 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
pH sensitive water-in-water emulsions based on the pullulan and poly(N,N-dimethylacrylamide) aqueous two-phase system†
Alexander
Plucinski
and
Bernhard V. K. J.
Schmidt
 *
*
School of Chemistry, University of Glasgow, Glasgow G12 8QQ, UK. E-mail: bernhard.schmidt@glasgow.ac.uk
First published on 28th June 2022
Abstract
Aqueous-two phase systems (ATPS) and water-in-water (w/w) emulsions are a major topic in chemistry and biology, mainly due to their biocompatibility and various applications. In here, the ATPS formed from poly(N,N-dimethylacrylamide) (PDMA) with three different molar masses (24k – 106 g mol−1) and commercial pullulan is investigated. Additionally, the ATPS with ultra-high molecular weight PDMA was transformed into a w/w emulsion using a pH-responsive block copolymer as stabiliser. The w/w emulsion could be destabilised or stabilised, depending on the current pH. The novel pH sensitive w/w emulsion will open up new pathways for example for the encapsulation of biomolecules.
Introduction
Water-based polymer systems, especially aqueous-two phase systems (ATPS) are important technologies in areas like medicine,1 food industry2,3 as well as extraction, purification, and separation of biomolecules,4 proteins,5 or metal ions.6 An ATPS forms by dissolving two incompatible compounds e.g. polymer/polymer or polymer/salt in water, which leads to a macroscopic liquid–liquid phase separation.7–10 The most common system, using two polymers is the ATPS formed from poly(ethylene glycol) (PEG) and dextran (Dex).5,11 The mixture of PEG and Dex forms an ATPS, when the concentration exceeds the critical polymer concentration. One homopolymer enriches in the upper phase and the second homopolymer enriches in the lower phase of the two-phase system. The driving force of the demixing process in an all aqueous system is the enthalpy associated with, for example, water–polymer and polymer–polymer interaction opposed by loss of entropy during phase separation. When the entropic contribution favouring mixing becomes smaller relative to the enthalpic contribution opposing it, phase separation occurs.12 Phase separation and behaviour in the ATPS depend among other factors on polymer concentration, polymer molar mass, pH, and temperature.13–16 One key factor for the critical polymer concentration and correspondingly a stable APTS is the molar mass of the used compounds. In recetn years, various studies showed a significant influence of the molar mass to the critical polymer concentration.13,17,18Due to the high biocompatibility, water-in-water (w/w) emulsions based on ATPS have received increased attention recently.19,20 The dispersion of two thermodynamically incompatible aqueous solutions of macromolecules, e.g. two hydrophilic polymers, forms a w/w emulsion. Different polymer mixtures were used in ATPS for w/w emulsion formation, for example, PEG and Dex21 or different variations of polyacrylamides.17 Nevertheless, the polymer combination PEG and Dex is most commonly utilised for the formation of w/w emulsions. Classic emulsions such as water-in-oil (w/o) or oil-in-water (o/w) emulsions can be stabilised by surfactants or larger particles.22 For w/w emulsions, stabilisation based on surfactants is not suitable, due to lower interfacial tension of the ATPS and a very broad interface between the aqueous phases where small surfactant molecules cannot align properly.23,24 Thus, various types of particles were introduced for the stabilisation of w/w emulsions e.g. latex particles,25 polydopamine particles26,27 or aggregated double hydrophilic block copolymers.28
One strategy for the stabilisation of w/w emulsions is to work with external triggers, for example, temperature28,29 or pH-value.30,31 That avenue leads to sensitive w/w emulsions, depending on a defined temperature or pH-value. For example, Nicolai and co-workers introduced linear polyelectrolytes, such as diethyl aminoethyl dextran, to stabilise a PEG and Dex w/w emulsion using different pH-values.30 Freitas and co-workers reported a pH-switchable aqueous emulsion of xyloglucan and amylopectin stabilised via polysaccharide-coated protein particles.31 Pavlovic et al. showed temperature sensitive w/w emulsions employing a double hydrophilic block copolymer that featured a thermoresponsive block and thus thermoresponsive aggregation.28 So far, the literature showed that w/w emulsions can be designed to be responsive to external triggers. Especially the use of defined polymer-based stabilisers with integrated pH or temperature switchable blocks enables a considerable control of the emulsion state.
In order to investigate novel ATPS systems and w/w emulsions including new polysaccharides, pullulan (Pull) is a viable choice.32,33 Pullulan consists of maltotriose units coupled via 1,6-α-bonds34 and is well-known for its application in biomedicine,35,36e.g. as a carrier for gene delivery36,37 and to form polysaccharide nanoparticles.38,39 Moreover, Nicolai and co-workers used Pull to form a w/w emulsion with amylopectin using protein particles to stabilise the emulsion.33 Pull shows an interesting aggregation behaviour as part of block copolymers in combination with various polymer blocks, e.g. poly(N,N-dimethylacrylamide) (PDMA).40,41 These studies on block copolymer self-assembly are an indication that polyacrylamides such as PDMA might be a good choice for an ATPS and the formation of w/w emulsions in combination with Pull.17,40,42 PDMA featuring different molar masses can be synthesised via reversible addition–fragmentation chain transfer (RAFT) polymerisation techniques e.g. photo-iniferter (PI)-RAFT polymerisation,43,44 which gives a handle towards tailored ATPS and w/w emulsion formation.
