], which was strongly influenced by the polymer concentration and molecular weight.174 As also shown for other polymers, the TCP value of PnBMA increased almost linearly with increasing length of the alkyl chain on the imidazolium cation. Additionally, in [C2mim][NTf2]/[C4mim][NTf2] mixtures, the TCP of 2 wt% PnBMA (48 kDa) decreased from 108 °C to room temperature when the [C4mim][NTf2] weight fraction decreased. A similar trend was also observed in [C2mim][NTf2]/[C6mim][NTf2] mixtures with TCP values increasing from 108 to 247 °C when the [C6mim][NTf2] weight fraction was increased from 0 to 75 wt%. In the case of PEO-b-PnBMA diblock copolymers containing a solvophilic PEO segment, a micellization process forming PnBMA-core and PEO-corona micelles in [C2mim][NTf2]/[C4mim][NTf2] mixtures was observed via DLS analyses, with LCMT values increasing with increasing weight fraction of [C4mim][NTf2].
The thermoresponsive behaviour of PEO-derivatives has also been investigated in various ionic liquids (Fig. 44). For example, poly(ethyl glycidyl ether) (PEGE) showed a LCST-type behaviour in various ILs, which was strongly dependent not only on the polymer molecular weight and concentration, but also on the nature and alkyl chain length of the IL cation and on the type on the IL anion.173,195 For ILs with [NTf2] anion, PEGE (10 wt%) underwent a phase transition in imidazolium ILs, while it was soluble in pyridinium ILs and insoluble in ammonium and phosphonium ILs. Therefore, the hydrogen bonding interactions between the oxygen atoms of PEGE and the acidic hydrogens in the aromatic ILs were essential to increase its miscibility. The TCP also increased with increasing alkyl chain length on the imidazolium cations. Furthermore, when the proton in the C2 position of the imidazolium cation was substituted with a methyl group, the TCP significantly decreased (e.g. PEGE/[C4dmim][NTf2] has a TCP 120 °C lower than PEGE/[C4mim][NTf2]). In contrast to PBzMA, the hydrogen bonding interactions between the ether oxygen atoms of PEGE and the hydrogen atoms of the IL cations played a crucial role in the temperature-induced phase transition. Also in this case, the TCP values increased with increasing length of the alkyl chain on the IL cation. For ILs with imidazolium cations, PEGE exhibited an LCST phase transition in ILs containing imide anions, while it was immiscible in ILs with [OTf] and [PF6] anions. However, for imidazolium cations with longer alkyl chain length and [PF6] anion, a LCST behaviour was observed. Therefore, the thermoresponsive behaviour was also slightly influenced by the nature of the anion of the IL. In this study, the temperature-induced solution behaviour of other PEO-derivatives was also investigated. For example, poly(glycidyl methyl ether) (PGME) and poly(ethoxyethyl glycidyl ether) (PEEGE) were found to be completely miscible in ILs with [NTf2] anion, however, a LCST phase transition was observed in [C4mim][PF6] at 53.5 and 56 °C, respectively, at a polymer concentration of 10 wt%. Additionally, poly(propylene oxide) (PPO) exhibited a LCST phase transition at 48 °C only in [C4mim][NTf2], while it was completely immiscible in all the other tested ILs. Therefore, the ether oxygen atom of the PEO-derivatives played a crucial role in increasing their miscibility in the ILs through hydrogen bonding interactions.
Finally, a diblock copolymer consisting of a PEGE block (DP = 104) and a PEO segment (DP = 178) synthesised via sequential anionic ring-opening polymerization exhibited a unimers-to-micelles transition in [C4mim][PF6] with a LCMT of 15 °C at a polymer concentration of 1 wt%.180
In this section, the thermoresponsive solution behaviour of polymers in ionic liquids has been thoroughly discussed. In ILs, polymers exhibit a broad range of temperature-induced behaviours, from LCST, to UCST, to micellization and sol–gel transitions. The type of phase transition and the temperature at which it occurs are strongly dependent not only on the polymer composition, structure, molecular weight, and concentration, but also on the type of ionic liquid used, the length of the alkyl chain and the substitutions on the imidazolium cation, the nature of the anion, as well as the ratio between ILs mixtures. These polymers, especially crosslinked gels, might have a crucial impact on the development of environmentally stable smart gel materials for various applications. The reader is also referred to exhaustive reviews for further information on thermoresponsive polymers in ILs and their potential applications.182,196–198
7. Other organic solvents
In this last section, examples of polymers exhibiting temperature-induced phase transitions in organic solvents not previously mentioned are discussed (Table 10, and Table S9†). The solvents are displayed in order of polarity.| Solvent | Type of polymer | Polymer conc. | Phase transition type (Temp. °C) | Ref. |
|---|---|---|---|---|
| Diethyl ether | Poly(octadecyl vinyl ether) | 1 wt% | UCST (∼12) | 109 |
| THF | Hyperbranched 3-ethyl-3-(hydroxymethyl)oxetane core/glycidyl-graft-PEG shell copolymers | 5 mg mL−1 | UCST (@13.4–17.4) | 199 |
| THF | Poly(N,N-diethylacrylamide)-b-poly(4-acryloylmorpholine) | 6 wt% | UCST (∼20–40) | 200 |
| THF | Poly-(p-biphenylylmethyl-L-glutamate | 0.62–1.12 mg mL−1 | Chiral optical transition (∼−2.5) | 201 |
| THF | Poly-γ-p-biphenylethoxy-L-glutamate and poly-γ-p-biphenylhexoxy-L-glutamate | ∼20 wt% | α-Helix reorientation (∼7–37) gelation (∼9–39) | 202 |
| 1,4-Dioxane | Poly(11-(4-((E)-4-butylstyryl)phenoxy)undecyl methacrylate) | 2 wt% | UCST (@52.3) | 140 |
| Methyl acetate | Polystyrene | 0.003–0.078 wt% | LCST (@124.55–129.35) and UCST (@26.10–32.70) | 21 |
| Ethyl acetate | Poly(octadecyl vinyl ether-random-isobutyl vinyl ether) | 1 wt% | UCST (∼15) | 109 |
| n-Butyl acetate | Poly(2-chloroethyl vinyl ether-alt-maleic anhydride) | 0.05 wt% | LCST (∼85) | 203 |
| tert-Butyl acetate | Polystyrene | 0.005–0.3 wt% | LCST (@117.9–165.5) and UCST (@−32.5–5.5) | 21 |
| Propyl acetate/DCE | Poly(2-chloroethyl vinyl ether-alt-maleic anhydride) | 0.1 wt% | LCST (∼42–70) | 149 |
| DME | Poly-(methacrylic acid-alt-hydroxyethyl acrylate) | 8 mg mL−1 | LCST (∼18) | 204 |
| DME | Poly(arylene ether sulfone) | 2.5 mg mL−1 | LCST (@37) | 163 |
| DME | poly(arylene ether sulfonate)-b-polylactide | 15 mg mL−1 | LCST (@27.4) | 163 |
| Acetone | Poly(octadecyl vinyl ether-random-isobutyl vinyl ether) | 1 wt% | UCST (∼38) | 109 |
| DMF | Comb-like copolymer of N-phenyl maleimide and n-octadecyl vinyl ether | 1 wt% | UCST (@51) | 117 |
| DMF | Poly(thioether)-b-polysiloxane-b-furfuryl poly(thioether)-bismaleimide elastomer | n/a | De-crosslinking (@150) | 205 |
| DMAc | 4,4′-Sulfonyldiphenol (SDP) based oligourethane derivative based on 1,6-hexamethylene diisocyanate | 8 wt% | Sol–gel transition (ND) | 206 |
| DMSO | 4,4′-Sulfonyldiphenol (SDP) based oligourethane derivative based on 1,6-hexamethylene diisocyanate | 8 wt% | Sol–gel transition (ND) | 206 |
| ACN | Poly(3-(3-butylureido)propyl acrylate-stat-N-[3-(acryloyloxy)propyl]-N,N,N-trihexylammonium tetrakis(3,5-bis(trifluoromethyl)phenyl)borate | 100 mg mL−1 | UCST (@57) | 164 |
The thermoresponsive behaviour of various polymers was also investigated in a wide range of non-chlorinated organic solvents of different polarities. The polymers will be classified based on increasing solvent polarity. While some of the polymers, such as poly(ODVE), have been mentioned in previous paragraphs due to their solution behaviour in other solvents, novel polymer types will also be introduced in this section.
As mentioned before, poly(vinyl ether)s have interesting solution properties depending on the nature of the pendant side chains. Both poly(ODVE) and poly(HDVE) exhibited an UCST phase transition in diethyl ether.109 Moreover, a UCST-type phase transition around 7 °C was observed for poly(ODVE) in tetrahydrofuran (THF).109,147 Similarly, the partially fluorinated poly(vinyl ether) poly(9FVE) also showed the same solution behaviour at around 8 °C, which was strongly affected by the polymer polydispersity and degree of polymerization.153 In another example, poly(vinyl ether)s having imidazolium salt pendants, namely poly[2-(vinyloxy)-ethyl-1-butylimidazolium chloride], showed a LCST at around 40 °C in THF/1-butanol mixtures (86/14% w/w), characterized by a large hysteresis between the heating and the cooling cycles in the transmittance plots.155
Additionally, alternating copolymers of 2-chloroethyl vinyl ether and maleic anhydride synthesized by free radical solution polymerization exhibited a LCST behaviour in THF/hexane mixtures, as shown by static light scattering (SLS) analyses.149,207 The TCP could be tuned by varying the solvents mixture composition, the polymer molecular weight, and the copolymer concentration.207 Since THF was a good solvent for the copolymer and hexane a non-solvent, an increasing amount of THF in the solvent mixture resulted in the shift of the cloud point to higher temperatures and in the widening of the homogeneous one-phase region below the TCP curve. Increasing the THF fraction above 85 wt% resulted in the absence of a phase transition due to the complete solubility of the copolymer in the solvents mixture.149
In another study, core–shell copolymers consisting of a hyperbranched 3-ethyl-3-(hydroxymethyl)oxetane core and a glycidyl-graft-PEG shell (PEG-g-POG) with different DPs of the glycidyl segment exhibited a UCST phase transition in THF, which was attributed to the crystallization of the PEG side chains (Fig. 45).199 The thermoresponsive behaviour was dependent on both the polymer concentration in solution and the DP of the glycidyl segment. To exemplify, the copolymers having PEG side chains with a Mn of 1900 g mol−1 and a glycidyl segment with DP = 5, 8, and 12 showed TCP values of 13.4, 14.5 and 17.4 °C, respectively. The authors believe that these hyperbranched copolymers will help in the design and production of new intelligent materials for specialized applications in the biomedical field.
 | ||
| Fig. 45 Schematic representation of the core–shell copolymers with a hyperbranched 3-ethyl-3-(hydroxymethyl)oxetane core and a glycidyl-graft-PEG shell. Reproduced from ref. 199 Copyright 2021 American Chemical Society. | ||
In a recent report, it has been shown that poly(4-acryloylmorpholine) (PAM) homopolymer with a molecular weight of 117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1 exhibited a UCST behaviour in THF around 10 °C at a polymer concentration of 3 wt%, which increased to 15 °C at 6 wt%.200 Similarly, it was observed that block copolymers consisting of a poly(N,N-diethylacrylamide) block and a poly(4-acryloylmorpholine) segment (PDEA-b-PAM) showed a UCST phase transition in the same solvent, as demonstrated by turbidity and DLS measurements However, the thermoresponsive behaviour was strongly dependent on the molecular weight and on the concentration of the block polymer (Fig. 46). For instance, PDEA-b-PAM with a final molecular weight of 403
300 g mol−1 exhibited a UCST behaviour in THF around 10 °C at a polymer concentration of 3 wt%, which increased to 15 °C at 6 wt%.200 Similarly, it was observed that block copolymers consisting of a poly(N,N-diethylacrylamide) block and a poly(4-acryloylmorpholine) segment (PDEA-b-PAM) showed a UCST phase transition in the same solvent, as demonstrated by turbidity and DLS measurements However, the thermoresponsive behaviour was strongly dependent on the molecular weight and on the concentration of the block polymer (Fig. 46). For instance, PDEA-b-PAM with a final molecular weight of 403![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 showed a UCST phase transition with a TCP of around 10–12 °C at 3 wt%, which increased up to 40 °C at 6 wt%, with a broadening of the transition temperature range from 20 to 40 °C. The broader phase transition range was explained by the presence of the stabilizing THF-soluble PDEA blocks. For other shorter block copolymers and at lower concentrations, a thermoresponsive behaviour in THF was not observed.
