Open Access Article
Open Access ArticleThioanhydride/isothiocyanate/epoxide ring-opening terpolymerisation: sequence selective enchainment of monomer mixtures and switchable catalysis†
Dorothee
Silbernagl
 a,
Heinz
Sturm
a,
Heinz
Sturm
 a and
Alex J.
Plajer
a and
Alex J.
Plajer
 *b
*b
aBAM Bundesanstalt für Materialforschung und -Prüfung, Unter den Eichen 87, 12205 Berlin, Germany
bIntitut für Chemie und Biochemie, Freie Universität Berlin, Fabeckstraße 34-36, 14195 Berlin, Germany. E-mail: plajer@zedat.fu-berlin.de
First published on 20th June 2022
Abstract
We report a new sequence selective terpolymerisation in which three monomers (butylene oxide (BO) A, PhNCS B and phtalic thioanhydride (PTA) C) are selectively enchained into an (ABA′C)n sequence. PTA/PhNCS/BO ring-opening terpolymerisation ROTERP can be coupled with CS2 ROTERP to generate tetrapolymers and with εDL ROP in switchable catalysis for blockpolymer synthesis.
Heteroatom-containing polymers have strong potential as degradable replacements for polymers based on saturated aliphatic backbones.1–3 Often such polymers are synthesised via the living ring opening polymerisation (ROP) of a heterocycle A to produce (A)n such as poly(thio)esters, poly(thio)carbonate and poly(thio)ethers.2,4 In some cases, the ROP of three or four-membered heterocycles A can be coupled with the insertion of heteroallenes or cyclic anhydrides B to generate alternating copolymers (AB)n in so called ring-opening copolymerisation (ROCOP).5,6 Prominent examples include CO2/epoxide ROCOP forming polycarbonates or cyclic anhydride/epoxide ROCOP forming polyesters.7–9 However, sulfur-containing variants also exist and relevant to this study are recent reports on isothiocyanate/epoxide ROCOP forming poly(monothioimidocarbonates), isothiocyanate/thiirane ROCOP forming poly(dithioimidocarbonates), cyclic thioanhydride/epoxide ROCOP forming poly(ester-alt-thioesters).10–18 Recently we realised alternating ring-opening terpolymerisation (ROTERP) of ternary monomer mixtures comprising thiophtatlic anhydride (PTA), CS2 and butylene oxide (BO).19 Here, a simple lithium catalyst (e.g. lithiumbenzyloxide LiOBn or lithiumhexamethyldisilazide LiHMDS) selectively forms poly(ester-alt-ester-alt-trithiocarbonates) in up to 98% selectivity with respect to the erroneous thioester links from PTA/BO ROCOP. The polymer shows an unusual “head-to-head-alt-tail-to-tail” selectivity, meaning that ester groups sit next to tertiary carbon centres while trithiocarbonates sit next to secondary carbon centres. Our results indicated that PTA/CS2/BO ROTERP formally derives from Li catalysed CS2/BO ROCOP reported by Werner and co-workers.17 Both polymerisations are enabled by a O/S exchange reaction step in which an alkoxide chain end from a ring opened epoxide A isomerizes into a thiolate through incorporation of the oxygen into the adjacent link and this isomerised incorporated epoxide (forming a link akin insertion of a virtual thiirane) has been termed A′.17,20 Thus, we hypothesized that other monomer combinations which undergo Li catalysed ROCOP could be suitable for sequence selective ROTERP. Xiong and coworkers very recently reported RNCS/epoxide ROCOP mediated by a simple LiOtBu catalyst and accordingly we hypothesised that isothiocyanates could undergo ROTERP (Fig. 1).12
 | ||
| Fig. 1 Comparison of different ROCOPs and ROTERP. [Li] = LiN(SiMe3) or LiOCH2Ph (LiOBn).12,17,19 | ||
Therefore, we investigated the terpolymerisation of PTA/PhNCS/BO at different monomer ratios and catalyst loadings at 80 °C with lithiumbenzyloxide (LiOBn) as the catalyst (Table 1). Gratifyingly, we find that mixtures comprising 15 eq. PhNCS and 5 eq. BO with LiOBn loadings of 1–8 mol% per equivalent of PTA form poly(ester-alt-ester-alt-dithioimidocarbonates) in quantitative polymer selectivity. The 1H NMR spectrum (Fig. 2) of the polymer shows two main aryl resonances corresponding to a symmetrically substituted terephthalate unit (δ = 7.67 and 7.47 ppm) in an approximate 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 integrative ratio with respect to the NPh aromatic resonances indicating a 1
5 integrative ratio with respect to the NPh aromatic resonances indicating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the two aromatic moieties.