Herein, the ATPS formation of commercial Pull and PDMA different PDMAs were synthesised via RAFT polymerisation and molar masses were varied between lower molar mass of 24 kg mol−1and ultra high molecular weight (UHMW) > 1 × 106 g mol−1. The mixtures of PDMA with Pull were analysed and revealed ATPS formation. Additionally, the ATPS formed by UHMW PDMA and Pull was used to form w/w emulsions stabilised with poly(styrene) (PS) nanoparticles or the pH responsive block copolymer poly(2-(dimethylamino)ethyl methacrylate)-b-poly(oligo(ethylene glycol) methyl ether methacrylate) (PDMAEMA-b-POEGMA).45 The emulsions were further analysed via bright-field microscopy and confocal laser scanning microscopy (CLSM). In order to localise the polymer in the emulsion, Rhodamine B (RhB)- and Fluorescein-labelled polymers were employed. The w/w emulsions stabilised with PDMAEMA-b-POEGMA showed a pH sensitive stability enabling to turn the emulsion state on or off via pH adjustment (Scheme 1).
Experimental
Materials
Acetone (Fisher, analytical grade), acetic acid (1.0 N, VWR), azobis(isobutyronitrile) (AIBN, 99%, Sigma Aldrich, recrystallised from methanol), dichloromethane (DCM, analytical grade, VWR), 2-bromisobutyric acid (98.5%, Sigma Aldrich), carbon disulfide (CS2, 99%, Sigma-Aldrich), 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid (Sigma Aldrich), 2-(dimethylamino)ethyl methacrylate (DMAEMA, 98%, Sigma Aldrich, passed over a column of basic aluminium oxide), dimethyl sulfoxide (DMSO; Merck Millipore, Emsure®, ACS), N,N-dimethyl formamide (DMF, SLS), N,N-dimethylacrylamide (DMA, Sigma Aldrich, passed over a column of basic aluminium oxide), ethanethiol (98%, Alfa Aesar), ethyl acetate (99.5%, VWR), Fluorescein isothiocyanate (FITC, Sigma Aldrich), n-hexane (95%, Sigma Aldrich), hydrochloric acid (conc., Fisher), Millipore water (obtained from an Sartorius Arium pro ultrapure water system), oligo(ethylene glycol methyl ether) methacrylate (OEGMA, Sigma Aldrich, Mn = 500 g mol−1, passed over a column of basic aluminium oxide), poly(styrene) (PS) latex nanoparticles (0.1 μm, negatively charged, Sigma Aldrich, 10% aqueous suspension), potassium phosphate (Sigma Aldrich), pullulan (Pull, TCI), Rhodamine B isothiocyanate (RITC, Sigma-Aldrich), sodium acetate (anhydrous, 98%, Fisher), sodium hydroxide (NaOH, Fisher), sodium sulfate (anhydrous, SLS) and tetrahydrofuran (THF, 99.85%, Acros Organics) were used as received unless otherwise noted. 2-(((Ethylthio)carbonothioyl)thio)-2-methylpropanoic acid (EMP),46,47 RITC-PDMA17 and FITC-Pull48,49 were synthesised according to the literature.Analytical methods
1H-NMR spectra were recorded in deuterium oxide (D2O, Aldrich) at ambient temperature at 400 MHz with a Bruker Ascend400. Size exclusion chromatography (SEC) of UHMW PDMA was conducted in 0.1 M aqueous NaNO3 buffer at 25 °C using a column system with a PL Aquagel-OH Guard and PL Aquagel-OH MIXED-H and Viscotek VE 3580 RI detector and Viscotek SEC-MALS 20 for the molar mass determination. The system was calibrated with Pull standards. SEC of low molar mass PDMA was conducted in NMP with 0.005 mol L−1 LiBr and methyl benzoate as internal standard at 70 °C using a column system with PSS GRAM VS, PSS GRAM 7 μm 100 A, PSS GRAM 7 μm 1000 A columns and PSS SECurity Refractive Index-1260 RID detector and calibration with PS standards. SEC of PDMAEMA and PDMAEMA-b-POEGMA was conducted in DMF at 25 °C using a column system of two Agilent PLgel 5 μm Mixed-D 300 × 7.5 mm columns and an Agilent PLgel Guard 50 × 7.5 mm column, a Shimadzu RID-20A refractive index detector and a calibration with PEG standards. A Brookhaven differential refractometer was used for the determination of dn/dc. Confocal laser scanning microscopy (CLSM) and bright field microscopy were performed on a Zeiss LSM710 confocal microscope (Zeiss, Göttingen, Germany) and software Carl Zeiss ZEN 2011 v7.0.3.286. LD EC Epiplan NEUFLUAR 50×, 0.55 DIC (Carl Zeiss, White Plains, NY, USA), NEUFLUAR 20×, 0.55 DIC (Carl Zeiss, White Plains, NY, USA) and N-Achroplan 10×/0.25 Ph 1 (Carl Zeiss, White Plains, NY, USA) objectives were used. All samples were prepared in a CELLview (Greiner Bio-One, Stonehouse, UK) 35 mm plastic cell culture dish with a borosilicate glass bottom. Dynamic light scattering (DLS) was performed on a ZetaSizer by Malvern with Millipore water as solvent. The size of the emulsion droplets was determined over 30 particles from bright field images and averaged. The error is based on the standard deviation. Partition coefficients were determined via the concentration calculated from the NMR using DMF as internal standard according to eqn (S1) (ESI†).PI-RAFT-polymerisation of DMA