500 g mol−1 showed a UCST phase transition with a TCP of around 10–12 °C at 3 wt%, which increased up to 40 °C at 6 wt%, with a broadening of the transition temperature range from 20 to 40 °C. The broader phase transition range was explained by the presence of the stabilizing THF-soluble PDEA blocks. For other shorter block copolymers and at lower concentrations, a thermoresponsive behaviour in THF was not observed.
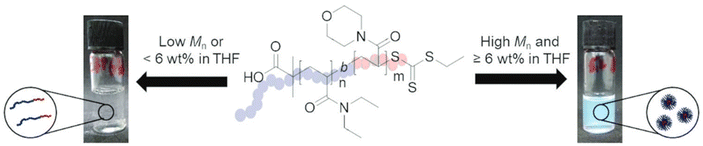 | ||
| Fig. 46 Schematic representation of the aggregation behaviour of poly(N,N-diethylacrylamide)-b-poly(4-acryloylmorpholine) (PDEA-b-PAM) in THF, depending on the polymer molecular weight and concentration. Reproduced from ref. 200 Copyright 2021 The Authors. Macromolecular Rapid Communications published by Wiley-VCH GmbH. | ||
Poly(p-biphenylylmethyl-L-glutamate), PBPMG, exhibited a temperature-induced phase transition in the helicogenic solvent THF at dilute concentrations.201 It has been demonstrated by static and dynamic LS analyses and circular dichroism measurements, that at low temperatures the increasingly poor solvent conditions led to the ordered aggregation of the polymer chains whose association increased in parallel with a decrease in the optical rotation in solution. The increase in temperature resulted in the dissolution of the polymer chains. Poly-γ-p-biphenylethoxy-L-glutamate and poly-γ-p-biphenylhexoxy-L-glutamate, two PBPMG derivatives, showed two thermoresponsive behaviours in THF: a 90° reorientation of the polymer α-helix with respect to the external magnetic field and a thermoreversible gelation, as demonstrated by polarized optical microscopy, NMR and rheometric measurements.202
In another example, it has been shown that linear and star poly(styrene) polymers analysed using thermal field-flow fractionation and DLS, exhibited a temperature-induced phase transition in THF at a concentration of 5 mg mL−1.148 Both techniques demonstrated that the star polymers were more temperature-sensitive than the linear counterparts, which increased with increasing number of arms rather than with the length of the arms. This was explained by a difference in the rearrangement in solution of the linear and star polymers with increasing temperature. To exemplify, linear polymers would expand in a 3D manner when the temperature is raised, leading to an overall smaller size change compared to star polymers, which arms would preferably swell in 1D from the core in solution, resulting in an overall greater change in size. A similar trend but with smaller changes in size was also observed in dimethylacetamide.
Interestingly, the liquid crystalline polymer poly(11-(4-((E)-4-butylstyryl)phenoxy)undecyl methacrylate) is the only example of thermoresponsive polymers in pure 1,4-dioxane that we could find in literature.140 The polymer exhibited a UCST-type phase transition at 52.3 °C at a polymer concentration of 2 wt%, which was demonstrated to be driven by the crystallization of the polymer in the solvent.
The thermoresponsive behaviour of polystyrene homopolymers has been investigated in methyl and ethyl acetate, in which both LCST and UCST-type transitions were observed.21 As an example, a 770 kDa polymer solution in methyl acetate with a concentration of 0.05 wt% exhibited TCP at around 125 and 30 °C for LCST and UCST-type behaviour, respectively, as measured by thermooptical analysis. It is important to notice that the apparatus used for the measurements required samples sealed by a vacuum in order to determine the cloud points of the polymer solutions at the saturated vapor pressure of the solvent, making possible the determination of temperature-induced phase transitions also in low boiling point solvents. Interestingly, 0.05 wt% ethyl acetate solutions of the polystyrene homopolymers only showed LCST-type phase transitions, with TCP decreasing from 165.5 to 147.7 °C with increasing molecular weight from 100 to 600 kDa. A similar thermoresponsive behaviour was also observed in tert-butyl acetate.
As expected, poly(vinyl ether)s present temperature-induced phase transitions also in alkyl acetate solvents. For example, poly(dodecyl vinyl ether) (poly(DDVE)) and poly(cholesteryl 2-(vinyloxy)ethyl carbonate) (poly(ChVE)(Et)) exhibited a UCST transition in ethyl acetate around 45 °C and at 67 °C, respectively (Fig. 47).109 Similarly, a UCST phase transition was observed for random copolymers of IBVE and ODVE at around 15 °C in the same solvent. In another example, the thermoresponsive behaviour of poly[2-(vinyloxy)-ethyl-1-butylimidazolium chloride] was achieved by combining ethyl acetate with a good solvent, 1-butanol (85/15% w/w).155 The polymer exhibited a highly sensitive LCST-type phase transition at around 65 °C in the solvents mixture.
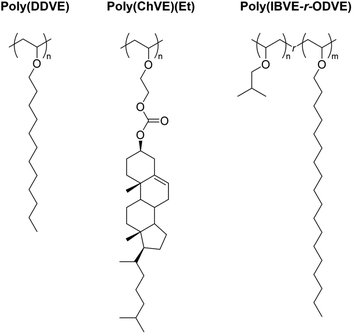 | ||
| Fig. 47 Chemical structures of poly(dodecyl vinyl ether) (poly(DDVE)), poly(cholesteryl 2-(vinyloxy)ethyl carbonate) (poly(ChVE)(Et)), and poly(isobutyl vinyl ether-random-octadecyl vinyl ether) (poly(IBVE-r-ODVE)).109 | ||
An LCST-type phase transition in n-butyl acetate was observed for poly(2-chloroethyl vinyl ether-alt-maleic anhydride) alternated copolymers obtained via free radical solution polymerization, which was attributed to specific polar interactions between the polymer and the solvent.203 The TCP was dependent on the polymer molecular weight and it could be tuned by adding co-solvents to the binary mixture. The addition of non-solvents (i.e. benzene, toluene, cyclohexane, 1,2-dichloroethane, n-octane) resulted in a decrease in the TCP, while it increased when a good solvent (i.e. 2-hexanone, propyl acetate) was added. Interestingly, even when a low amount of DMSO was added (2 wt%), no thermoresponsive behaviour was observed up to 100 °C. These alternated copolymers also exhibited an LCST-type behaviour in propyl acetate/DCE mixtures.149 Since DCE was a non-solvent for the polymer, the increase in the amount of DCE resulted in a decrease in the TCP of the polymer and in the broadening of the hysteresis between heating and cooling cycles in the turbidity plot.
In another example, an alternating copolymer of methacrylic acid (MAA) and hydroxyethyl acrylate (HEA) synthesised via cyclopolymerization of AB-type divinyl monomer with hemiacetal ester spacer followed by hydrolysis, revealed an interesting thermoresponsive behaviour in dimethoxyethane (DME) (Fig. 48).204 It is important to notice that, in the paper, dimethyl ether is mentioned as the solvent used for the analyses. However, due to the low boiling point of dimethyl ether (i.e. – 24 °C) and the absence of information about the sample preparation, we suggest that the authors probably meant dimethoxyethane. The copolymer exhibited a LCST-type phase transition in DME at around 18 °C at a concentration of 8 mg mL−1, suggested to be caused by the balance of two hydrogen bonding interactions: (i) the intra-chain interactions between neighbouring pendants (–COOH and –OH), and (ii) the inter-chain interactions between –COOH pendants. The adjacent hydroxyl group could affect the inter-chain interaction between carboxylic functional groups via H bonding with the –COOH group and/or its solvation, and the interaction preference might be altered with temperature changes, resulting in the thermoresponsive behaviour.
 | ||
| Fig. 48 Schematic depiction of the cyclopolymerization of AB-type divinyl monomer with hemiacetal ester spacer.204 | ||
In order to better understand the thermoresponsiveness of the alternating copolymer, the solution behaviour of pMAA and pHEA homopolymers was investigated.208 While pHEA was completely soluble in DME and failed to show thermoresponsiveness, pMAA exhibited a LCST around 25 °C. At temperatures lower than the LCST, the polymer chains were solvated through the formation of hydrogen bonds between the carboxylic acid groups and the solvent molecules that led to a reduction of the rotational and conformational degrees of freedom of the solvent. When the temperature was increased above the LCST, an entropy-driven desolvation occurred and the polymer chains aggregated through inter and intramolecular hydrogen bonding, resulting in the polymer precipitation.
Poly(arylene ether sulfone) and its diblock copolymers with DL-lactide also underwent LCST-type phase transitions in dimethoxyethane.163 The TCP of the homopolymers increased from 37 to more than 60 °C with increasing molecular weight and decreasing polymer concentration. Moreover, the TCP of the diblock copolymers increased from 27.4 to 45 °C with the decrease in the DP of the PES block from 15 to 10. Reducing the polymer concentration from 15 to 5 mg mL−1 resulted in a further rise of the TCP above 60 °C.
Vinyl ether polymers are intensively studied in various organic solvents and show thermoresponsiveness also in more polar solvents. For example, when a small amount of ODVE was polymerized with IBVE, the resulting random copolymer exhibited a UCST around 38 °C in acetone.109 Similarly, the partially fluorinated poly(9FVE) underwent a UCST around 14 °C in the same polar solvent.153 The comb-like copolymer resulting from the copolymerization of ODVE with N-phenyl maleimide showed a UCST behaviour at 51 °C in DMF due to the aggregation of the long alkyl chains of the vinyl ether monomer at low temperatures, as demonstrated by high resolution 1H NMR analyses in DMF-d7.117 The UCST could be easily tuned by varying the polymer concentration and by adding co-solvents in the binary mixture.