1 ratio of the two aromatic moieties.
 | ||
| Fig. 2 Reaction Scheme, 1H, 13C{1H} NMR and IR spectra of PTA/PhNCS/BO terpolymer (polymer of Table 1, run #4). | ||
LiOBn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTA PTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BO BO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PhNCSa PhNCSa |
Time [min] | PTA conversionb | Coupling selectivityc | Ester selectivityd | M n ,e [kDa] (Đ) |
|---|---|---|---|---|---|
| a Copolymerisation at T = 80 °C, LiOBn generated in situ from LiHMDS and BnOH (see ESI†). b Determined by comparison of the relative integrals, in the normalised 1H NMR spectrum (CDCl3, 25 °C, 400 MHz), of terepthalate CH resonances due to (co/ter)polymer and PTA. c Determined by comparison of the relative integrals, in the normalised 1H NMR spectrum (CDCl3, 25 °C, 400 MHz), of tertiary CH resonances due to (co/ter)polymer and cyclic thioimidocarbonate and polyether. d Determined by comparison of the relative integrals, in the normalised the 13C{1H} NMR spectrum (CDCl3, 25 °C) of resonances due to ester relative to thioester links. e Determined by SEC (size-exclusion chromatography) measurements conducted in THF, using narrow MW polystyrene standards to calibrate the instrument. f NaHMDS was employed in place of LiHMDS. g KHMDS was employed in place of LiHMDS. h CS2. | |||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 93 93 |
2 | 99% | >99% | 97.5% | 2.51 (1.15) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 187 187 |
3 | 98% | >99% | 97.5% | 5.5 (1.17) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375 375 |
6 | 98% | >99% | 97.5% | 9.94 (1.20) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
30 | 95% | >99% | 97.5% | 13.61 (1.33) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500 1500 |
480 | 86% | >99% | 97.5% | 14.0 (1.49) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125 125 |
30 | >99% | >99% | 91% | 22.18 (1.32) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
30 | >99% | >99% | 94% | 21.6 (1.29) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
30 | >99% | >99% | 95% | 17.61 (1.28) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750f 750f |
30 | 10% | >99% | n.d. | n.d. |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750g 750g |
30 | 0% | — | — | — |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375 375![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375h 375h |
30 | >99% | 95% | 96% | 18.09 (1.38) |
Furthermore, there is one main resonance for the CHR3 protons (δ = 5.23 ppm) in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 integrative ratio and one main signal for the CH2R2 protons (δ = 3.80–2.90 ppm) in a 4
4 integrative ratio and one main signal for the CH2R2 protons (δ = 3.80–2.90 ppm) in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 integrative ratio to the aromatic terephtalate signals respectively. The 13C{1H} NMR spectrum (Fig. 2) reveals that the links formed are primarily dithioimidocarbonates R–S–C(
4 integrative ratio to the aromatic terephtalate signals respectively. The 13C{1H} NMR spectrum (Fig. 2) reveals that the links formed are primarily dithioimidocarbonates R–S–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh)–S–R (δ = 159.1 ppm, ≈31% of all links) and arylesters R–C(
NPh)–S–R (δ = 159.1 ppm, ≈31% of all links) and arylesters R–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O–R (δ = 166.6 ppm, ≈64% of all links), alongside minor thioester R–C(
O)–O–R (δ = 166.6 ppm, ≈64% of all links), alongside minor thioester R–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–S–R (δ = 192.7 ppm, ≈2.5% of all links) and monothioimidocarbonate R-O–C(