Destabilised DMA (1.0 g, 10 mmol, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 151 eq.), EMP (146 μL, 0.06 μmol, 1.0 eq. from a stock of 1 mg mL−1 DMSO), and acetate buffer (1 mL, 0.2 M, and pH = 5) were mixed in a vial (7 mL) containing a stirring bar and sealed with a septum. The solution was bubbled with nitrogen for 30 min and the polymerisation was initiated using a UV lamp (nail lamp, 4 × 9 W, λ ≈ 365 nm). The polymerisation was stopped after 24 h. Subsequently, the polymer was dialysed against deionised water (Spectra/Por 3500 Da) for 3 days. Finally, the sample was freeze-dried and a white solid (780 mg, Mn = 1.07 × 106 g mol−1, Đ = 1.40) was obtained.
151 eq.), EMP (146 μL, 0.06 μmol, 1.0 eq. from a stock of 1 mg mL−1 DMSO), and acetate buffer (1 mL, 0.2 M, and pH = 5) were mixed in a vial (7 mL) containing a stirring bar and sealed with a septum. The solution was bubbled with nitrogen for 30 min and the polymerisation was initiated using a UV lamp (nail lamp, 4 × 9 W, λ ≈ 365 nm). The polymerisation was stopped after 24 h. Subsequently, the polymer was dialysed against deionised water (Spectra/Por 3500 Da) for 3 days. Finally, the sample was freeze-dried and a white solid (780 mg, Mn = 1.07 × 106 g mol−1, Đ = 1.40) was obtained.
Preparation of ATPS and phase diagram
PDMA (50 mg) was dissolved in deionised water (450 mg) to obtain a 10 wt% solution. A 10 wt% solution of pullulan was prepared in the same way. Afterwards both solutions were mixed to receive a 5.0 wt%/5.0 wt% mixture. Subsequently, the solution was equilibrated at ambient temperature in order to demix, investigated and diluted (100 mg of deionised water each cycle). The process was repeated, until no phase separation was observed, which was recorded as the data point of the binodal curve. All other concentration combinations were conducted in a similar way.Preparation of w/w emulsions using PDMAEMA-b-POEGMA
UHMW PDMA (15 mg) and Pull (15 mg) were dissolved in water (470 mg, pH = 5 or 9) to form a 3.0/3.0 wt% solution. PDMAEMA-b-POEGMA (10 mg) was dispersed in water (490 mg, pH = 5 or 9) to generate a 2.0 wt% dispersion. Both solutions were combined to obtain a concentration of PDMA/Pull 1.5/1.5 wt% and 1.0 wt% PDMAEMA-b-POEGMA in the mixture. The mixture was subjected to ultrasonic treatment for 2 min, shaken by hand for 1 min, and subsequently analysed via CLSM. After 24 h, phase separation was observed and the sample was analysed again via CLSM.Preparation of w/w emulsions using PS latex nanoparticles
UHMW PDMA (15 mg) and pullulan (15 mg) were dissolved in water (470 mg, pH = 5 or 9) to form a 3.0/3.0 wt% solution. PS latex nanoparticles (10 μL of a 10% stock solution) were dispersed in water (490 mg, pH = 5 or 9) to generate a 0.2 wt% dispersion. Both solutions were combined to obtain a concentration of PDMA/Pull 1.5/1.5 wt% and 0.1 wt% PS nanoparticles in the mixture. The mixture was subjected to ultrasonic treatment for 2 min, shaken by hand for 1 min, and subsequently analysed via CLSM. After 24 h, phase separation was observed and the sample was analysed again via CLSM.Preparation of w/w emulsions with additional labelled PDMA and Pull
PDMA (12 mg), RITC–PDMA (3 mg), pullulan (12 mg), and FITC–pullulan (3 mg) were dissolved in water (470 mg, pH = 5 or 9) to form a 3.0/3.0 wt% solution. PDMAEMA-b-POEGMA (10 mg) were dispersed in water (490 mg, pH = 5 or 9) to generate a 2.0 wt% dispersion. Both mixtures were combined to obtain a concentration of PDMA/Pull 1.5/1.5 wt% and 1.0 wt% PDMAEMA-b-POEGMA in the mixture. The mixture was subjected to ultrasonic treatment for 2 min, shaken by hand for 1 min, and subsequently analysed via CLSM. After 24 h, phase separation was observed and the sample was analysed again via CLSM.Results and discussion