The thermoresponsive behaviour of a series of blue-emitting 4,4′-sulfonyldiphenol (SDP)-based oligourethane derivatives (OUs) based on 1,6-hexamethylene diisocyanate (OUHDI), 4,4′-methylenebis(cyclohexyl isocyanate) (OUHMDI), and 2,4-diisocyanatotoluene (OUTDI) synthesised via a single-pot reaction has been investigated in several organic solvents.206 For example, in solvents with hydrogen-bonding receptor units, such as DMF, dimethyl acetamide-DMAc, and DMSO, the polymers showed a spontaneous thermoreversible gelation, which facilitated the self-assembly of the polymer chains into nanotubes. As a result of the aggregation, these oligomers showed a blue fluorescence, while an enhanced bright deep-blue emission was achieved upon gelation due to inter-chain hydrogen bonding and oxygen cluster interactions. Among all the investigated polymers, OUHDI showed the most efficient thermoreversible gelation at 63 °C in DMF due to the greater ability of the higher symmetric linear structure to crystallize. Furthermore, the temperature-induced gelation was found to be both concentration and time-dependent. It was demonstrated that the gelation occurred more rapidly at high polymer concentrations due to increased inter-chain interactions, and the polymer solution changed from a viscous solution to a gel upon cooling over a period of 48 h. The synthetic approach used to introduce heteroatoms into the SDP-based thermoresponsive polymers might offer a new direction for the design and fabrication of blue luminescent gelators, which could be used in the development of high-quality displays and organic light-emitting diodes.
A thermoresponsive dielectric elastomer, poly(thioether)-b-polysiloxane-b-furfuryl poly(thioether) triblock copolymer, was obtained from the silicon alkoxide catalysed ROP of furfuryl glycidyl ether and carbonyl sulfide followed by the Diels–Alder reaction with bismaleimide (PSiFGE-BMI).205 The triblock copolymer underwent a reversible de-crosslinking reaction when heated up from room temperature to 160 °C in DMF. DSC analyses revealed the appearance of an endothermic peak in the range 120–180 °C, with a maximum heat flow at around 150 °C, indicating the rupture of the crosslinking network. During the cooling process, the appearance of an exothermic peak at around 70 °C confirmed the reversible re-association of the crosslinking network. This study provides a new strategy for the synthesis of homogenous dielectric elastomers, which could have promising applications as actuated devices.
Poly(3-(3-butylureido)propyl acrylate-stat-N-[3-(acryloyloxy)propyl]-N,N,N-trihexylammonium tetrakis(3,5-bis(trifluoromethyl)phenyl)borate copolymers containing self-attractive urea units and self-repulsive lipophilic moieties exhibited a UCST behaviour in acetonitrile (ACN).164 Also in this solvent, the TCP decreased from 57 to 52 °C by increasing the content of the TFPB ion pair from 4 to 5 mol%, due to a more efficient dissolution of the polymer by ionic dissociation.
Finally, thermoresponsive polysiloxanes (TRPSis) synthesised via the catalyst-free aza-Michael addition of poly(aminopropylmethylsiloxane) to NIPAM have shown to undergo a UCST phase transition in ACN, with TCP increasing by lowering the N-isopropyl content due to enhanced hydrogen bonding interactions of the polysiloxanes caused by the presence of more amino groups.96 Moreover, TRPSis also exhibited a UCST in ACN/ethylene carbonate and in ACN/ethyl acetate mixtures. In the former solvent mixture, TRPSis showed higher UCST compared to pure solvents (Fig. 49A), while in the latter case the measured UCST was lower (Fig. 49C). This result was attributed to the co-effect of two Hansen solubility parameters during the mixing process: the energy from the dipolar inter-molecular force between molecules (δp), and the energy from hydrogen bonds between molecules (δh). As can be observed in Fig. 49B, the increase in nethvlene carbonate resulted in decreased δp and increased δh values. On the contrary, upon increasing nethylacetate, δp increased, while δh decreased (Fig. 49D). These polymers could be promising candidates for the application as intelligent catalysts for extraction in non-aqueous conditions.
 | ||
| Fig. 49 (A) UCST and (B) and δp and δh of TRPSis in acetonitrile/ethylene carbonate mixed solvents as a function of the ethylene carbonate content; (C) UCST and (D) δp and δh of TRPSis in acetonitrile/ethyl acetate mixed solvents as a function of the ethyl acetate content. Reproduced from ref. 96 Copyright 2017 Royal Society of Chemistry and the Centre National de la Recherche Scientifique. | ||
In this last section, the temperature-induced solution behaviour of polymers in various non-halogenated organic solvents with different polarities has been discussed. Also in this case, the thermoresponsive behaviour depends on various parameters, including, but not limited to, the polymer concentration and molecular weight, as well as the presence of specific functional groups or atoms, and the resulting intra- and inter-molecular interactions between polymer chains and/or solvent molecules. Using temperature to trigger different solution behaviours in a wide range of solvents with different polarities opens the possibility to design and develop new intelligent materials for a variety of potential applications in, for example, the electronic and biomedical fields.
8. Conclusions
The aim of this review article was to highlight the importance and variety of thermoresponsive polymers in non-aqueous systems present in literature. While the temperature-induced solution behaviour of polymers has been extensively investigated and reported in pure water or water/organic solvent mixtures, polymers showing thermoresponsiveness in other organic solvents are still not very common.As explained above, the terms LCST and UCST refer to exact values corresponding to the minimum or the maximum of the binodal curve in the phase diagram of a polymer/solvent mixture and they should not be confused with cloud point temperatures. TCPs correspond to any value on the binodal curve and refer to the temperature at which phase separation occurs at a specific polymer concentration in solution. For both water and organic solvents, these values are influenced by many parameters, such as the polymer molecular weight and polydispersity, its chemical composition, the presence of specific functional groups on the polymer chains, the addition and ratio of co-solvents, additives, and effectors, the type of solvent, as well as inter- and intra-molecular interactions between macromolecules and solvent molecules. Furthermore, the determination of the TCP values strongly depends on the experimental method used, which makes the comparison of reported data more difficult. Many characterization approaches are used to evaluate the thermoresponsive behaviour of polymers in solution. Among all, the evaluation of the turbidity of a polymer solution upon the increase (or decrease) of temperature is the most common technique used for the determination of the TCP value. Other widely employed methods for the characterization of thermoresponsive polymers are DLS, (variable temperature) NMR spectroscopy, FTIR spectroscopy, SANS, SAXS, and DSC. Additional less common techniques include circular dichroism, rheology and dynamic viscoelasticity measurements. Furthermore, microscopy methods, such as SEM, TEM, cryo-TEM and AFM, are also frequently used for the visualization of morphological changes in the self-assembly of polymers driven by temperature variations.
This detailed summary proves the versatility and potential of thermoresponsive polymers in non-aqueous systems, which show unique solution behaviour in a large variety of solvents with different polarities. However, while several types of potential applications have been proposed and reported for polymers in water and water/organic solvent mixtures, especially in the biomedical and personal care areas, only a few examples for non-aqueous systems are present in literature, mainly in the design of smart functional polymer materials for industrial applications as friction modifiers, and oil thickeners. Nevertheless, the easy synthesis, high stability and the tuneable thermoresponsive properties of these polymers make them promising candidates for their potential application not only in the lubricant industry, but also as smart materials for electronics and for the development of new sensors. Therefore, the understanding of the polymer/solvent relationship, the interactions involved, and the parameters affecting the behaviour in solution is crucial for the design of thermoresponsive polymers in non-aqueous systems. However, apart from the fundamental understanding, the focus should also be aimed to study the potential applications of these systems. Therefore, despite the lack in the literature of potential applications of these systems, we believe that this collection will help the chemistry community to better understand the chemistry required for the design of thermoresponsive polymers in non-aqueous systems and their emerging potential, with the hope that new ideas can arise from scientists reading this article.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.References
- M. A. C. Stuart, W. T. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban and F. Winnik, Emerging applications of stimuli-responsive polymer materials, Nat. Mater., 2010, 9(2), 101–113 CrossRef PubMed.
- P. Theato, B. S. Sumerlin, R. K. O'Reilly and T. H. Epps III, Stimuli responsive materials, Chem. Soc. Rev., 2013, 42(17), 7055–7056 RSC.
- C. De las Heras Alarcón, S. Pennadam and C. Alexander, Stimuli responsive polymers for biomedical applications, Chem. Soc. Rev., 2005, 34(3), 276–285 RSC.
- D. Roy, W. L. Brooks and B. S. Sumerlin, New directions in thermoresponsive polymers, Chem. Soc. Rev., 2013, 42(17), 7214–7243 RSC.
- A. Gandhi, A. Paul, S. O. Sen and K. K. Sen, Studies on Thermoresponsive Polymers: Phase Behaviour, Drug Delivery and Biomedical Applications, Asian J. Pharm. Sci., 2015, 10(2), 99–107 CrossRef.
- V. Aseyev, H. Tenhu and F. M. Winnik, Non-ionic thermoresponsive polymers in water, in Self Organized Nanostructures of Amphiphilic Block Copolymers II. Advances in Polymer Science, ed. A. Müller and O. Borisov, Springer, Berlin, Heidelberg, 2010, vol. 242, pp. 29–89 Search PubMed.
- M. A. Ward and T. K. Georgiou, Thermoresponsive Polymers for Biomedical Applications, Polymers, 2011, 3(3), 1215–1242 CrossRef CAS.
- Y. J. a. M. Kim and Y. T. Matsunaga, Thermo-responsive polymers and their application as smart biomaterials, J. Mater. Chem. B, 2017, 5(23), 4307–4321 RSC.
- F. Doberenz, K. Zeng, C. Willems, K. Zhang and T. Groth, Thermoresponsive polymers and their biomedical application in tissue engineering, J. Mater. Chem. B, 2020, 8(4), 607–628 RSC.
- R. Liu, M. Fraylich and B. R. Saunders, Thermoresponsive copolymers: from fundamental studies to applications, Colloid Polym. Sci., 2009, 287(6), 627–643 CrossRef CAS.
- D. a. R. Crespy and R. M. Rossi, Temperature–responsive polymers with LCST in the physiological range and their applications in textiles, Polym. Int., 2007, 56(12), 1461–1468 CrossRef CAS.
- Y. Kohno, S. Saita, Y. Men, J. Yuan and H. Ohno, Thermoresponsive polyelectrolytes derived from ionic liquids, Polym. Chem., 2015, 6(12), 2163–2178 RSC.
- D. Schmaljohann, Thermo-and pH-responsive polymers in drug delivery, Adv. Drug Delivery Rev., 2006, 58(15), 1655–1670 CrossRef CAS PubMed.
- W. J. Work, K. Horie, M. Hess and R. F. T. Stepto, Definition of terms related to polymer blends, composites, and multiphase polymeric materials (IUPAC Recommendations 2004), Pure Appl. Chem., 2004, 76(11), 1985–2007 CrossRef CAS.