O)–S–R (δ = 192.7 ppm, ≈2.5% of all links) and monothioimidocarbonate R-O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NPh)–S–R (δ = 156.0 ppm, ≈2.5% of all links) linkages (95% ROTERP links and 5% ROCOP errors). Due to overlapping 1H NMR resonances for the respective linkages, the linkage ratios were approximated by integration of the relative integrals of the quaternary carbon resonances which proved to correspond well to the linkage ratios determined by integration of the 1H NMR spectra in related terpolymers.19 The thioester and monothioimidocarbonate links represent the links of the parent ROCOP reactions between PTA/BO and PhNCS/BO and are inferred to result from incomplete O/S exchange and insertion of PTA or PhNCS into alkoxide chain ends or from insertion of thiolate chain ends into PTA as previously shown.19 2D NMR spectroscopy (Fig. S3 and S4†) further substantiates that dithioimidocarbonate units are positioned adjacent to CH2 groups while arylesters are connected to the tertiary CHMe groups. Hence, we propose a similar “head-to-head-alt-tail-to-tail” selectivity connectivity as for the previously reported ROTERP involving CS2 in place of PhNCS. Furthermore, no ether links were detected in the polymer, which are a common side products formed in related ROCOPs.5,21 The respective resonance ratios remain unchanged after multiple precipitations from DCM
NPh)–S–R (δ = 156.0 ppm, ≈2.5% of all links) linkages (95% ROTERP links and 5% ROCOP errors). Due to overlapping 1H NMR resonances for the respective linkages, the linkage ratios were approximated by integration of the relative integrals of the quaternary carbon resonances which proved to correspond well to the linkage ratios determined by integration of the 1H NMR spectra in related terpolymers.19 The thioester and monothioimidocarbonate links represent the links of the parent ROCOP reactions between PTA/BO and PhNCS/BO and are inferred to result from incomplete O/S exchange and insertion of PTA or PhNCS into alkoxide chain ends or from insertion of thiolate chain ends into PTA as previously shown.19 2D NMR spectroscopy (Fig. S3 and S4†) further substantiates that dithioimidocarbonate units are positioned adjacent to CH2 groups while arylesters are connected to the tertiary CHMe groups. Hence, we propose a similar “head-to-head-alt-tail-to-tail” selectivity connectivity as for the previously reported ROTERP involving CS2 in place of PhNCS. Furthermore, no ether links were detected in the polymer, which are a common side products formed in related ROCOPs.5,21 The respective resonance ratios remain unchanged after multiple precipitations from DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH or THF
MeOH or THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pentane confirming that all links are part of the same polymer. Linkage identity could be further substantiated by the ATR-IR spectrum (Fig. 2) showing an arylester C
pentane confirming that all links are part of the same polymer. Linkage identity could be further substantiated by the ATR-IR spectrum (Fig. 2) showing an arylester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch at ṽ = 1716 cm−1 as well as a dithioimidocarbonate C
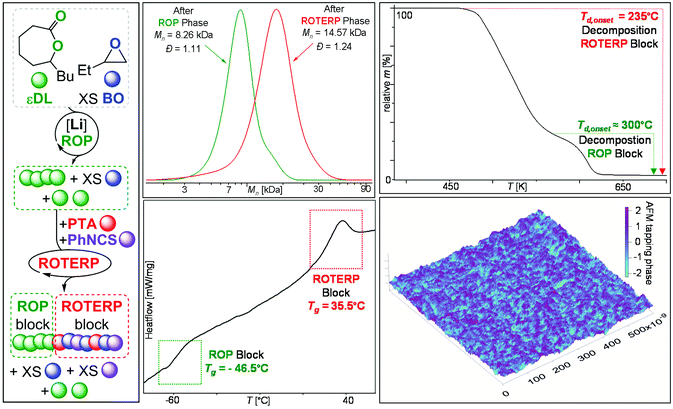
O stretch at ṽ = 1716 cm−1 as well as a dithioimidocarbonate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretch at ṽ = 1563 cm−1.10,19 MALDI-TOF analysis unfortunately only led to decomposition of the materials and no signals could be identified as previously reported for sulfur-rich polymers.22,23 However the OBn initiator can be identified to be part of the purified polymers (Fig. S5†) suggesting the formation of linear as opposed to cyclic chains. The polymers are colourless amorphous solids (Tg = 30.6 °C, Fig. S8†) with good thermal stability (Td,5% = 230.1 °C, Fig. S10†). SEC analysis of the obtained materials at catalyst loadings of 1–8 mol% vs. PTA shows that the methodology can yield polymers with molecular weights ranging from Mn = 2.55 to 14.00 kg mol−1 (Đ = 1.15–1.49, Table 1 and Fig. S19†). Attempting lower LiOBn loadings did not result in any polymerisation. Employing Na or K in place of Li resulted in unappreciable turnover supporting that Li acts as a catalyst rather than a spectator countercation for the OBn initiator (Table 1 run #9 and #10). Decreasing