Synthesis of various poly(N,N-dimethylacrylamides) via RAFT polymerisation
For ATPS formation, PDMA with various molar masses was synthesised at first. RAFT polymerisation is a superb avenue for the synthesis of polyacrylamides such as PDMA. For the lower and medium molar mass range, PDMA was synthesised via classical RAFT polymerisation. EMP was used as the chain transfer agent and the reaction was performed at 65 °C with AIBN as initiator in DMF. In the case of the UHMW PDMA, EMP was used as photoiniferter in acetate buffer via UV light (nail lamp, λ = 365 nm). Conversions were determined by 1H-NMR (Fig. S1–3†) revealing quantitative monomer conversion for low molar mass PDMA, 75% monomer conversion for medium molar mass PDMA and quantitative monomer conversion for UHMW PDMA. The low and medium molar mass PDMAs were analysed via GPC in NMP against PS standards (Fig. S4a and Table S1†) indicating in molar mass of Mn = 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1 and Mn = 80
900 g mol−1 and Mn = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a dispersity of Đ = 1.11 and Đ = 1.06, respectively. UHMW PDMA was obtained with a molar mass of Mn = 1.07 × 106 g mol−1 and a dispersity of Đ = 1.40, as analysed via SEC with MALS detection in 0.1 N NaNO3 (Fig. S4b and Table S1†).
000 g mol−1 and a dispersity of Đ = 1.11 and Đ = 1.06, respectively. UHMW PDMA was obtained with a molar mass of Mn = 1.07 × 106 g mol−1 and a dispersity of Đ = 1.40, as analysed via SEC with MALS detection in 0.1 N NaNO3 (Fig. S4b and Table S1†).
ATPS of PDMA and pullulan
In order to study the phase behaviour of PDMA and pullulan in water, ATPS formation of pullulan and PDMA of different molar mass in aqueous solution was investigated. For that, phase diagrams were elucidated for three different molar masses of PDMA (24k, 80k and 1 × 106 g mol−1) with commercial pullulan.To develop the ATPS phase diagram, start solutions of total 10 wt% polymer (PDMA/Pull) concentration (9/1, 7.5/2.5, 5/5, 2.5/7.5, 1/9 wt%, respectively) were prepared. Subsequently, the solutions were mixed, equilibrated at ambient temperature to demix, investigated, and diluted to find the concentration at which only one phase is observed (Fig. 1a). The last concentration with visible phase separation, was used as a data point for the binodal, which is the line separating the one- and two-phase area in the phase diagram (Fig. 1b and Fig. S7†). A shift of the binodal was observed depending on PDMA molar mass. For the combination with low and medium molar mass PDMA the binodal is located at higher concentration. The lowest concentration for an observed phase separation on equal polymer concentration were at 4.0/4.0 wt% for PDMA24k and 3.1/3.1 wt% for PDMA80k. However, for the ATPS using UHMW PDMA the binodal is located at significantly lower concentrations with a lowest concentration for an observed phase separation on equal concentrations of polymer of 1.25/1.25 wt%. These results show the significant influence of the molar mass of the polymers for the minimum required polymer content for a phase separation. The higher the molar mass, the lower the required concentration for a stable ATPS, which is an effect known from literature.13,18 In order to quantify the demixing of the individual polymer types in the ATPS, the location and concentration of each polymer was detected via1H-NMR of each phase (Fig. S5 and S6†) employing DMF as internal standard. The results showed a clear separation of the polymers after 24 h. PDMA was enriched in the upper phase and pullulan was enriched in the lower phase of the ATPS. However, in each phase a residual amount of the opposite polymer was present in the respective depleted phases. After 24 h the partition coefficients (eqn (S1)†) for the ATPS of PDMA106 and Pull were for 17.9 for PDMA106 and 0.067 for Pull in the upper phase as well as 0.056 and 14.87 for PDMA106 and 0.067 for Pull in the lower phase.
