 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nitroxide mediated radical polymerization for the preparation of poly(vinyl chloride) grafted poly(acrylate) copolymers†
Aurélien
Vebr
 a,
Magali
Dallegre
a,
Laurent
Autissier
a,
Magali
Dallegre
a,
Laurent
Autissier
 a,
Charlotte
Drappier
a,
Charlotte
Drappier
 *b,
Karel
Le Jeune
*b,
Karel
Le Jeune
 *b,
Didier
Gigmes
*b,
Didier
Gigmes
 *a and
Anthony
Kermagoret
*a and
Anthony
Kermagoret
 *a
*a
aAix Marseille Univ, CNRS, ICR, Marseille, France. E-mail: didier.gigmes@univ-amu.fr; anthony.kermagoret@univ-amu.fr
bWESTLAKE CATALYSE, Zone Athélia 3, 139 Voie Atlas, 13600 La Ciotat, France. E-mail: Charlotte.Drappier@westlake.com; Karel.Lejeune@westlake.com
First published on 13th April 2022
Abstract
In view to control the thermal properties of PVC without the use of toxic phthalate derivatives, alkoxyamines were grafted onto an azide modified PVC, through copper catalyzed azide–alkyne cycloaddition (CuAAC), to initiate a radical polymerization of acrylate monomers including butyl acrylate (BA) and polyethylglycol acrylate (PEGA) monomers. A series of PVC-g-PBA copolymers having various glass transition temperatures (Tg) between 63.5 to −10.5 °C were obtained. The PVC-g-PEGA copolymers presented a semi-crystalline behavior with a Tg of −4 °C and a Tm of 43 °C. This method, combining CuAAC click reaction and radical polymerization, was efficient to covalently link a plasticizing group on PVC. This “grafting from” strategy using alkoxyamine functionalized PVC can be expanded to produce new materials via nitroxide mediated polymerization of various monomers.
1. Introduction
With a global production of around 44 million tons per year, poly(vinyl chloride) PVC is one of the most industrially produced polymer in the world. While pure PVC is a highly rigid thermoplastic, its high tolerance to various plasticizers, lowering the glass transition temperature Tg of the material, allows to control its flexibility and ductility for a wide range of applications.1,2Usually plasticizers are introduced during the extrusion of the polymer. However, not covalently linked to the polymer, plasticizers can migrate outside the material over time, leading to contamination of the environment and loss of material properties.
For a long time, phthalate plasticizers were mostly used as additives to tune the PVC properties. But, for many of them, a high toxicity has been clearly established, and they were classified as carcinogenic, mutagenic or toxic to reproduction CMR products.3–8 In particular, the most used one, di-2-ethylhexylphthalate DEHP, causes endocrine disruption with dramatic consequences for the development of children.9,10
While they are progressively banned for sensitive applications, and the use of bio-sourced and non-toxic plasticizers is currently in progress,11–15 phthalates are still expected to represent around 80% of the global plasticizer demand in 2022.16 However the safest plasticizers are still able to leach, reducing the lifespan of products.
PVC business is increasingly challenging high-performance and sustainable applications for demanding markets such as medical, building and construction, or automotive, and the covalent grafting of plasticizers is an efficient and elegant way to prevent the plasticizer migration,1,17,18 affording even more safe and robust flexible PVC materials.
Different methods to functionalize PVC have been explored.17,19–21 One way consisted in the substitution of a chloride atom of the PVC chain22 in order to graft a pendant group. Following this strategy, Michel et al. and Reinecke et al. developed the grafting of thiolate group bearing phthalate derivatives and produced PVC with tunable Tg.23–25 Similarly, the PVC grafting of various groups, such as poly(styrene),26 lauraldehyde-derived Mannich base,27 cardanol alkyl ether,28 dehydroabietic group,29via a pending amine group or epoxidized aliphatic methyl ester30 has been explored to tune the properties of the polymer.