the amount of PhNCS in the initial monomer mixture (and therefore increasing the PTA concentration as PhNCS acts as a cosolvent) led to more erroneous thioester links (Table 1 run #6–#8) and a similar amount of monothioimidocarbonate links (ca. 3% of all links). This can be rationalized by kinetic competition between O/S rearrangement and propagation from alkoxide intermediates. In these cases, we also observe the onset of PhNCS/BO ROCOP to form poly(monothioimidocarbonates) once all PTA is consumed as confirmed by 1H (Fig. S6†) and 13C NMR (Fig. S7†) spectroscopy. This leads to poly(monothioimidocarbonate) blocks forming adjacent to the ROTERP blocks which also explains the higher molecular weights obtained at the same PTA loadings. The new monomer combination is furthermore compatible with PTA/CS2/BO ROTERP. Tetrapolymerisation of PTA and BO with PhNCS and CS2 results in poly(ester-alt-ester-alt-heterocarbonate) formation in which both RNCS (forming dithioimidocarbonates) and CS2 (forming trithiocarbonates) are incorporated into the polymer. NMR (Fig. S11–S15†) and FTATR-IR (Fig. S17†) clearly show the presence of trithiocarbonate (δ = 222.8 ppm, ṽ = 1063 cm−1) links in addition to the other links from PTA/PhNCS/BO ROTERP. Corresponding to Table 1 run #11, a polymer comprising 67% arylester, 23% trithiocarbonate, 6% dithioimidocarbonate, 3.5% thioester and 0.5% monothioimidocarbonate links is produced (96% ROTERP links and 4% ROCOP errors). Apparently, PTA/PhNCS/BO ROTERP follows a similar polymerisation mechanism (Fig. S31†) as the one proposed for PTA/CS2/BO ROTERP. It involves a central O/S rearrangement step and preferential insertion of PTA into alkoxide chain ends and of the heteroallene into thiolate chain ends at which point a mixture of heteroallenes can be employed which are then both incorporated at this insertion step. Interestingly, although PhNCS and CS2 are employed in an equimolar ratio, CS2 is incorporated preferentially in 77% selectivity. We could confirm this observation by varying the initial PhNCS:CS2 ratio in which CS2 was always incorporated to a greater degree than employed in the initial monomer feed (see Fig. S18†). In ROCOP, switchable catalysis has been established as an elegant and valuable tool to synthesise blockpolymers with useful material properties.24–26 Here a suitable catalyst first mediates the ROP of for example cyclic esters (e.g. εDL forming PDL) with epoxides present in the mixture until the second ROCOP monomer (e.g. CO2) is added causing immediate termination of ROP and the onset of (e.g. CO2/epoxide forming polycarbonate) ROCOP to form a ROCOP block connected to the ROP polymer. We recently showed that ROTERP is also suitable for switchable catalysis in combination with the lithium catalysed ROP of εDL.19 Having identified a new heteroallene that undergoes ROTERP, we were intrigued whether this monomer combination is also suitable for the construction of blockpolymers via switchable catalysis. Accordingly, we added PhNCS (750 eq. per LiOBn) and PTA (50 eq.) to polymerising εDL (50 eq.) in BO (250 eq.) after 15 min at room temperature which completely and immediately stops the occurrence of εDL ROP. Heating to 80 °C initiates ROTERP and a poly(ester-alt-ester-alt-dithioiminocarbonate) block grows from the PDL-chain-end until the reaction is stopped after 30 min. Under these conditions the ROTERP block consists of 64% arylester links, 31% dithioimidocarbonate links and 2.5% erroneous thioester and monothioimidocarbonate links, respectively (95% ROTERP links and 5% ROCOP errors). Switchable catalysis and block polymer formation were established by various methods: (i) no εDL is consumed after ROTERP starts (Fig. S21†) and the 13C{1H} PDL (δ = 173.2 ppm, Fig. S23†) remains unchanged showing that ROP stops and that no transesterification between blocks occurs; (ii) the number averaged molecular weight shifts from Mn = 8.26 (Đ = 1.11) to 14.57 kg mol−1 (Đ = 1.24, Fig. 3), which shows the growth