W/w emulsion stabilised by polymer particles
To form a w/w emulsion, the ATPS of UHMW PDMA and pullulan was chosen due to the low polymer concentration required for formation of a stable ATPS. An ATPS of UHMW PDMA and commercial pullulan was prepared at a concentration of 1.5/1.5 wt%, which was selected because it is placed far enough in the two-phase area of the phase diagram to assure a stable ATPS. To stabilise the w/w emulsion, negatively charged PS latex nanoparticles and PDMAEMA-b-POEGMA aggregate particles were used (Scheme 2).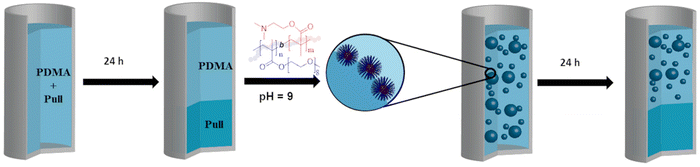 | ||
| Scheme 2 ATPS formation of PDMA and pullulan and w/w-emulsion stabilised with the block copolymer PDMAEMA-b-POEGMA at pH = 9 (POEGMA block shown in blue; PDMAEMA block shown in red). | ||
In order to form w/w emulsions, negatively charged PS latex nanoparticles with around 100 nm diameter were added to the ATPS to give a final stabiliser concentration of 0.1 wt%. Subsequently, the mixture was subjected to ultrasonic treatment for 2 min and shaken by hand for 1 min. The mixture turned cloudy, which indicated the formation of an emulsion stabilised with PS nanoparticles. In the following, the emulsion was analysed directly after preparation via bright-field microscopy displaying droplet formation (Fig. S8†). The average droplet size directly after preparation was 32 ± 5 μm at pH = 6 and 76 ± 26 μm at pH = 9. After approximately 3 h the mixture started to phase-separate, which was completed after 24 h. The upper phase remained cloudy, and the lower phase turned clear. Both phases were analysed via bright-field microscopy (Fig. S8†), revealing droplets in the upper phase and no droplets in the lower phase. The average droplet size in the cloudy phase after phase separation was around 49 ± 22 μm at pH = 6 and 118 ± 53 μm at pH = 9. The results revealed droplet size increase during phase separation for the emulsion stabilised by PS. Additionally, the average droplet size of the emulsion in basic solution was significantly higher in comparison to the emulsion in acidic solution. One key factor for the droplet size is the preparation of the emulsion as it was treated ultrasound and shaken by hand only. More defined droplets could be generated e.g. via microfluidics.
To investigate a pH sensitive w/w emulsion stabiliser, PDMAEMA-b-POEGMA was employed. PDMAEMA-b-POEGMA was synthesised via RAFT polymerisation using 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid as a chain transfer agent. The block copolymer was obtained with a molar mass of 108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a dispersity of Đ = 1.37 (Fig. S9†). The block copolymer PDMAEMA-b-POEGMA was employed due to the pH sensitivity of the PDMAEMA block leading to aggregate formation under basic conditions.45,50 The pKa of PDMAEMA is in a range between 7 and 7.5.45,51 Due the tertiary amine in PDMAEMA, charge density can be adjusted via protonating and deprotonating of the amine using different pH.50,51 Dynamic-light scattering (DLS) revealed aggregates with a hydrodynamic diameter of 20 nm in basic aqueous solution (pH = 9) and a hydrodynamic diameter of 3 nm in acidic aqueous solution indicating particle formation and free block copolymer chains, respectively (Fig. S10†).
000 g mol−1 and a dispersity of Đ = 1.37 (Fig. S9†). The block copolymer PDMAEMA-b-POEGMA was employed due to the pH sensitivity of the PDMAEMA block leading to aggregate formation under basic conditions.45,50 The pKa of PDMAEMA is in a range between 7 and 7.5.45,51 Due the tertiary amine in PDMAEMA, charge density can be adjusted via protonating and deprotonating of the amine using different pH.50,51 Dynamic-light scattering (DLS) revealed aggregates with a hydrodynamic diameter of 20 nm in basic aqueous solution (pH = 9) and a hydrodynamic diameter of 3 nm in acidic aqueous solution indicating particle formation and free block copolymer chains, respectively (Fig. S10†).
For emulsion formation, PDMAEMA-b-POEGMA was dissolved at pH = 9 and used at a concentration of 1 wt% in combination with the ATPS formed by PDMA106 and Pull at a concentration of 1.5/1.5 wt%. Afterwards, the mixture was treated like the PS nanoparticle stabilised emulsion employing ultrasound and shaking by hand. In contrast to the PS emulsion, the mixture stayed clear, which might be explained with the difference in stabiliser particle size (100 nm vs. 20 nm). Similar to the PS nanoparticle-stabilsed emulsion, phase separation started after around 3 h, which was completed again after 24 h. The mixture was analysed directly after preparation and after 24 h via bright-field microscopy. Bright-field microscopy and CLSM showed droplets directly after preparation and in the upper phase after 24 h (Fig. 2, 3 and S11†). No droplets were observed in the lower phase. The average droplet size directly after preparation was 86 ± 56 μm at pH = 9 and after 24 h, in the cloudy phase the average droplet size was around 107 ± 31 μm at pH = 9. Bright field microscopy and CLSM images indicated successful w/w emulsion formation from the ATPS formed by UHMW PDMA and pullulan using PS nanoparticles or PDMAEMA-b-POEGMA as stabiliser at pH = 9.