Otherwise, the introduction of azide groups via the Cl substitution of PVC can be a first step to the grafting of pending functions.20,31,32 Then the copper catalyzed azide–alkyne cycloaddition (CuAAC) of alkynyl groups allowed the introduction of plasticizers including phthalate derivatives,33,34 cardanol35 or polyglycerol.36
Other strategies based on “grafting from” methods have been explored to initiate a polymerization process from the PVC chain. Atom transfer radical polymerization37 is an attractive method to prepare functionalized PVC regarding the control of the polymerization of a wide range of monomers, and the reactive chloride atom of the PVC could be potential initiators since the dormant ATRP species is based on an activated carbon-halide site.38,39 As demonstrated by Percec et al., the PVC defects (allylic defects or chloride atom on tertiary carbon) present the best ATRP initiator sites.40,41 However the very low amount of reactive chloride atoms of the PVC toward ATRP activation limits the number of potential polymerization sites of the PVC backbone.42 Despite this strong limitation, the modification of PVC materials was achieved via ATRP with various monomers.38,43–51 In contrast, the radical polymerization from reversible addition fragmentation chain-transfer RAFT agents grafted on PVC remains limited.52
Nitroxide mediated polymerization NMP is an efficient reversible-deactivation radical polymerization RDRP method for macromolecular engineering.53–55 In contrast to ATRP or RAFT, NMP does not require a metal catalyst or sulfur compound, which is a strong advantage to produce safe plastics. Entezami et al. used harsh conditions combining Friedel–Crafts, bromination and nucleophilic substitution reactions to graft TEMPO on PVC and to initiate the styrene NMP from the modified chains.56
Here, we choose to introduce SG1 alkoxyamines onto the PVC backbone to initiate a NMP process and to build pending polymer chains to tune the Tg of the graft PVC. Our strategy consists of (i) the controlled substitution of chloride atoms with azide functions, followed by (ii) the introduction of the alkoxyamine possessing an alkynyl group via CuAAC click chemistry and, finally, (iii) initiation of a radical polymerization of acrylate monomers.
2. Results and discussion
2.1. Alkoxyamine
The PVC properties were tuned by a “grafting from” polymerization of acrylate monomers via a grafted alkoxyamine on the polymer chain and the initiation of a NMP process. In this contribution, the linking of the alkoxyamines through a triazole group was achieved via a CuAAC click reaction between a pendant alkynyl group and a PVC-N3 polymer.Here, we focus on nitroxide SG1 which is a versatile NMP controlling agent.53,57–59 In particular, the BlocBuilder™ alkoxyamine or MAMA-SG1,60 resulting from the trapping of a 2-methyl-propionic acid radical with SG1, is a highly efficient polymerization initiator.
Firstly, we chose to exploit the terminal acid group of MAMA-SG1 to introduce an alkynyl group. Following the literature, we first performed the reaction of hydroxysuccinimide on MAMA-SG1 to modify the terminal acid group into a N-acetoxy-succinimide function and then the resulting MAMASG1-NHS was reacted with propargyl amine to yield the alkoxyamine 1 (Fig. 1). The alkoxyamine 1 was fully characterized by 1H and 13C NMR (Fig. S2–S6†), mass spectrometry and IR (Fig. S7†).
Another strategy to introduce the alkynyl group consisted of a 1,2 intermolecular radical addition (IRA) of MAMA-SG161 onto a propargyl acrylate (Fig. 1). Under thermal activation, MAMA-SG1 liberated the 2-methyl-propionic acid radical which reacted on the double bond of the propargyl acrylate and the resulting radical was trapped with SG1, leading to alkoxyamine 2 (Fig. 1). The alkoxyamine 2 was fully characterized by 1H and 13C NMR (Fig. S8 and S9†), mass spectrometry and IR (see below). In addition, the activation energy (Ea) of the C–O bond homolysis62 of alkoxyamine 2 was evaluated to 117 kJ mol−1 by electron spin resonance (ESR) spectroscopy at 80 °C in tert-butyl benzene (see ESI†).
2.2. PVC-N3
The azide substitution on a chloride atom of the PVC units is a well-known technique to introduce functionalities via copper catalyzed azide–alkyne cycloaddition (CuAAC) click reactions.20 However, a fine control of the concentration of azide groups on the PVC chains is a dramatic issue since this parameter directs the number of functionalities on the graft polymer.In this contribution, we utilized a PVC provided by Aldrich (MW = 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and Mn = 22
000 g mol−1 and Mn = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, according to Aldrich). PVC and sodium azide (PVC/NaN3 wt-ratio of 1/1) reacted in DMF at 50 or 60 °C for 0.33–16.5 h (Table S1†). The introduction of the azide groups on the PVC was confirmed by 1H NMR (Fig. S10†) and IR with N3 characteristic strong signal33 at around 2100 cm−1. The presence of –Cl group on PVC was confirmed with the C–Cl stretching signal at 610 cm−1 (see IR spectrum below).63 The concentrations of azide modified monomeric units were determined by elemental analysis (Table S1†).