- T. Baltes, F. Garret–Flaudy and R. Freitag, Investigation of the LCST of polyacrylamides as a function of molecular parameters and the solvent composition, J. Polym. Sci., Part A: Polym. Chem., 1999, 37(15), 2977–2989 CrossRef CAS.
- R. E. Bernstein, C. A. Cruz, D. R. Paul and J. W. Barlow, LCST behavior in polymer blends, Macromolecules, 1977, 10(3), 681–686 CrossRef CAS.
- K. Van Durme, H. Rahier and B. Van Mele, Influence of additives on the thermoresponsive behavior of polymers in aqueous solution, Macromolecules, 2005, 38(24), 10155–10163 CrossRef CAS.
- Y. Zhang, S. Furyk, L. B. Sagle, Y. Cho, D. E. Bergbreiter and P. S. Cremer, Effects of Hofmeister anions on the LCST of PNIPAM as a function of molecular weight, J. Phys. Chem. C, 2007, 111(25), 8916–8924 CrossRef CAS PubMed.
- Q. Zhang, C. Weber, U. S. Schubert and R. Hoogenboom, Thermoresponsive polymers with lower critical solution temperature: from fundamental aspects and measuring techniques to recommended turbidimetry conditions, Mater. Horiz., 2017, 4(2), 109–116 RSC.
- A. Halperin, M. Kröger and F. M. Winnik, Poly (N–isopropylacrylamide) phase diagrams: fifty years of research, Angew. Chem., Int. Ed., 2015, 54(51), 15342–15367 CrossRef CAS PubMed.
- Y. C. Bae, S. M. Lambert, D. S. Soane and J. M. Prausnitz, Cloud-point curves of polymer solutions from thermooptical measurements, Macromolecules, 1991, 24(15), 4403–4407 CrossRef CAS.
- C. E. G. C. Boutris, E. G. Chatzi and C. Kiparissides, Characterization of the LCST behaviour of aqueous poly (N-isopropylacrylamide) solutions by thermal and cloud point techniques, Polymer, 1997, 38(10), 2567–2570 CrossRef CAS.
- H. Mao, C. Li, Y. Zhang, S. Furyk, P. S. Cremer and D. E. Bergbreiter, High-throughput studies of the effects of polymer structure and solution components on the phase separation of thermoresponsive polymers, Macromolecules, 2004, 37(3), 1031–1036 CrossRef CAS.
- D. E. Bergbreiter and H. Fu, Thermodynamic cloud point assays, J. Polym. Sci., Part A: Polym. Chem., 2008, 46(1), 186–193 CrossRef CAS.
- S. Jana, S. P. Rannard and A. I. Cooper, Structure–LCST relationships for end-functionalized water-soluble polymers: an “accelerated” approach to phase behaviour studies, Chem. Commun., 2007, 28, 2962–2964 RSC.
- J. C. Meredith, A. Karim and E. J. Amis, High-throughput measurement of polymer blend phase behavior, macromolecules, 2000, 33(16), 5760–5762 CrossRef CAS.
- P. Luchette, N. Abiy and H. Mao, Microanalysis of clouding process at the single droplet level, Sens. Actuators, B, 2007, 128(1), 154–160 CrossRef CAS.
- X. Zhou, J. Li, C. Wu and B. Zheng, Constructing the Phase Diagram of an Aqueous Solution of Poly (N–isopropyl acrylamide) by Controlled Microevaporation in a Nanoliter Microchamber, Macromol. Rapid Commun., 2008, 29(16), 1363–1367 CrossRef CAS.
- M. V. Deshmukh, A. A. Vaidya, M. G. Kulkarni, P. R. Rajamohanan and S. Ganapathy, LCST in poly (N-isopropylacrylamide) copolymers: high resolution proton NMR investigations, Polymer, 2000, 41(22), 7951–7960 CrossRef CAS.
- F. M. Winnik, Fluorescence studies of aqueous solutions of poly (N-isopropylacrylamide) below and above their LCST, Macromolecules, 1990, 23(1), 233–242 CrossRef CAS.
- H. Yamauchi and Y. Maeda, LCST and UCST behavior of poly (N-isopropylacrylamide) in DMSO/water mixed solvents studied by IR and micro-Raman spectroscopy, J. Phys. Chem. B, 2007, 111(45), 12964–12968 CrossRef CAS PubMed.
- S. Y. Lin, K. S. Chen and R. C. Liang, Thermal micro ATR/FT-IR spectroscopic system for quantitative study of the molecular structure of poly (N-isopropylacrylamide) in water, Polymer, 1999, 40(10), 2619–2624 CrossRef CAS.
- M. Sahn, T. Yildirim, M. Dirauf, C. Weber, P. Sungur, S. Hoeppener and U. S. Schubert, LCST behavior of symmetrical PNiPAm-b-PEtOx-b-PNiPAm triblock copolymers, Macromolecules, 2016, 49(19), 7257–7267 CrossRef CAS.
- C. Wu and X. Wang, Globule-to-coil transition of a single homopolymer chain in solution, Phys. Rev. Lett., 1998, 80(18), 4092–4094 CrossRef CAS.
- K. Kanta Sharker, Y. Ohara, Y. Shigeta, S. Ozoe and S. I. Yusa, Upper Critical Solution Temperature (UCST) Behavior of Polystyrene-Based Polyampholytes in Aqueous Solution, Polymer, 2019, 11(2), 265 Search PubMed.
- T. Ougizawa, T. Inoue and H. W. Kammer, UCST and LCST behavior in polymer blends, Macromolecules, 1985, 18(10), 2089–2092 CrossRef CAS.
- K. Van Durme, G. Van Assche and B. Van Mele, Kinetics of demixing and remixing in poly (N-isopropylacrylamide)/water studied by modulated temperature DSC, Macromolecules, 2004, 37(25), 9596–9605 CrossRef CAS.
- S. A. Vshivkov and A. P. Safronov, The conformational coil–globule transition of polystyrene in cyclohexane solution, Macromol. Chem. Phys., 1997, 198(10), 3015–3023 CrossRef CAS.
- J. S. Scarpa, D. D. Mueller and I. M. Klotz, Slow hydrogen-deuterium exchange in a non-. alpha.-helical polyamide, J. Am. Chem. Soc., 1967, 89(24), 6024–6030 CrossRef CAS.
- M. Heskins and J. E. Guillet, Solution properties of poly (N-isopropylacrylamide), J. Macromol. Sci., Part A: Pure Appl.Chem., 1968, 2(8), 1441–1455 CrossRef CAS.
- S. Lanzalaco and E. Armelin, Poly (n-isopropylacrylamide) and copolymers: A review on recent progresses in biomedical applications, Gels, 2017, 3(4), 3 CrossRef PubMed.
- Y. Zhang, S. Furyk, D. E. Bergbreiter and P. S. Cremer, Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series, J. Am. Chem. Soc., 2005, 127(41), 14505–14510 CrossRef CAS PubMed.
- M. A. Haq, Y. Su and D. Wang, Mechanical properties of PNIPAM based hydrogels: A review, Mater. Sci. Eng., C, 2017, 70, 842–855 CrossRef PubMed.
- P. Alexandridis and T. A. Hatton, Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modeling, Colloids Surf., A, 1995, 96(1–2), 1–46 CrossRef CAS.
- J. F. Lutz, Polymerization of oligo (ethylene glycol)(meth) acrylates: Toward new generations of smart biocompatible materials, J. Polym. Sci., Part A: Polym. Chem., 2008, 46(11), 3459–3470 CrossRef CAS.
- J. F. Lutz, Ö. Akdemir and A. Hoth, Point by point comparison of two thermosensitive polymers exhibiting a similar LCST: is the age of poly (NIPAM) over?, J. Am. Chem. Soc., 2006, 128(40), 13046–13047 CrossRef CAS PubMed.
- N. Badi, Non-linear PEG-based thermoresponsive polymer systems, Prog. Polym. Sci., 2017, 66, 54–79 CrossRef CAS.
- C. R. Becer, S. Hahn, M. W. Fijten, H. M. Thijs, R. Hoogenboom and U. S. Schubert, Libraries of methacrylic acid and oligo (ethylene glycol) methacrylate copolymers with LCST behavior, J. Polym. Sci., Part A: Polym. Chem., 2008, 46(21), 7138–7147 CrossRef CAS.
- J. Huang and A. Heise, Stimuli responsive synthetic polypeptides derived from N-carboxyanhydride (NCA) polymerisation, Chem. Soc. Rev., 2013, 42(17), 7373–7390 RSC.
- R. Hoogenboom, H. M. Thijs, M. J. Jochems, B. M. van Lankvelt, M. W. Fijten and U. S. Schubert, Tuning the LCST of poly(2-oxazoline) s by varying composition and molecular weight: alternatives to poly(N-isopropylacrylamide)?, Chem. Commun., 2008,(44), 5758–5760 RSC.
- R. Hoogenboom and H. Schlaad, Thermoresponsive poly(2-oxazoline) s, polypeptoids, and polypeptides, Polym. Chem., 2017, 8(1), 24–40 RSC.
- F. A. Plamper, M. Ruppel, A. Schmalz, O. Borisov, M. Ballauff and A. H. Müller, Tuning the thermoresponsive properties of weak polyelectrolytes: aqueous solutions of star-shaped and linear poly (N, N-dimethylaminoethyl methacrylate), Macromolecules, 2007, 40(23), 8361–8366 CrossRef CAS.
- H. Uyama and S. Kobayashi, A novel thermo-sensitive polymer. Poly(2-iso-propyl-2-oxazoline), Chem. Lett., 1992, 21(9), 1643–1646 CrossRef.
- N. A. Cortez-Lemus and A. Licea-Claverie, Poly (N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular, Prog. Polym. Sci., 2016, 53, 1–51 CrossRef CAS.
- J. Niskanen and H. Tenhu, How to manipulate the upper critical solution temperature (UCST)?, Polym. Chem., 2017, 8(1), 220–232 RSC.
- J. Seuring and S. Agarwal, Polymers with upper critical solution temperature in aqueous solution, Macromol. Rapid Commun., 2012, 33(22), 1898–1920 CrossRef CAS PubMed.
- F. M. Winnik, H. Ringsdorf and J. Venzmer, Methanol-water as a co-nonsolvent system for poly (N-isopropylacrylamide), Macromolecules, 1990, 23(8), 2415–2416 CrossRef CAS.
- H. G. Schild, M. Muthukumar and D. A. Tirrell, Cononsolvency in mixed aqueous solutions of poly (N-isopropylacrylamide), Macromolecules, 1991, 24(4), 948–952 CrossRef CAS.
- S. Li, Y. Su, M. Dan and W. Zhang, Thermo-responsive ABA triblock copolymer of PVEA-b-PNIPAM-b-PVEA showing solvent-tunable LCST in a methanol–water mixture, Polym. Chem., 2014, 5(4), 1219–1228 RSC.