In order to localise the polymers in the emulsion, PDMA was labelled with RITC and pullulan was labelled with FITC. The emulsions were prepared for both stabilisers as described before and analysed via CLSM directly after preparation and after 24 h (Fig. 2). For the system stabilised with PS nanoparticles, PDMA was located over the entire sample directly after the preparation. However, pullulan was only present inside the emulsion droplets. After 24 h, similar to the bright field images, emulsion droplets were observed only in the upper phase of the two-phase system (Fig. 2a and b). In the upper phase, PDMA was located again over the entire sample and pullulan was enriched inside the droplets (Fig. 2e and f). The CLSM images for the system stabilised with PDMAEMA-b-POEGMA displayed, directly after preparation, PDMA located over the entire sample and pullulan enriched inside the droplets. After 24 h emulsion droplets were observed in the upper phase only. Similar to the w/w emulsion stabilised with PS-nanoparticles, PDMA was located over the entire sample and the pullulan enriched inside the droplets. Overall, the CLSM results showed that for both stabilisers the polymers PDMA and pullulan are predominately present in different phases. Pullulan enriched inside the droplets and the UHMW PDMA enriched outside the droplets. Nevertheless, the CLSM images of the PS nanoparticle system indicate the presence of PDMA inside the droplets as well. The reason for the increased amount of PDMA in the Pull enriched droplets after phase separation could be the higher concentration of PDMA in the upper phase and shift of the polymer ratio after phase separation. Another reason could be the non-perfect phase separation of the ATPS system PDMA and pullulan (Fig. S6†). Even after a period of 24 h there are approximately 10% of each polymer present in the opposite enriched phase. All CLSM images were prepared with only one dye present at a time. Furthermore, the results indicate the dye functionalisation does not have an influence on the partitioning of the polymers in the emulsion.
pH-Sensitive w/w emulsions
In order to prove the pH influence and sensitivity of the emulsion stabilised by PDMAEMA-b-POEGMA, the emulsion was prepared under acidic and basic conditions. The emulsions were prepared with a polymer concentration of 1.5/1.5 wt% and stabilised with 1.0 wt% PDMAEMA-b-POEGMA. Two different emulsions were prepared, one at pH = 5 and one at pH = 9. The mixture was subjected to ultrasonic treatment for 2 min and shaken by hand for 1 min. The emulsion was analysed directly after preparation via bright-field microscopy (Fig. 3a and b). Furthermore, both samples were analysed by bright-field microscopy after 24 h. For the mixture prepared at pH = 5, bright-field microscopy shows the formation of large droplets (>200 μm) directly after preparation. The significant larger droplets indicate that PDMAEMA-b-POEGMA could not successfully stabilise the w/w emulsion at pH = 5. The larger droplets indicate coalescence on the way to a complete phase separation of the mixture. The bright-field images after 24 h showed no droplets at all, which substantiate the unsuccessful stabilisation of the emulsion. The insufficient stabilisation is indicated by DLS showing only small particle diameters of the block copolymer under acidic conditions corresponding to single polymer coils (Fig. S10†) that are not capable of w/w emulsion stabilisation. However, at pH = 9 bright-field images display droplet formation directly after preparation, which indicates a presence of an emulsion stabilised by PDMAEMA-b-POEGMA aggregates.Furthermore, droplets were only observed in the upper phase after 24 h (Fig. 3d). Overall, the w/w emulsion using the ATPS of UHMW PDMA and pullulan could be stabilised using the block copolymer PDMAEMA-b-POEGMA. The emulsion is only stable in basic aqueous solution (pH = 9), due to the aggregation of the block copolymer under those conditions. In acidic solution however, the emulsion could not be stabilised by the block copolymer PDMAEMA-b-POEGMA. In contrast, non-pH responsive PS nanoparticles were capable of stabilising the w/w emulsion in basic and acidic medium (Fig. S8†).
Our results showed the influence of the pH value on the stabilisation of the w/w emulsion of UHMW PDMA and pullulan stabilised by PDMAEMA-b-POEGMA. In order to prove the sensitivity and switchability of stabilisation using PDMAEMA-b-POEGMA, the pH value was changed in the mixture. The emulsion was prepared with a polymer concentration of 1.5/1.5 wt%, a block copolymer concentration of 1.0 wt% and a start pH value of pH = 10. The mixture was subjected to ultrasonic treatment for 2 min and shaken by hand for 1 min. A small sample was taken for analysis. Afterwards, the pH was changed to pH = 5 using conc. HCl and the sample subjected to ultrasonic treatment and shaking by hand again. A sample was retrieved for analysis and the pH was changed to pH = 10 using NaOH solution and the sample was redispersed. All samples were analysed via bright-field microscopy (Fig. 4). For the first sample at pH = 10 the microscope images showed droplet formation (Fig. 4a). After the pH change to acidic, the displayed droplets were significantly larger due to an unstable w/w emulsion and onset of phase separation in the sample (Fig. 4b). However, after a pH change to basic, the emulsion droplets were stable again (Fig. 4c). Overall the results show that the w/w emulsion of the UHMW PDMA and pullulan, stabilised by PDMAEMA-b-POEGMA is pH sensitive. In basic aqueous solution the emulsion is stable. If the pH value is changed to acidic, the block copolymer is not stabilising the emulsion anymore. The w/w emulsion can be stabilised again with a change of the pH back to basic. However, pH switches are limited by the change in concentration as the sample is diluted by a small amount of acidic or basic medium during the pH switch. If the polymer concentration will drop under the limit of the ATPS binodal the emulsion is not stable anymore.