000 g mol−1, according to Aldrich). PVC and sodium azide (PVC/NaN3 wt-ratio of 1/1) reacted in DMF at 50 or 60 °C for 0.33–16.5 h (Table S1†). The introduction of the azide groups on the PVC was confirmed by 1H NMR (Fig. S10†) and IR with N3 characteristic strong signal33 at around 2100 cm−1. The presence of –Cl group on PVC was confirmed with the C–Cl stretching signal at 610 cm−1 (see IR spectrum below).63 The concentrations of azide modified monomeric units were determined by elemental analysis (Table S1†).
At 60 °C in DMF, between 4.4% and 43% of the –(CH2–CHCl)– units were converted into –(CH2–CHN3)– units, depending on the reaction time and temperature (Table 1). The concentration of grafted N3 increased with reaction time. However, when reactions longer than 6 h were performed, we did not observe a significant increase of grafted N3 and a plateau of around 40% of modified PVC units was reached (Fig. S11†). Under very similar conditions, Chung, Kwak et al. reported a degree of azidation of 3.6%,36 close to our results. Similarly, Braslau et al. reported a degree of azidation of 15% for a reaction time of 2.5 h at 62 °C in DMF.33 The Cl substitution with N3 would depend on the PVC structure (molar weight, number of defects, etc.).64
| Entry | Time [h] | Temperature (°C) | % of N3 unita |
|---|---|---|---|
| Conditions: DMF, PVC/NaN3 wt-ratio of 1/1.a % of Cl atom substituted by azide in PVC, determined by elemental analysis.b PVC provided by Aldrich. | |||
| 1b | 1 | 60 | 4.4 |
| 2 | 1 | 60 | 4.7 |
| 3 | 2.75 | 60 | 18 |
| 4 | 4 | 60 | 27 |
| 5 | 5.5 | 60 | 38 |
| 6 | 8.25 | 60 | 32 |
| 7 | 11 | 60 | 44 |
| 8 | 16.5 | 60 | 33 |
| 9 | 0.33 | 50 | 1.4 |
| 10 | 1 | 50 | 3.4 |
To reduce the number of chloride substitution with the azide group, the reaction was performed at 50 °C and 1.4% and 3.4% of the PVC units were functionalized after 20 min and 1 h, respectively (Table 1).
Analyzed by SEC, the PVC containing 4.4% of azide monomeric units presented very similar molar weight and dispersity (Mw = 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1, Đ = 2.0, SEC analyses using PS standard) to those of starting PVC, showing that the grafting of azide groups did not affect the structure of the PVC.
700 g mol−1, Đ = 2.0, SEC analyses using PS standard) to those of starting PVC, showing that the grafting of azide groups did not affect the structure of the PVC.
2.3. Grafting of alkoxyamine via click reaction
The grafting of alkoxyamine 1 on PVC-N3 (containing 4.4% of N3 units, see ESI†) was performed via a CuAAC click reaction (Fig. 2) in DMF at 30 °C for 24 h using CuBr/2,2′-bipyridine as catalyst. ATR-IR analysis (Fig. S12†) of the resulting polymer confirmed the disappearance of N3 signal and the presence of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N signals at 1650 cm−1, attributed to the conversion of azide into triazole, and the presence of amide signals (1760 cm−1) and diethylphosphonate signals (1034, 1010 and 958 cm−1) of the alkoxyamine 1. However, the modified polymer was almost insoluble in organic solvents (CDCl3, THF, DMF, DMSO)
N signals at 1650 cm−1, attributed to the conversion of azide into triazole, and the presence of amide signals (1760 cm−1) and diethylphosphonate signals (1034, 1010 and 958 cm−1) of the alkoxyamine 1. However, the modified polymer was almost insoluble in organic solvents (CDCl3, THF, DMF, DMSO)
It might be possible that during the reaction, the grafting alkoxyamine could liberate the nitroxide via the NO–CMe2 bond homolysis and made a radical coupling, leading to a cross-linked PVC insoluble in organic solvents. Furthermore, the use of polar solvents, such as DMF, dramatically decreases the bond dissociation energy of SG1 alkoxyamines,65 which also supports the theory of the bond homolysis, leading to a cross-linked and insoluble PVC.