- L. Liu, T. Wang, C. Liu, K. Lin, G. Liu and G. Zhang, Specific Anion Effect in Water–Nonaqueous Solvent Mixtures: Interplay of the Interactions between Anion, Solvent, and Polymer, J. Phys. Chem. B, 2013, 117(37), 10936–10943 CrossRef CAS PubMed.
- F. Tanaka, T. Koga, H. Kojima, N. Xue and F. M. Winnik, Preferential adsorption and co-nonsolvency of thermoresponsive polymers in mixed solvents of water/methanol, Macromolecules, 2011, 44(8), 2978–2989 CrossRef CAS.
- M. J. Hore, B. Hammouda, Y. Li and H. Cheng, Co-nonsolvency of poly (n-isopropylacrylamide) in deuterated water/ethanol mixtures, Macromolecules, 2013, 46(19), 7894–7901 CrossRef CAS.
- I. Bischofberger, D. C. E. Calzolari, P. De Los Rios, I. Jelezarov and V. Trappe, Hydrophobic hydration of poly-N-isopropyl acrylamide: a matter of the mean energetic state of water, Sci. Rep., 2014, 4(1), 1–7 Search PubMed.
- R. Takahashi, X. P. Qiu, N. Xue, T. Sato, K. Terao and F. M. Winnik, Self-Association of the thermosensitive block copolymer poly (2-isopropyl-2-oxazoline)-b-poly (N-isopropylacrylamide) in water–methanol mixtures, Macromolecules, 2014, 47(19), 6900–6910 CrossRef CAS.
- D. Mukherji, M. Wagner, M. D. Watson, S. Winzen, T. E. de Oliveira, C. M. Marques and K. Kremer, Relating side chain organization of PNIPAm with its conformation in aqueous methanol, Soft Matter, 2016, 12(38), 7995–8003 RSC.
- H. A. Pérez-Ramírez, C. Haro-Pérez and G. Odriozola, Effect of Temperature on the Cononsolvency of Poly (N-isopropylacrylamide)(PNIPAM) in Aqueous 1-Propanol, ACS Appl. Polym. Mater., 2019, 1(11), 2961–2972 CrossRef.
- W. M. Wan, P. Zhou, F. Cheng, X. L. Sun, X. H. Lv, K. K. Li, H. Xu, M. Sun and F. Jäkle, Thermo-responsive behavior of borinic acid polymers: experimental and molecular dynamics studies, Soft Matter, 2015, 11(36), 7159–7164 RSC.
- T. Munk, S. Hietala, K. Kalliomäki, M. Nuopponen, H. Tenhu, F. Tian, J. Rantanen and S. Baldursdóttir, Behaviour of stereoblock poly (N-isopropyl acrylamide) in acetone–water mixtures, Polym. Bull., 2011, 67(4), 677–692 CrossRef CAS.
- G. Dalkas, K. Pagonis and G. Bokias, Control of the lower critical solution temperature—type cononsolvency properties of poly (N-isopropylacrylamide) in water—dioxane mixtures through copolymerisation with acrylamide, Polymer, 2006, 47(1), 243–248 CrossRef CAS.
- K. Pagonis and G. Bokias, Temperature-and solvent-sensitive hydrogels based on N-isopropylacrylamide and N, N-dimethylacrylamide, Polym. Bull., 2007, 58(1), 289–294 CrossRef CAS.
- K. Pagonis and G. Bokias, Simultaneous lower and upper critical solution temperature–type co–nonsolvency behaviour exhibited in water–dioxane mixtures by linear copolymers and hydrogels containing N-isopropylacrylamide and N, N–dimethylacrylamide, Polym. Int., 2006, 55(11), 1254–1258 CrossRef CAS.
- K. Pagonis and G. Bokias, Upper critical solution temperature—type cononsolvency of poly(N, N-dimethylacrylamide) in water—organic solvent mixtures, Polymer, 2004, 45(7), 2149–2153 CrossRef CAS.
- A. Asadujjaman, V. Ahmadi, M. Yalcin, N. ten Brummelhuis and A. Bertin, Thermoresponsive functional polymers based on 2, 6-diaminopyridine motif with tunable UCST behaviour in water/alcohol mixtures, Polym. Chem., 2017, 8(20), 3140–3153 RSC.
- N. Lucht, S. Eggers and V. Abetz, Cononsolvency in the ‘drunken'state: the thermoresponsiveness of a new acrylamide copolymer in water–alcohol mixtures, Polym. Chem., 2017, 8(7), 1196–1205 RSC.
- A. Asadujjaman, T. E. de Oliveira, D. Mukherji and A. Bertin, Polyacrylamide “revisited”: UCST-type reversible thermoresponsive properties in aqueous alcoholic solutions, Soft Matter, 2018, 14(8), 1336–1343 RSC.
- J. M. Cowie, M. A. Mohsin and I. J. McEwen, Alcohol-water cosolvent systems for poly (methyl methacrylate), Polymer, 1987, 28(9), 1569–1572 CrossRef CAS.
- R. Hoogenboom, C. R. Becer, C. Guerrero-Sanchez, S. Hoeppener and U. S. Schubert, Solubility and thermoresponsiveness of PMMA in alcohol-water solvent mixtures, Aust. J. Chem., 2010, 63(8), 1173–1178 CrossRef CAS.
- Q. Zhang, P. Schattling, P. Theato and R. Hoogenboom, Tuning the upper critical solution temperature behavior of poly (methyl methacrylate) in aqueous ethanol by modification of an activated ester comonomer, Polym. Chem., 2012, 3(6), 1418–1426 RSC.
- Q. Zhang, P. Schattling, P. Theato and R. Hoogenboom, UV-tunable upper critical solution temperature behavior of azobenzene containing poly (methyl methacrylate) in aqueous ethanol, Eur. Polym. J., 2015, 62, 435–441 CrossRef CAS.
- R. Hoogenboom, S. Rogers, A. Can, C. R. Becer, C. Guerrero-Sanchez, D. Wouters, S. Hoeppener and U. S. Schubert, Self-assembly of double hydrophobic block copolymers in water–ethanol mixtures: from micelles to thermoresponsive micellar gels, Chem. Commun., 2009,(37), 5582–5584 RSC.
- Q. Fang, T. Chen, Q. Zhong and J. Wang, Thermoresponsive polymers based on oligo (ethylene glycol) methyl ether methacrylate and modified substrates with thermosensitivity, Macromol. Res., 2017, 25(3), 206–213 CrossRef CAS.
- P. J. Roth, F. D. Jochum and P. Theato, UCST-type behavior of poly [oligo (ethylene glycol) methyl ether methacrylate](POEGMA) in aliphatic alcohols: solvent, co-solvent, molecular weight, and end group dependences, Soft Matter, 2011, 7(6), 2484–2492 RSC.
- P. J. Roth, M. Collin and C. Boyer, Advancing the boundary of insolubility of non-linear PEG-analogues in alcohols: UCST transitions in ethanol–water mixtures, Soft Matter, 2013, 9(6), 1825–1834 RSC.
- A. Can, Q. Zhang, T. Rudolph, F. H. Schacher, J. F. Gohy, U. S. Schubert and R. Hoogenboom, Schizophrenic thermoresponsive block copolymer micelles based on LCST and UCST behavior in ethanol–water mixtures, Eur. Polym. J., 2015, 69, 460–471 CrossRef CAS.
- A. Kuila, N. Maity, D. P. Chatterjee and A. K. Nandi, Phase Behavior of Poly (vinylidene fluoride)-graft-poly (diethylene glycol methyl ether methacrylate) in Alcohol–Water System: Coexistence of LCST and UCST, J. Phys. Chem. B, 2016, 120(9), 2557–2568 CrossRef CAS PubMed.
- H. M. L. Lambermont-Thijs, H. P. C. van Kuringen, J. P. W. van der Put, U. S. Schubert and R. Hoogenboom, Temperature Induced Solubility Transitions of Various Poly(2-Oxazoline)s in Ethanol-Water Solvent Mixtures, Polymers, 2010, 2(3), 188–199 CrossRef CAS.
- C. Diehl, I. Dambowsky, R. Hoogenboom and H. Schlaad, Self–Assembly of Poly (2−alkyl–2−oxazoline)s by Crystallization in Ethanol–Water Mixtures Below the Upper Critical Solution Temperature, Macromol. Rapid Commun., 2011, 32(21), 1753–1758 CrossRef CAS PubMed.
- R. Hoogenboom, H. M. Thijs, D. Wouters, S. Hoeppener and U. S. Schubert, Tuning solution polymer properties by binary water–ethanol solvent mixtures, Soft Matter, 2008, 4(1), 103–107 RSC.
- R. Hoogenboom, H. M. Lambermont-Thijs, M. J. Jochems, S. Hoeppener, C. Guerlain, C. A. Fustin, J. F. Gohy and U. S. Schubert, A Schizophrenic Gradient Copolymer: Switching and Reversing Poly(2-Oxazoline) Micelles Based on UCST and Subtle Solvent Changes, Soft Matter, 2009, 5(19), 3590–3592 RSC.
- H. M. L. Lambermont–Thijs, R. Hoogenboom, C. A. Fustin, C. Bomal–D'Haese, J. F. Gohy and U. S. Schubert, Solubility Behavior of Amphiphilic Block and Random Copolymers Based on 2-Ethyl-2-Oxazoline and 2-Nonyl-2-Oxazoline in Binary Water–Ethanol Mixtures, J. Polym. Sci., Part A: Polym. Chem., 2009, 47(2), 515–522 CrossRef.
- W. Liu, M. Zhu, J. Xiao, Y. Ling and H. Tang, Synthesis and UCST–type phase behavior of polypeptide with alkyl side–chains in alcohol or ethanol/water solvent mixtures, J. Polym. Sci., Part A: Polym. Chem., 2016, 54(21), 3425–3435 CrossRef CAS.
- M. Li, X. Wang, Y. Xu, Y. Ling and H. Tang, Preparation of glycopolypeptides bearing mannose moieties and biphenyl pendants and their upper–critical–solution–temperature–type thermoresponsive properties in alcohol/water solvent mixtures, Polym. Int., 2016, 65(12), 1493–1500 CrossRef CAS.
- C. Ge, W. Liu, Y. Ling and H. Tang, Synthesis and thermoresponsive properties of OEGylated polypeptide with a LCST at body temperature in water and with a UCST in alcohol or ethanol/water solvent mixture, J. Polym. Sci., Part A: Polym. Chem., 2018, 56(2), 163–173 CrossRef CAS.
- W. M. Wan, F. Cheng and F. Jäkle, A Borinic Acid Polymer with Fluoride Ion–and Thermo–responsive Properties that are Tunable over a Wide Temperature Range, Angew. Chem., Int. Ed., 2014, 53(34), 8934–8938 CrossRef CAS PubMed.
- R. Zhu, M. K. Baraniak, F. Jäkle and G. Liu, Anion Specificity in Dimethyl Sulfoxide–Water Mixtures Exemplified by a Thermosensitive Polymer, J. Phys. Chem. B, 2018, 122(34), 8293–8300 CrossRef CAS PubMed.