Conclusions
ATPS and w/w emulsions are a major topic in current polymer chemistry. In here, a new ATPS consisting of PDMA and pullulan was investigated. The stability of the PDMA/pullulan ATPS is depending on the molar mass of the polymers. PI-RAFT polymerisation allows an easy access to higher molar mass PDMA, which enables a significant decrease in the required concentration for a stable ATPS. In addition, the ATPS was used to form w/w emulsions stabilised by PS nanoparticles or the block copolymer PDMAEMA-b-POEGMA in basic aqueous solution. The emulsion was stable in the mixture and after phase separation in the upper phase. Pullulan was enriched inside the droplets and PDMA was located all over the sample. Furthermore, the w/w emulsion stabilised by PDMAEMA-b-POEGMA was pH sensitive, i.e. depending on the pH, the emulsion could be stabilised or not stabilised by the block copolymer. The development of this novel pH sensitive w/w emulsion will be open new pathways for various applications, for example for the encapsulation of biomolecules or pH sensitive biomolecule purification.Author contributions
AP: conceptualisation, data collection, writing original draft. BS: conceptualisation, review and editing the draft, supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge Marlies Gräwert and June Southall for SEC measurements. B. S. and A. P. acknowledge funding from the University of Glasgow and the German Research Foundation (grant no. SCHM 3282/3-1).Notes and references
- R. Haag and F. Kratz, Angew. Chem., Int. Ed., 2006, 45, 1198–1215 CrossRef CAS.
- J.-W. Rhim, H.-M. Park and C.-S. Ha, Prog. Polym. Sci., 2013, 38, 1629–1652 CrossRef CAS.
- M. Rito-Palomares, A. Negrete, E. Galindo and L. Serrano-Carreon, J. Chromatogr. B: Biomed. Sci. Appl., 2000, 743, 403–408 CrossRef CAS.
- C. D. Keating, Acc. Chem. Res., 2012, 45, 2114–2124 CrossRef CAS PubMed.
- J. A. Asenjo and B. A. Andrews, J. Chromatogr. A, 2011, 1218, 8826–8835 CrossRef CAS PubMed.
- P. da Rocha Patrício, M. C. Mesquita, L. H. M. da Silva and M. C. H. da Silva, J. Hazard. Mater., 2011, 193, 311–318 CrossRef PubMed.
- J. F. Pereira, M. G. Freire and J. A. Coutinho, Fluid Phase Equilib., 2020, 505, 112341 CrossRef CAS.
- P. Albertsson, Biochem. Pharmacol., 1961, 5, 351–358 CrossRef CAS PubMed.
- R. Hatti-Kaul, Mol. Biotechnol., 2001, 19, 269–277 CrossRef CAS.
- Y. Chao and H. C. Shum, Chem. Soc. Rev., 2020, 49, 114–142 RSC.
- J. Ryden and P.-a. Albertsson, J. Colloid Interface Sci., 1971, 37, 219–222 CrossRef CAS.
- Å. Gustafsson, H. Wennerström and F. Tjerneld, Polymer, 1986, 27, 1768–1770 CrossRef.
- D. Forciniti, C. Hall and M.-R. Kula, Fluid Phase Equilib., 1991, 61, 243–262 CrossRef CAS.
- L. H. M. d. Silva, J. S. Coimbra and A. J. d. A. Meirelles, J. Chem. Eng. Data, 1997, 42, 398–401 CrossRef.
- W. J. Frith, Adv. Colloid Interface Sci., 2010, 161, 48–60 CrossRef CAS PubMed.
- A. P. Constantinou, A. Tall, Q. Li and T. K. Georgiou, J. Polym. Sci., 2022, 60, 188–198 CrossRef CAS.
- A. Plucinski, M. Pavlovic and B. V. K. J. Schmidt, Macromolecules, 2021, 5366–5375 CrossRef CAS.
- Y.-T. Wu, D.-Q. Lin and Z.-Q. Zhu, Fluid Phase Equilib., 1998, 147, 25–43 CrossRef CAS.
- J. Esquena, Curr. Opin. Colloid Interface Sci., 2016, 25, 109–119 CrossRef CAS.
- T. Nicolai and B. Murray, Food Hydrocolloids, 2017, 68, 157–163 CrossRef CAS.
- D. M. A. Buzza, P. D. Fletcher, T. K. Georgiou and N. Ghasdian, Langmuir, 2013, 29, 14804–14814 CrossRef CAS PubMed.
