Photoswitchable block copolymers based on main chain α-bisimines†
Abstract
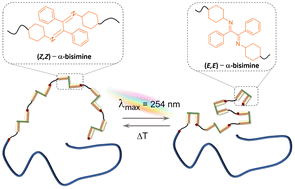
We introduce linear diblock copolymers (BCPs) consisting of readily accessable and photoswitchable α-bisimine units in the polymer backbone. The BCPs are prepared via a combination of activators regenerated by electron transfer (ARGET) atom transfer radical polymerization (ATRP) and head-to-tail acyclic diene metathesis (ADMET) polymerization. The selective head-to-tail ADMET polymerization of α-bisimine-based asymmetrical monomers in the presence of macro-chain stoppers allows for the construction of well-defined block copolymers. Acrylate-endcapped polystyrenes synthesized from ARGET ATRP and commercial poly(ethylene glycol) methyl ether acrylate were used as macro-chain stoppers. 1H NMR spectroscopy and Size-Exclusion Chromatography (SEC) confirmed the light-responsive properties of the resulting block copolymers (BCPs) in solution. An unambigeous reversible (Z,Z)/(E,E)-isomerization of the α-bisimines was observed for PS- and PEG-based BCPs upon UV irradiation (λmax = 254 nm). The advanced synthetic approach paves the way towards the preparation of efficient light-responsive materials, where in-chain morphological changes are required.


 Please wait while we load your content...
Please wait while we load your content...