- S. Li, L. Feng, H. Lu and S. Feng, From LCST to UCST: the phase separation behaviour of thermo-responsive polysiloxanes with the solubility parameters of solvents, New J. Chem., 2017, 41(5), 1997–2003 RSC.
- Q. Zhang and R. Hoogenboom, Polymers with upper critical solution temperature behavior in alcohol/water solvent mixtures, Prog. Polym. Sci., 2015, 48, 122–142 CrossRef CAS.
- M. Zhu, Y. Xu, C. Ge, Y. Ling and H. Tang, Synthesis and UCST–type phase behavior of OEG ylated poly (γ–benzyl–l–glutamate) in organic media, J. Polym. Sci., Part A: Polym. Chem., 2016, 54(10), 1348–1356 CrossRef CAS.
- M. Li, Y. Xu, T. Liu, Y. Li, Y. Ling and H. Tang, Preparation and Thermoresponsive Properties of UCST–Type Polypeptide Bearing p–Tolyl Pendants and 3−Methyl–1, 2, 3−triazolium Linkages in Methanol or Ethanol/Water Solvent Mixtures, Macromol. Chem. Phys., 2017, 218(10), 1700006 CrossRef.
- X. Wang, C. Ge, Y. Ling and H. Tang, Preparation and UCST-type phase behavior of glycopolypeptides in alcoholic solvents, RSC Adv., 2015, 5(130), 108023–108029 RSC.
- K. Wang, Q. Liu, G. Liu and Y. Zeng, Novel thermoresponsive homopolymers of Poly [oligo (ethylene glycol)(acyloxy) methacrylate] s: LCST-type transition in water and UCST-type transition in alcohols, Polymer, 2020, 122746 CrossRef CAS.
- Y. Xiong, J. Liu, Y. Wang, H. Wang and R. Wang, One–Step Synthesis of Thermosensitive Nanogels Based on Highly Cross–Linked Poly(ionic liquid)s, Angew. Chem., Int. Ed., 2012, 51(36), 9114–9118 CrossRef CAS PubMed.
- D. H. Seuyep N., G. A. Luinstra and P. Theato, Post-polymerization modification of reactive polymers derived from vinylcyclopropane: 1. synthesis and thermo-responsive behaviour, Polym. Chem., 2013, 4(9), 2724–2730 RSC.
- A. Sehlinger, O. Kreye and M. A. Meier, Tunable polymers obtained from Passerini multicomponent reaction derived acrylate monomers, Macromolecules, 2013, 46(15), 6031–6037 CrossRef CAS.
- L. Zhao, L. Zhang, Z. Zheng, Y. Ling and H. Tang, Synthesis and Properties of UCST–Type Thermo–and Light–Responsive Homopolypeptides with Azobenzene Spacers and Imidazolium Pendants, Macromol. Chem. Phys., 2019, 220(12), 1900061 CrossRef.
- K. Wang, Q. Liu, G. Lu, Y. Zhang, Y. Zhou, S. Chen, Q. Ma, G. Liu and Y. Zeng, Acid-Labile Temperature-Responsive Homopolymers and a Diblock Copolymer Bearing the Pendent Acetal Group, Macromolecules, 2021, 54(8), 3725–3734 CrossRef CAS.
- Q. Zhang and R. Hoogenboom, UCST behavior of polyampholytes based on stoichiometric RAFT copolymerization of cationic and anionic monomers, Chem. Commun., 2015, 51(1), 70–73 RSC.
- Y. Pei, N. C. Dharsana, J. A. Van Hensbergen, R. P. Burford, P. J. Roth and A. B. Lowe, RAFT dispersion polymerization of 3-phenylpropyl methacrylate with poly [2-(dimethylamino) ethyl methacrylate] macro-CTAs in ethanol and associated thermoreversible polymorphism, Soft Matter, 2014, 10(31), 5787–5796 RSC.
- K. I. Seno, A. Date, S. Kanaoka and S. Aoshima, Synthesis and solution properties of poly (vinyl ether) s with long alkyl chain, biphenyl, and cholesteryl pendants, J. Polym. Sci., Part A: Polym. Chem., 2008, 46(13), 4392–4406 CrossRef CAS.
- M. Zhu, W. Liu, J. Xiao, Y. Ling and H. Tang, Synthesis and UCST–type phase behaviors of OEGylated random copolypeptides in alcoholic solvents, J. Polym. Sci., Part A: Polym. Chem., 2016, 54(21), 3444–3453 CrossRef CAS.
- K. Dušek, Solubility of poly (2-hydroxyethyl methacrylate) in some aliphatic alcohols, Collect. Czech. Chem. Commun., 1969, 34(11), 3309–3317 CrossRef.
- M. Dan, Y. Su, X. Xiao, S. Li and W. Zhang, A new family of thermo-responsive polymers based on poly [N-(4-vinylbenzyl)-N, N-dialkylamine], Macromolecules, 2013, 46(8), 3137–3146 CrossRef CAS.
- Á. Szabó, G. Bencskó, G. Szarka and B. Iván, Thermoresponsive UCST–Type Behavior of Interpolymer Complexes of Poly (ethylene glycol) and Poly (poly (ethylene glycol) methacrylate) Brushes with Poly (acrylic acid) in Isopropanol, Macromol. Chem. Phys., 2017, 218(5), 1600466 CrossRef.
- T. Terashima, M. Ouchi, T. Ando, M. Kamigaito and M. Sawamoto, Amphiphilic, thermosensitive ruthenium(II)-bearing star polymer catalysts: one-pot synthesis of PEG armed star polymers with ruthenium(II)-enclosed microgel cores via metal-catalyzed living radical polymerization, Macromolecules, 2007, 40(10), 3581–3588 CrossRef CAS.
- P. J. Roth, T. P. Davis and A. B. Lowe, UCST-driven self-assembly and crosslinking of diblock copolymer micelles, Polym. Chem., 2012, 3(8), 2228–2235 RSC.
- S. Cheng, Y. Xue, Y. Lu, X. Li and J. Dong, Thermoresponsive pyrrolidone block copolymer organogels from 3D micellar networks, ACS Omega, 2017, 2(1), 105–112 CrossRef CAS PubMed.
- Z. Liu, K. Inomata and Y. Guo, Thermoreversible UCST-type phase behavior of comb-like poly (N-phenyl maleimide-co-n-octadecyl vinyl ether) in organic media, Colloid Polym. Sci., 2011, 289(17–18), 1917–1925 CrossRef CAS.
- V. García Sakai, J. S. Higgins and J. P. M. Trusler, Cloud curves of polystyrene or poly (methyl methacrylate) or poly (styrene-co-methyl methacrylate) in cyclohexanol determined with a thermo-optical apparatus, J. Chem. Eng. Data, 2006, 51(2), 743–748 CrossRef.
- S. Sasaki, M. Hikata, C. Shiraki and I. Uematsu, Molecular Aggregation and Gelation in Poly (y-benzyl L-glutamate) Solutions, Polym. J., 1982, 14(3), 205–213 CrossRef CAS.
- X. Fu, C. Xing and J. Sun, Tunable LCST/UCST-Type Polypeptoids and Their Structure–Property Relationship, Biomacromolecules, 2020, 21(12), 4980–4988 CrossRef CAS PubMed.
- Y. Di, X. Ma, C. Li, H. Liu, X. Fan, M. Wang, H. Deng, T. Jiang, Z. Yin and K. Deng, A New Thermosensitive Poly(N–propionyl–aspartic acid/ethylene glycol) with No Cytotoxicity and Tunable UCST, Macromol. Chem. Phys., 2014, 215(4), 365–371 CrossRef CAS.
- T. Gelbrich, M. Feyen and A. M. Schmidt, Magnetic thermoresponsive core−shell nanoparticles, Macromolecules, 2006, 39(9), 3469–3472 CrossRef CAS.
- Y. Su, M. Dan, X. Xiao, X. Wang and W. Zhang, A new thermo–responsive block copolymer with tunable upper critical solution temperature and lower critical solution temperature in the alcohol/water mixture, J. Polym. Sci., Part A: Polym. Chem., 2013, 51(20), 4399–4412 CrossRef CAS.
- D. L. Ho, B. Hammouda, S. R. Kline and W. R. Chen, Unusual phase behavior in mixtures of poly(ethylene oxide) and ethyl alcohol, J. Polym. Sci., Part B: Polym. Phys., 2006, 44(3), 557–564 CrossRef CAS.
- A. Can, S. Hoeppener, P. Guillet, J. F. Gohy, R. Hoogenboom and U. S. Schubert, Upper critical solution temperature switchable micelles based on polystyrene–block–poly (methyl acrylate) block copolymers, J. Polym. Sci., Part A: Polym. Chem., 2011, 49(17), 3681–3687 CrossRef CAS.
- D. H. Seuyep N., D. Szopinski, G. A. Luinstra and P. Theato, Post-polymerization modification of reactive polymers derived from vinylcyclopropane: a poly (vinylcyclopropane) derivative with physical gelation and UCST behaviour in ethanol–water mixtures, Polym. Chem., 2014, 5(19), 5823–5828 RSC.
- G. B. Chua, P. J. Roth, H. T. Duong, T. P. Davis and A. B. Lowe, Synthesis and Thermoresponsive Solution Properties of Poly[oligo (ethylene glycol)(meth) acrylamide]s: Biocompatible PEG Analogues, Macromolecules, 2012, 45(3), 1362–1374 CrossRef CAS.
- P. J. Roth, T. P. Davis and A. B. Lowe, Comparison between the LCST and UCST transitions of double thermoresponsive diblock copolymers: insights into the behavior of POEGMA in alcohols, Macromolecules, 2012, 45(7), 3221–3230 CrossRef CAS.
- Y. Gu, L. Liu, Y. Wang, C. Zhang, H. Dong and T. Aoki, Thermotropic, Reversible, and Highly Selective One-Handed Helical Structure of Hydroxyl Group-Containing Poly (phenylacetylene) s and Its Static Memory, Macromolecules, 2021, 54(21), 10216–10223 CrossRef CAS.
- S. Saeki, N. Kuwahara, S. Konno and M. Kaneko’, Upper and lower critical solution temperatures in polystyrene solutions, Macromolecules, 1973, 6(2), 246–250 CrossRef CAS.
- I. LaRue, M. Adam, M. Pitsikalis, N. Hadjichristidis, M. Rubinstein and S. S. Sheiko, Reversible Morphological Transitions of Polystyrene-b-Polyisoprene Micelles, Macromolecules, 2006, 39(1), 309–314 CrossRef CAS.
- Y. Pei, O. R. Sugita, L. Thurairajah and A. B. Lowe, Synthesis of Poly(Stearyl Methacrylate-b-3-Phenylpropyl Methacrylate) Nanoparticles in n-Octane and Associated Thermoreversible Polymorphism, RSC Adv., 2015, 5(23), 17636–17646 RSC.
- L. A. Fielding, J. A. Lane, M. J. Derry, O. O. Mykhaylyk and S. P. Armes, Thermo-Responsive Diblock Copolymer Worm Gels in Non-Polar Solvents, J. Am. Chem. Soc., 2014, 136(15), 5790–5798 CrossRef CAS PubMed.