of existing chains rather than the initiation of new ones; (iii) 31P end group analysis shows the consumption of all PDL end groups (Fig. S30†);27 (iv) the composition of the resulting blockpolymer remains unchanged through multiple precipitations from DCM/MeOH and THF/pentane supporting that the blocks are joint; (vii) DSC analysis exhibits two Tg's at − 46.5 °C for the ROP block and 35.5 °C for the ROTERP block suggesting microphase separation in the solid-state which could be confirmed by AFM (Fig. 3);28 (viii) TGA analysis shows a stepwise thermal decomposition profile with two Td,onset at approximately 235 °C for the ROTERP block and 300 °C for the ROP block (Fig. 3). In conclusion, we have
identified a new monomer combination that undergoes lithium catalysed sequence selective terpolymerisation. Mixtures of PTA/PhNCS/BO forming poly(ester-alt-ester-alt-dithioimidocarbonate)s in up to 95% selectivity with respect to the erroneous links from PhNCS/BO and PTA/BO ROCOP. This has enabled the synthesis of complex tetrapolymers from quarternary monomer mixture or via switchable catalysis. Our results establish ROTERP as a valuable methodology for the synthesis of heteroatom containing blockpolymers.
N stretch at ṽ = 1563 cm−1.10,19 MALDI-TOF analysis unfortunately only led to decomposition of the materials and no signals could be identified as previously reported for sulfur-rich polymers.22,23 However the OBn initiator can be identified to be part of the purified polymers (Fig. S5†) suggesting the formation of linear as opposed to cyclic chains. The polymers are colourless amorphous solids (Tg = 30.6 °C, Fig. S8†) with good thermal stability (Td,5% = 230.1 °C, Fig. S10†). SEC analysis of the obtained materials at catalyst loadings of 1–8 mol% vs. PTA shows that the methodology can yield polymers with molecular weights ranging from Mn = 2.55 to 14.00 kg mol−1 (Đ = 1.15–1.49, Table 1 and Fig. S19†). Attempting lower LiOBn loadings did not result in any polymerisation. Employing Na or K in place of Li resulted in unappreciable turnover supporting that Li acts as a catalyst rather than a spectator countercation for the OBn initiator (Table 1 run #9 and #10). Decreasing the amount of PhNCS in the initial monomer mixture (and therefore increasing the PTA concentration as PhNCS acts as a cosolvent) led to more erroneous thioester links (Table 1 run #6–#8) and a similar amount of monothioimidocarbonate links (ca. 3% of all links). This can be rationalized by kinetic competition between O/S rearrangement and propagation from alkoxide intermediates. In these cases, we also observe the onset of PhNCS/BO ROCOP to form poly(monothioimidocarbonates) once all PTA is consumed as confirmed by 1H (Fig. S6†) and 13C NMR (Fig. S7†) spectroscopy. This leads to poly(monothioimidocarbonate) blocks forming adjacent to the ROTERP blocks which also explains the higher molecular weights obtained at the same PTA loadings. The new monomer combination is furthermore compatible with PTA/CS2/BO ROTERP. Tetrapolymerisation of PTA and BO with PhNCS and CS2 results in poly(ester-alt-ester-alt-heterocarbonate) formation in which both RNCS (forming dithioimidocarbonates) and CS2 (forming trithiocarbonates) are incorporated into the polymer. NMR (Fig. S11–S15†) and FTATR-IR (Fig. S17†) clearly show the presence of trithiocarbonate (δ = 222.8 ppm, ṽ = 1063 cm−1) links in addition to the other links from PTA/PhNCS/BO ROTERP. Corresponding to Table 1 run #11, a polymer comprising 67% arylester, 23% trithiocarbonate, 6% dithioimidocarbonate, 3.5% thioester and 0.5% monothioimidocarbonate links is produced (96% ROTERP links and 4% ROCOP errors). Apparently, PTA/PhNCS/BO ROTERP follows a similar polymerisation mechanism (Fig. S31†) as the one proposed for PTA/CS2/BO ROTERP. It involves a central O/S rearrangement step and preferential insertion of PTA into alkoxide chain ends and of the heteroallene into thiolate chain ends at which point a mixture of heteroallenes can be employed which