Under the same conditions, the alkoxyamine 2 was successfully grafted using a PVC-N3 (Fig. 2) containing 4.7% of azide units (Table 1) and the resulting modified PVC bearing alkoxyamine 2 was soluble in DMF. Actually, alkoxyamine 2, having a NO–CH– bond, would have a higher bond dissociation energy for the C–O homolysis (estimated to 117 kJ mol−1 in tert-butylbenzene) compared to alkoxyamines which present a nitroxide NO–CMe2– bond (bond dissociation energy of MAMA-SG1 of 112.3 kJ mol−1 in tert-butylbenzene).66–68 The broad dispersity (Đ = 3.9, see ESI†) of PVC-Alk2 would suggest minor cross-linking side reactions occurring by radical coupling. During the purification process, the use of an aqueous solution of EDTA was important to remove the residual copper catalysts.69 The IR spectrum of the resulting material confirmed the consumption of azide groups (signals at 2100 cm−1) and the presence of new signals at 1600 cm−1 attributed to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bonds of triazole (Fig. 3). The presence of a broad signal at 1700 cm−1 and three signals at 1050, 1020 and 920 cm−1, attributed to –C
N– bonds of triazole (Fig. 3). The presence of a broad signal at 1700 cm−1 and three signals at 1050, 1020 and 920 cm−1, attributed to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of acrylate and the diethylphosphonate group of MAMA-SG1 respectively (Fig. 3), confirmed the grafting of alkoxyamine 2 on PVC. The 1H NMR spectrum showed the presence of –CH3 signals of grafted alkoxyamine 2 between 1.4 and 1.0 ppm of the PVC signals (–CHCl– broad signals between 4.6 and 3.9 ppm and –CH2– broad signals between 2.5 and 1.9 ppm, Fig. 4 and Fig. S13†). However, the –CH2– signals of alkoxyamine 2 remained very weak on the modified polymer and not clearly identified. According to integrals of 1H NMR spectrum and elemental analysis (Table S2†), 3.8% of units were functionalized with an alkoxyamine (Fig. S13†).
O of acrylate and the diethylphosphonate group of MAMA-SG1 respectively (Fig. 3), confirmed the grafting of alkoxyamine 2 on PVC. The 1H NMR spectrum showed the presence of –CH3 signals of grafted alkoxyamine 2 between 1.4 and 1.0 ppm of the PVC signals (–CHCl– broad signals between 4.6 and 3.9 ppm and –CH2– broad signals between 2.5 and 1.9 ppm, Fig. 4 and Fig. S13†). However, the –CH2– signals of alkoxyamine 2 remained very weak on the modified polymer and not clearly identified. According to integrals of 1H NMR spectrum and elemental analysis (Table S2†), 3.8% of units were functionalized with an alkoxyamine (Fig. S13†).
 | ||
| Fig. 3 ATR-IR spectra of PVC, PVC-N3 (4.7%), alkoxyamine 2, PVC-g-Alk2 and PVC-g-PBA (91 wt% of BA, initiated from PVC-g-Alk2). | ||
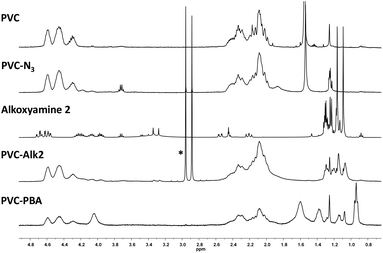 | ||
| Fig. 4 1H NMR spectra of PVC, PVC-N3 (4.7%), alkoxyamine 2, PVC-g-Alk2 and PVC-g-PBA (91 wt% of BA, initiated from PVC-g-Alk2), (CDCl3, 400 MHz, traces of DMF are marked with *). | ||
A second PVC-N3 containing a low concentration of 1.4% of azide units (entry 10 Table 1) was used to synthesize alkoxyamine-grafted PVC.