- M. T. Savoji, D. Zhao, R. J. Muisener, K. Schimossek, K. Schoeller, T. P. Lodge and M. A. Hillmyer, Poly(Alkyl Methacrylate)-Grafted Polyolefins as Viscosity Modifiers for Engine Oil: A New Mechanism for Improved Performance, Ind. Eng. Chem. Res., 2018, 57(6), 1840–1850 CrossRef CAS.
- L. P. Ratcliffe, B. E. McKenzie, G. M. Le Bouëdec, C. N. Williams, S. L. Brown and S. P. Armes, Polymerization-Induced Self-Assembly of All-Acrylic Diblock Copolymers via RAFT Dispersion Polymerization in Alkanes, Macromolecules, 2015, 48(23), 8594–8607 CrossRef CAS.
- Y. Pei, L. Thurairajah, O. R. Sugita and A. B. Lowe, RAFT dispersion polymerization in nonpolar media: Polymerization of 3-phenylpropyl methacrylate in n-tetradecane with poly (stearyl methacrylate) homopolymers as macro chain transfer agents, Macromolecules, 2015, 48(1), 236–244 CrossRef CAS.
- R. R. Gibson, A. Fernyhough, O. M. Musa and S. P. Armes, RAFT dispersion polymerization of N, N-dimethylacrylamide in a series of n-alkanes using a thermoresponsive poly (tert-octyl acrylamide) steric stabilizer, Polym. Chem., 2021, 12(14), 2165–2174 RSC.
- M. Naya, K. Kokado and K. Sada, Triple Thermoresponsiveness of a TADDOL-Based Homopolymer through the Formation of Supramolecular Complexes with Chiral Guest Molecules at Variable Ratios, ACS Appl. Polym. Mater., 2020, 2(11), 4415–4424 CrossRef CAS.
- K. Nishimori, E. Cazares-Cortes, J. M. Guigner, F. Tournilhac and M. Ouchi, Physical gelation of AB-alternating copolymers made of vinyl phenol and maleimide units: cooperation between precisely incorporated phenol and long alkyl pendant groups, Polym. Chem., 2019, 10(18), 2327–2336 RSC.
- Y. Yu, G. Shao and W. Zhang, A crystallization driven thermoresponsive transition in a liquid crystalline polymer, Polym. Chem., 2021, 12(39), 5662–5667 RSC.
- S. Abbas, Z. Li, H. Hassan and T. P. Lodge, Thermoreversible Morphology Transitions of Poly(Styrene-b-Dimethylsiloxane) Diblock Copolymer Micelles in Dilute Solution, Macromolecules, 2007, 40(11), 4048–4052 CrossRef CAS.
- N. Merlet-Lacroix, E. Di Cola and M. Cloitre, Swelling and Rheology of Thermoresponsive Gradient Copolymer Micelles, Soft Matter, 2010, 6(5), 984–993 RSC.
- M. J. Derry, O. O. Mykhaylyk and S. P. Armes, A Vesicle-to-Worm Transition Provides a New High-Temperature Oil Thickening Mechanism, Angew. Chem., Int. Ed., 2017, 56(7), 1746–1750 CrossRef CAS PubMed.
- W. Fu, W. Bai, S. Jiang, B. T. Seymour and B. Zhao, UCST-Type Thermoresponsive Polymers in Synthetic Lubricating Oil Polyalphaolefin (PAO), Macromolecules, 2018, 51(5), 1674–1680 CrossRef CAS.
- M. J. Rymaruk, C. T. O'Brien, S. L. Brown, C. N. Williams and S. P. Armes, Effect of Core Cross-Linking on the Physical Properties of Poly(Dimethylsiloxane)-Based Diblock Copolymer Worms Prepared in Silicone Oil, Macromolecules, 2019, 52(18), 6849–6860 CrossRef CAS.
- M. Concilio, N. Nguyen and C. R. Becer, Oxazoline-Methacrylate Graft-Copolymers with Upper Critical Solution Temperature Behaviour in Yubase Oil, Polym. Chem., 2021, 12(30), 4359–4371 RSC.
- T. Yoshida, K. I. Seno, S. Kanaoka and S. Aoshima, Stimuli-Responsive Reversible Physical Networks. I. Synthesis and Physical Network Properties of Amphiphilic Block and Random Copolymers with Long Alkyl Chains by Living Cationic Polymerization, J. Polym. Sci., Part A: Polym. Chem., 2005, 43(6), 1155–1165 CrossRef CAS.
- G. Greyling, A. Lederer and H. Pasch, Thermal Field–Flow Fractionation for the Investigation of the Thermoresponsive Nature of Star and Linear Polystyrene, Macromol. Chem. Phys., 2018, 219(24), 1800417 CrossRef.
- Z. Liu, Y. Guo and K. Inomata, Lower critical solution temperature behavior of poly (2-chloroethyl vinyl ether-alt-maleic anhydride) in organic media, Polym. J., 2010, 42(11), 901–904 CrossRef CAS.
- Y. Guo, Z. Liu and K. Inomata, Thermoresponsive phase behavior of poly (2-chloroethyl vinyl ether-alt-maleic anhydride) solutions in propyl acetate/n-alkane mixed solvents, Fluid Phase Equilib., 2012, 315, 29–34 CrossRef CAS.
- C. Clamor, B. N. Cattoz, P. M. Wright, R. K. O'Reilly and A. P. Dove, Controlling the crystallinity and solubility of functional PCL with efficient post-polymerisation modification, Polym. Chem., 2021, 12(13), 1983–1990 RSC.
- A. Salonen, D. Langevin and P. Perrin, Light and temperature bi-responsive emulsion foams, Soft Matter, 2010, 6(21), 5308–5311 RSC.
- H. Shimomoto, D. Fukami, S. Kanaoka and S. Aoshima, Fluorinated vinyl ether homopolymers and copolymers: Living cationic polymerization and temperature–induced solubility transitions in various organic solvents including perfluoro solvents, J. Polym. Sci., Part A: Polym. Chem., 2011, 49(9), 2051–2058 CrossRef CAS.
- A. Niehoff, A. Mantion, R. McAloney, A. Huber, J. Falkenhagen, C. M. Goh, A. F. Thünemann, M. A. Winnik and H. Menzel, Elucidation of the structure of poly(γ-benzyl-l-glutamate) nanofibers and gel networks in a helicogenic solvent, Colloid Polym. Sci., 2013, 291(6), 1353–1363 CrossRef CAS PubMed.
- K. I. Seno, S. Kanaoka and S. Aoshima, Synthesis and LCST–type phase separation behavior in organic solvents of poly (vinyl ethers) with pendant imidazolium or pyridinium salts, J. Polym. Sci., Part A: Polym. Chem., 2008, 46(17), 5724–5733 CrossRef CAS.
- S. Montolio, L. Gonzáez, B. Altava, H. Tenhu, M. I. Burguete, E. García-Verdugo and S. V. Luis, LCST-type polymers based on chiral-polymeric ionic liquids, Chem. Commun., 2014, 50(73), 10683–10686 RSC.
- E. Sato, Y. Masuda, J. Kadota, T. Nishiyama and H. Horibe, Dual stimuli-responsive homopolymers: Thermo-and photo-responsive properties of coumarin-containing polymers in organic solvents, Eur. Polym. J., 2015, 69, 605–615 CrossRef CAS.
- X. Guan, S. Wang, G. Shi, J. Zhang and X. Wan, Thermoswitching of Helical Inversion of Dynamic Polyphenylacetylenes through cis-trans Isomerization of Amide Pendants, Macromolecules, 2021, 54(10), 4592–4600 CrossRef CAS.
- S. Amemori, K. Kokado and K. Sada, Fundamental molecular design for precise control of thermoresponsiveness of organic polymers by using ternary systems, J. Am. Chem. Soc., 2012, 134(20), 8344–8347 CrossRef CAS PubMed.
- M. Naya, K. Kokado, K. B. Landenberger, S. Kanaoka, S. Aoshima and K. Sada, Supramolecularly Designed Thermoresponsive Polymers in Different Polymer Backbones, Macromol. Chem. Phys., 2020, 221(5), 1900455 CrossRef CAS.
- S. Amemori, K. Kokado and K. Sada, Polymer Phase–Transition Behavior Driven by a Charge–Transfer Interaction, Angew. Chem., Int. Ed., 2013, 52(15), 4174–4178 CrossRef CAS PubMed.
- Z. Cao, G. Ma, M. Leng, S. Zhang, J. Chen, C. Do, K. Hong, L. Fang and X. Gu, Variable-Temperature Scattering and Spectroscopy Characterizations for Temperature-Dependent Solution Assembly of PffBT4T-Based Conjugated Polymers, ACS Appl. Polym. Mater., 2022, 4(5), 3023–3033 CrossRef CAS.
- J. Lee, B. Lee, J. Park, J. Oh, T. Kim, M. Seo and S. Y. Kim, Synthesis and phase transition behavior of well-defined Poly (arylene ether sulfone) s by chain growth condensation polymerization in organic media, Polymer, 2018, 153, 430–437 CrossRef CAS.
- S. Amemori, K. Iseda, S. Anan, T. Ono, K. Kokado and K. Sada, Thermoresponsivity of polymer solution derived from a self-attractive urea unit and a self-repulsive lipophilic ion unit, Polym. Chem., 2017, 8(26), 3921–3925 RSC.
- T. Ueki, A. A. Arai, K. Kodama, S. Kaino, N. Takada, T. Morita, K. Nishikawa and M. Watanabe, Thermodynamic study on phase transitions of poly (benzyl methacrylate) in ionic liquid solvents, Pure Appl. Chem., 2009, 81(10), 1829–1841 CrossRef CAS.
- T. Ueki and M. Watanabe, Lower critical solution temperature behavior of linear polymers in ionic liquids and the corresponding volume phase transition of polymer gels, Langmuir, 2007, 23(3), 988–990 CrossRef CAS PubMed.
- S. Tamura, T. Ueki, K. Ueno, K. Kodama and M. Watanabe, Thermosensitive self-assembly of diblock copolymers with lower critical micellization temperatures in an ionic liquid, Macromolecules, 2009, 42(16), 6239–6244 CrossRef CAS.
- T. Ueki, T. Karino, Y. Kobayashi, M. Shibayama and M. Watanabe, Difference in lower critical solution temperature behavior between random copolymers and a homopolymer having solvatophilic and solvatophobic structures in an ionic liquid, J. Phys. Chem. B, 2007, 111(18), 4750–4754 CrossRef CAS PubMed.
- T. Ueki, A. Yamaguchi, N. Ito, K. Kodama, J. Sakamoto, K. Ueno, H. Kokubo and M. Watanabe, Photoisomerization-induced tunable LCST phase separation of azobenzene-containing polymers in an ionic liquid, Langmuir, 2009, 25(16), 8845–8848 CrossRef CAS PubMed.