are then both incorporated at this insertion step. Interestingly, although PhNCS and CS2 are employed in an equimolar ratio, CS2 is incorporated preferentially in 77% selectivity. We could confirm this observation by varying the initial PhNCS:CS2 ratio in which CS2 was always incorporated to a greater degree than employed in the initial monomer feed (see Fig. S18†). In ROCOP, switchable catalysis has been established as an elegant and valuable tool to synthesise blockpolymers with useful material properties.24–26 Here a suitable catalyst first mediates the ROP of for example cyclic esters (e.g. εDL forming PDL) with epoxides present in the mixture until the second ROCOP monomer (e.g. CO2) is added causing immediate termination of ROP and the onset of (e.g. CO2/epoxide forming polycarbonate) ROCOP to form a ROCOP block connected to the ROP polymer. We recently showed that ROTERP is also suitable for switchable catalysis in combination with the lithium catalysed ROP of εDL.19 Having identified a new heteroallene that undergoes ROTERP, we were intrigued whether this monomer combination is also suitable for the construction of blockpolymers via switchable catalysis. Accordingly, we added PhNCS (750 eq. per LiOBn) and PTA (50 eq.) to polymerising εDL (50 eq.) in BO (250 eq.) after 15 min at room temperature which completely and immediately stops the occurrence of εDL ROP. Heating to 80 °C initiates ROTERP and a poly(ester-alt-ester-alt-dithioiminocarbonate) block grows from the PDL-chain-end until the reaction is stopped after 30 min. Under these conditions the ROTERP block consists of 64% arylester links, 31% dithioimidocarbonate links and 2.5% erroneous thioester and monothioimidocarbonate links, respectively (95% ROTERP links and 5% ROCOP errors). Switchable catalysis and block polymer formation were established by various methods: (i) no εDL is consumed after ROTERP starts (Fig. S21†) and the 13C{1H} PDL (δ = 173.2 ppm, Fig. S23†) remains unchanged showing that ROP stops and that no transesterification between blocks occurs; (ii) the number averaged molecular weight shifts from Mn = 8.26 (Đ = 1.11) to 14.57 kg mol−1 (Đ = 1.24, Fig. 3), which shows the growth of existing chains rather than the initiation of new ones; (iii) 31P end group analysis shows the consumption of all PDL end groups (Fig. S30†);27 (iv) the composition of the resulting blockpolymer remains unchanged through multiple precipitations from DCM/MeOH and THF/pentane supporting that the blocks are joint; (vii) DSC analysis exhibits two Tg's at − 46.5 °C for the ROP block and 35.5 °C for the ROTERP block suggesting microphase separation in the solid-state which could be confirmed by AFM (Fig. 3);28 (viii) TGA analysis shows a stepwise thermal decomposition profile with two Td,onset at approximately 235 °C for the ROTERP block and 300 °C for the ROP block (Fig. 3). In conclusion, we have
identified a new monomer combination that undergoes lithium catalysed sequence selective terpolymerisation. Mixtures of PTA/PhNCS/BO forming poly(ester-alt-ester-alt-dithioimidocarbonate)s in up to 95% selectivity with respect to the erroneous links from PhNCS/BO and PTA/BO ROCOP. This has enabled the synthesis of complex tetrapolymers from quarternary monomer mixture or via switchable catalysis. Our results establish ROTERP as a valuable methodology for the synthesis of heteroatom containing blockpolymers.
Conflicts of interest
There are no conflicts of interests.Acknowledgements
The VCI is acknowledged for a Liebig Fellowship for A. J. P. Prof. Dr Christian Müller and Prof. Dr Rainer Haag are thanked for continuous support. Thomas Rybak and Prof. Dr Bernhard Schartel are thanked for DSC measurements.Notes and references
- Y. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354–362 CrossRef CAS PubMed.
- G. Becker and F. R. Wurm, Chem. Soc. Rev., 2018, 47, 7739–7782 RSC.
- T. P. Haider, C. Völker, J. Kramm, K. Landfester and F. R. Wurm, Angew. Chem., Int. Ed., 2019, 58, 50–62 CrossRef CAS PubMed.