- J. W. Kim, D. Lee, H. C. Shum and D. A. Weitz, Adv. Mater., 2008, 20, 3239–3243 CrossRef CAS.
- D. Forciniti, C. Hall and M. Kula, J. Biotechnol., 1990, 16, 279–296 CrossRef CAS.
- E. Scholten, J. E. Visser, L. M. Sagis and E. van der Linden, Langmuir, 2004, 20, 2292–2297 CrossRef CAS.
- H. Firoozmand, B. S. Murray and E. Dickinson, Langmuir, 2009, 25, 1300–1305 CrossRef CAS PubMed.
- J. Zhang, J. Hwang, M. Antonietti and B. V. K. J. Schmidt, Biomacromolecules, 2018, 20, 204–211 CrossRef PubMed.
- J. Zhang, B. Kumru and B. V. K. J. Schmidt, Langmuir, 2019, 35, 11141–11149 CrossRef CAS PubMed.
- M. Pavlovic, A. Plucinski, L. Zeininger and B. V. K. J. Schmidt, Chem. Commun., 2020, 56, 6814–6817 RSC.
- T. Merland, L. Waldmann, O. Guignard, M.-C. Tatry, A.-L. Wirotius, V. Lapeyre, P. Garrigue, T. Nicolai, L. Benyahia and V. Ravaine, J. Colloid Interface Sci., 2022, 608, 1191–1201 CrossRef CAS PubMed.
- L. Tea, T. Nicolai and F. Renou, Langmuir, 2019, 35, 9029–9036 CrossRef CAS PubMed.
- R. A. de Freitas, T. Nicolai, C. Chassenieux and L. Benyahia, Langmuir, 2016, 32, 1227–1232 CrossRef CAS PubMed.
- J. P. Machado, L. Benyahia and T. Nicolai, J. Colloid Interface Sci., 2021, 603, 633–640 CrossRef CAS PubMed.
- J. P. Machado, I. Capron, R. A. de Freitas, L. Benyahia and T. Nicolai, Food Hydrocolloids, 2022, 124, 107320 CrossRef CAS.
- I. W. Sutherland, Trends Biotechnol., 1998, 16, 41–46 CrossRef CAS.
- R. S. Singh, N. Kaur, M. Hassan and J. F. Kennedy, Int. J. Biol. Macromol., 2021, 166, 694–706 CrossRef CAS PubMed.
- M. Rekha and C. P. Sharma, Trends Biomater. Artif. Organs, 2007, 20, 116–121 Search PubMed.
- H. Hosseinkhani, T. Aoyama, O. Ogawa and Y. Tabata, J. Controlled Release, 2002, 83, 287–302 CrossRef CAS PubMed.
- A. Plucinski, Z. Lyu and B. V. K. J. Schmidt, J. Mater. Chem. B, 2021, 9, 7030–7062 RSC.
- L. T. Carvalho, R. M. Moraes, A. J. R. Teixeira, D. B. Tada, G. M. Alves, T. M. Lacerda, J. C. Santos, A. M. Santos and S. F. Medeiros, J. Appl. Polym. Sci., 2021, 138, 51344 CrossRef CAS.
- A. Plucinski, J. Willersinn, R. B. Lira, R. Dimova and B. V. K. J. Schmidt, Macromol. Chem. Phys., 2020, 2000053 CrossRef CAS.
- J. Willersinn, A. Bogomolova, M. B. Cabré and B. V. K. J. Schmidt, Polym. Chem., 2017, 8, 1244–1254 RSC.
- B. V. K. J. Schmidt, Macromol. Chem. Phys., 2018, 219, 1700494 CrossRef.
- R. N. Carmean, T. E. Becker, M. B. Sims and B. S. Sumerlin, Chem, 2017, 2, 93–101 CAS.
- R. N. Carmean, M. B. Sims, C. A. Figg, P. J. Hurst, J. P. Patterson and B. S. Sumerlin, ACS Macro Lett., 2020, 9, 613–618 CrossRef CAS PubMed.
- D. de Morais Zanata and M. I. Felisberti, Eur. Polym. J., 2022, 111069 CrossRef.
- B. V. K. J. Schmidt, M. Hetzer, H. Ritter and C. Barner-Kowollik, Macromolecules, 2011, 44, 7220–7232 CrossRef CAS.
- J. Skey and R. K. O'Reilly, Chem. Commun., 2008, 4183–4185 RSC.
- A. De Belder and K. Granath, Carbohydr. Res., 1973, 30, 375–378 CrossRef CAS.
- J. Liu and Y. Tabata, J. Drug Targeting, 2010, 18, 602–610 CrossRef CAS PubMed.
- V. Bütün, S. Armes and N. Billingham, Polymer, 2001, 42, 5993–6008 CrossRef.
- Z. Dong, H. Wei, J. Mao, D. Wang, M. Yang, S. Bo and X. Ji, Polymer, 2012, 53, 2074–2084 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py00469k |
| This journal is © The Royal Society of Chemistry 2022 |