- T. Ueki and M. Watanabe, Upper critical solution temperature behavior of poly (N-isopropylacrylamide) in an ionic liquid and preparation of thermo-sensitive nonvolatile gels, Chem. Lett., 2006, 35(8), 964–965 CrossRef CAS.
- T. Ueki, M. Watanabe and T. P. Lodge, Doubly thermosensitive self-assembly of diblock copolymers in ionic liquids, Macromolecules, 2009, 42(4), 1315–1320 CrossRef CAS.
- Y. He and T. P. Lodge, A thermoreversible ion gel by triblock copolymer self-assembly in an ionic liquid, Chem. Commun., 2007,(26), 2732–2734 RSC.
- K. Kodama, R. Tsuda, K. Niitsuma, T. Tamura, T. Ueki and H. Kokubo, Watanabe, M., Structural effects of polyethers and ionic liquids in their binary mixtures on lower critical solution temperature liquid-liquid phase separation, Polym. J., 2011, 43(3), 242–248 CrossRef CAS.
- H. N. Lee and T. P. Lodge, Poly (n-butyl methacrylate) in ionic liquids with tunable lower critical solution temperatures (LCST), J. Phys. Chem. B, 2011, 115(9), 1971–1977 CrossRef CAS PubMed.
- K. Kodama, H. Nanashima, T. Ueki, H. Kokubo and M. Watanabe, Lower critical solution temperature phase behavior of linear polymers in imidazolium-based ionic liquids: effects of structural modifications, Langmuir, 2009, 25(6), 3820–3824 CrossRef CAS PubMed.
- C. Wang, K. Hashimoto, R. Tamate, H. Kokubo and M. Watanabe, Controlled Sol–Gel Transitions of a Thermoresponsive Polymer in a Photoswitchable Azobenzene Ionic Liquid as a Molecular Trigger, Angew. Chem., Int. Ed., 2018, 57(1), 227–230 CrossRef CAS PubMed.
- H. N. Lee, Z. Bai, N. Newell and T. P. Lodge, Micelle/inverse micelle self-assembly of a PEO− PNIPAm block copolymer in ionic liquids with double thermoresponsivity, Macromolecules, 2010, 43(22), 9522–9528 CrossRef CAS.
- H. N. Lee, N. Newell, Z. Bai and T. P. Lodge, Unusual lower critical solution temperature phase behavior of poly (ethylene oxide) in ionic liquids, Macromolecules, 2012, 45(8), 3627–3633 CrossRef CAS.
- T. Ueki, Y. Nakamura, R. Usui, Y. Kitazawa, S. So, T. P. Lodge and M. Watanabe, Photoreversible gelation of a triblock copolymer in an ionic liquid, Angew. Chem., 2015, 127(10), 3061–3065 CrossRef.
- Y. Kobayashi, Y. Kitazawa, T. Komori, K. Ueno, H. Kokubo and M. Watanabe, Self–Assembly of Polyether Diblock Copolymers in Water and Ionic Liquids, Macromol. Rapid Commun., 2016, 37(14), 1207–1211 CrossRef CAS PubMed.
- K. Fujii, T. Ueki, K. Niitsuma, T. Matsunaga, M. Watanabe and M. Shibayama, Structural aspects of the LCST phase behavior of poly (benzyl methacrylate) in room-temperature ionic liquid, Polymer, 2011, 52(7), 1589–1595 CrossRef CAS.
- T. Ueki and M. Watanabe, Polymers in ionic liquids: dawn of neoteric solvents and innovative materials, Bull. Chem. Soc. Jpn., 2012, 85(1), 33–50 CrossRef CAS.
- C. Zhang and M. Maric, Fluorescent, thermoresponsive copolymers via nitroxide–mediated polymerization: Synthesis and effect of fluorescent groups on phase transitions in an ionic liquid, J. Polym. Sci., Part A: Polym. Chem., 2013, 51(21), 4702–4715 CrossRef CAS.
- C. Wang, X. Ma, Y. Kitazawa, Y. Kobayashi, S. Zhang, H. Kokubo and M. Watanabe, From Macromolecular to Small–Molecular Triggers: Facile Method toward Photoinduced LCST Phase Behavior of Thermoresponsive Polymers in Mixed Ionic Liquids Containing an Azobenzene Moiety, Macromol. Rapid Commun., 2016, 37(23), 1960–1965 CrossRef CAS PubMed.
- C. Wang, K. Hashimoto, J. Zhang, Y. Kobayashi, H. Kokubo and M. Watanabe, Micellization/Demicellization self-Assembly change of ABA triblock copolymers induced by a photoswitchable ionic liquid with a small molecular trigger, Macromolecules, 2017, 50(14), 5377–5384 CrossRef CAS.
- Y. Kitazawa, T. Ueki, K. Niitsuma, S. Imaizumi, T. P. Lodge and M. Watanabe, Thermoreversible high-temperature gelation of an ionic liquid with poly (benzyl methacrylate-b-methyl methacrylate-b-benzyl methacrylate) triblock copolymer, Soft Matter, 2012, 8(31), 8067–8074 RSC.
- Y. Kitazawa, T. Ueki, L. D. McIntosh, S. Tamura, K. Niitsuma, S. Imaizumi, T. P. Lodge and M. Watanabe, Hierarchical sol–gel transition induced by thermosensitive self-assembly of an ABC triblock polymer in an ionic liquid, Macromolecules, 2016, 49(4), 1414–1423 CrossRef CAS.
- Y. Kitazawa, T. Ueki, S. Imaizumi, T. P. Lodge and M. Watanabe, Tuning of sol–gel transition temperatures for thermoreversible ion gels, Chem. Lett., 2014, 43(2), 204–206 CrossRef CAS.
- T. Ueki, Y. Nakamura, A. Yamaguchi, K. Niitsuma, T. P. Lodge and M. Watanabe, UCST phase transition of azobenzene-containing random copolymer in an ionic liquid, Macromolecules, 2011, 44(17), 6908–6914 CrossRef CAS.
- R. Tamate, R. Usui, K. Hashimoto, Y. Kitazawa, H. Kokubo and M. Watanabe, Photo/thermoresponsive ABC triblock copolymer-based ion gels: photoinduced structural transitions, Soft Matter, 2018, 14(45), 9088–9095 RSC.
- H. N. Lee and T. P. Lodge, Lower critical solution temperature (LCST) phase behavior of poly (ethylene oxide) in ionic liquids, J. Phys. Chem. Lett., 2010, 1(13), 1962–1966 CrossRef CAS.
- Y. He and T. P. Lodge, Thermoreversible ion gels with tunable melting temperatures from triblock and pentablock copolymers, Macromolecules, 2008, 41(1), 167–174 CrossRef CAS.
- T. Ueki, R. Usui, Y. Kitazawa, T. P. Lodge and M. Watanabe, Thermally reversible ion gels with photohealing properties based on triblock copolymer self-assembly, Macromolecules, 2015, 48(16), 5928–5933 CrossRef CAS.
- X. Ma, R. Usui, Y. Kitazawa, R. Tamate, H. Kokubo and M. Watanabe, Physicochemical characterization of a photoinduced sol–gel transition of an azobenzene-containing ABA triblock copolymer/ionic liquid system, Macromolecules, 2017, 50(17), 6788–6795 CrossRef CAS.
- R. Tsuda, K. Kodama, T. Ueki, H. Kokubo, S. Imabayashi and M. Watanabe, LCST-type liquid–liquid phase separation behaviour of poly (ethylene oxide) derivatives in an ionic liquid, Chem. Commun., 2008,(40), 4939–4941 RSC.
- T. Ueki and M. Watanabe, Macromolecules in ionic liquids: progress, challenges, and opportunities, Macromolecules, 2008, 41(11), 3739–3749 CrossRef CAS.
- T. Ueki, Stimuli-responsive polymers in ionic liquids, Polym. J., 2014, 46(10), 646–655 CrossRef CAS.
- R. Tamate, K. Hashimoto, T. Ueki and M. Watanabe, Block copolymer self-assembly in ionic liquids, Phys. Chem. Chem. Phys., 2018, 20(39), 25123–25139 RSC.
- J. Zhang, B. Jin, G. Tang, Y. Luo and X. Li, Core–Shell Copolymers with Brush-on-Hyperbranched Arm Architecture: Synthesis, Dual Thermoresponsive Behaviors, and Nanocarriers, Macromolecules, 2021, 54(18), 8810–8821 CrossRef CAS.
- A. Plucinski, M. Pavlovic, M. Clarke, D. Bhella and B. V. K. J. Schmidt, Stimuli-Responsive Aggregation of High Molar Mass Poly(N,N-Diethylacrylamide)-b-Poly(4-Acryloylmorpholine) in Tetrahydrofuran, Macromol. Rapid Commun., 2021, e2100656 Search PubMed.
- S. Yue, G. C. Berry and M. M. Green, Intermolecular association and supramolecular organization in dilute solution. 2. Light scattering and optical activity of poly(p-biphenylylmethyl L-glutamate), Macromolecules, 1996, 29(19), 6175–6182 CrossRef CAS.
- D. S. Schirra, S. Jeziorowski, M. Lehmann and C. M. Thiele, Thermoreversible Gelation of Homopolyglutamates PBPMLG, PBPELG, and PBPHLG: Another Step toward de Novo RDC-Based Structure Elucidation, Macromolecules, 2022, 55, 3430–3436 CrossRef CAS.
- Z. Liu, Y. Guo and K. Inomata, LCST-type phase behavior of poly (2-chloroethyl vinyl ether-alt-maleic anhydride) in n-butyl acetate, Polym. J., 2011, 43(8), 676–682 CrossRef CAS.
- M. Ouchi, M. Nakano, T. Nakanishi and M. Sawamoto, Alternating sequence control for carboxylic acid and hydroxy pendant groups by controlled radical cyclopolymerization of a divinyl monomer carrying a cleavable spacer, Angew. Chem., Int. Ed., 2016, 55(47), 14584–14589 CrossRef CAS PubMed.
- Z. Feng, J. Guo, X. Cao, G. Feng, Z. Chen and X. H. Zhang, A thermo-reversible furfuryl poly(thioether)-b-polysiloxane-b-furfuryl poly(thioether) triblock copolymer as a promising material for high dielectric applications, Polym. Chem., 2022, 13(10), 1376–1386 RSC.
- N. Jiang, D. Zhu, Z. Su and M. R. Bryce, Blue-emitting thermoreversible oligourethane gelators with aggregation-induced emission properties, J. Mater. Chem. C, 2020, 8(15), 5137–5142 RSC.
- Z. Liu, Y. Guo and K. Inomata, Reversible thermoresponsive behavior of poly (2-chloroethyl vinyl ether-alt-maleic anhydride) in mixed solvent of tetrahydrofuran/hexane, Colloid Polym. Sci., 2011, 289(7), 791–798 CrossRef CAS.
- E. Cazares-Cortes, B. C. Baker, K. Nishimori, M. Ouchi and F. Tournilhac, Polymethacrylic Acid Shows Thermoresponsivity in an Organic Solvent, Macromolecules, 2019, 52(15), 5995–6004 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py01147f |
| This journal is © The Royal Society of Chemistry 2022 |