- N. E. Kamber, W. Jeong, R. M. Waymouth, R. C. Pratt, B. G. G. Lohmeijer and J. L. Hedrick, Chem. Rev., 2007, 107, 5813–5840 CrossRef CAS PubMed.
- A. J. Plajer and C. K. Williams, Angew. Chem., Int. Ed., 2022 DOI:10.1002/anie.202104495.
- S. Paul, Y. Zhu, C. Romain, R. Brooks, P. K. Saini and C. K. Williams, Chem. Commun., 2015, 51, 6459–6479 RSC.
- J. M. Longo, M. J. Sanford and G. W. Coates, Chem. Rev., 2016, 116, 15167–15197 CrossRef CAS PubMed.
- B. Grignard, S. Gennen, C. Jérôme, A. W. Kleij and C. Detrembleur, Chem. Soc. Rev., 2019, 48, 4466–4514 RSC.
- C. M. Kozak, K. Ambrose and T. S. Anderson, Coord. Chem. Rev., 2018, 376, 565–587 CrossRef CAS.
- X.-F. Zhu, R. Xie, G.-W. Yang, X.-Y. Lu and G.-P. Wu, ACS Macro Lett., 2021, 10, 135–140 CrossRef CAS PubMed.
- T. Lai, P. Zhang, J. Zhao and G. Zhang, Macromolecules, 2021, 54, 11113–11125 CrossRef CAS.
- L. Song, M. Liu, D. You, W. Wei and H. Xiong, Macromolecules, 2021, 54, 10529–10536 CrossRef CAS.
- C. Chen, Y. Gnanou and X. Feng, Macromolecules, 2021, 54, 9474–9481 CrossRef CAS.
- X.-F. Zhu, G.-W. Yang, R. Xie and G.-P. Wu, Angew. Chem., Int. Ed., 2022 DOI:10.1002/anie.202115189.
- L.-Y. Wang, G.-G. Gu, B.-H. Ren, T.-J. Yue, X.-B. Lu and W.-M. Ren, ACS Catal., 2020, 10, 6635–6644 CrossRef CAS.
- L.-Y. Wang, G.-G. Gu, T.-J. Yue, W.-M. Ren and X.-B. Lu, Macromolecules, 2019, 52, 2439–2445 CrossRef CAS.
- J. Diebler, H. Komber, L. Häußler, A. Lederer and T. Werner, Macromolecules, 2016, 49, 4723–4731 CrossRef CAS.
- T.-J. Yue, M.-C. Zhang, G.-G. Gu, L.-Y. Wang, W.-M. Ren and X.-B. Lu, Angew. Chem., Int. Ed., 2019, 58, 618–623 CrossRef CAS PubMed.
- S. M. Rupf, P. Pröhm and A. J. Plajer, Chem. Sci., 2022, 13, 6355–6365 RSC.
- J. Diebler, A. Spannenberg and T. Werner, Org. Biomol. Chem., 2016, 14, 7480–7489 RSC.
- A. J. Plajer and C. K. Williams, Angew. Chem., Int. Ed., 2021, 60, 13372–13379 CrossRef CAS PubMed.
- T. M. McGuire and A. Buchard, Polym. Chem., 2021, 12, 4253–4261 RSC.
- W.-M. Ren, J.-Y. Chao, T.-J. Yue, B.-H. Ren, G.-G. Gu and X.-B. Lu, Angew. Chem., Int. Ed., 2022, DOI:10.1002/anie.202115950.
- A. C. Deacy, G. L. Gregory, G. S. Sulley, T. T. D. Chen and C. K. Williams, J. Am. Chem. Soc., 2021, 143, 10021–10040 CrossRef CAS PubMed.
- C. Romain and C. K. Williams, Angew. Chem., Int. Ed., 2014, 53, 1607–1610 CrossRef CAS PubMed.
- C. Hu, X. Pang and X. Chen, Macromolecules, 2022, 55, 1879–1893 CrossRef CAS.
- A. Spyros, D. S. Argyropoulos and R. H. Marchessault, Macromolecules, 1997, 30, 327–329 CrossRef CAS.
- F. S. Bates, M. A. Hillmyer, T. P. Lodge, C. M. Bates, K. T. Delaney and G. H. Fredrickson, Science, 2012, 336, 434–440 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py00629d |
| This journal is © The Royal Society of Chemistry 2022 |