The IR spectrum confirmed the complete conversion of the azide sites and 1H NMR and the oxygen concentration (Table S2†), determined by elemental analysis, was in good agreement with a concentration of 1.4% of PVC units converted into a triazole.
2.4. Radical polymerization
Regarding its low Tg value of around −53 °C, poly(butyl acrylate) PBA is a prime candidate for graft PVC in view to prepare a migration-free soft material with tunable thermal properties.Firstly, we evaluated the ability of alkoxyamine 2 to initiate the radical polymerization of BA. At 100 °C in DMF, the polymerization process showed an increase of molar masses with BA conversion with a conversion up to 85% of BA achieved after 6 h but the dispersity remained relatively high (Đ between 1.7 and 2.3, Table S3†). The sudden increase of BA conversion was due to a hot spot consecutive to a gel effect observed between 2 and 4 h (Table S3†).
Since alkoxyamine 2 proved to be an efficient BA polymerization initiator, we exploited the PVC-alkoxyamine 2 having 3.8% of pending alkoxyamine units to prepare ten PVC-g-PBA copolymers (Fig. 5) containing a wide range of BA concentration (Table 2). The polymerization was performed at 100 °C in DMF, using a BA/alkoxyamine ratio of 67/1. The reactions times were between 6 and 12 h and the BA conversions were measured by 1H NMR from aliquots of the reaction media. BA conversions between 11% and 15% were achieved for a reaction time of 6 h, between 22% and 24% for 9 h, and up to 52% (Table 2 and Table S4†).
| Entry | Reaction time (h) | Conversion of BAa | BAa [wt%] |
T
g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [°C]
[°C] |
|---|---|---|---|---|
| Conditions: 100 °C, BA/DMF ratio of 1/4, BA/alkoxyamine ratio of 67/1.a Determined by 1H NMR.b Determined by DCS.c Aldrich PVC.d A low BA conv. was measured but the material contains a high BA wt%, maybe due to an experimental issue. | ||||
| 1c | — | — | 0 | 82 |
| 2 | 6 | 0.15 | 20 | 63.5 |
| 3 | 6 | 0.11 | 25 | 56 |
| 4 | 6 | 0.11 | 40 | 42 |
| 5 | 6 | 0.15 | 44 | 38 |
| 6 | 9 | 0.22 | 51 | 12.5 |
| 7 | 7 | 0.19 | 53 | 7.5 |
| 8 | 12 | 0.35 | 60 | 4.5 |
| 9d | 9 | 0.24 | 66 | 0.5 |
| 10 | 11 | 0.37 | 71 | −1 |
| 11 | — | 0.52 | 88 | −10.5 |
Under these reaction conditions, a series of ten PVC-g-PBA copolymers containing between 20–88 wt% of PBA (determined by 1H NMR, Table 2 and Fig. 4) was prepared.
The strong –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal observed at 1720 cm−1 on IR spectra of the copolymers confirmed the incorporation of BA monomers (Fig. 3). The IR spectra showed three signals at 1070, 1020 and 970 cm−1 due to the presence of diethylphophonate group of SG1 on the graft copolymers (Fig. 3). However, the presence of the nitroxide moiety was not clearly confirmed by 1H NMR probably due to its too low concentration (Fig. S13†). The glass transition temperatures (Tg) of the grafted polymers were determined by DSC (see ESI Fig. S14a–j†) and a wide range of Tg values between 63.5 to −10.5 °C, for PBA concentration of 20 and 88 wt% respectively, was achieved (Table 2). As shown in Fig. 6, the concentration of grafted PBA had a strong effect on the Tg of the modified PVC. The decrease of Tg values seemed to be linearly affected by the increase of the weight of grafted BA but a shift to lower Tg values was observed when more than 50 wt% BA were achieved (Fig. 6). The lowest Tg value was −10.5 °C despite the high concentration of grafted BA (88 wt%).
O signal observed at 1720 cm−1 on IR spectra of the copolymers confirmed the incorporation of BA monomers (Fig. 3). The IR spectra showed three signals at 1070, 1020 and 970 cm−1 due to the presence of diethylphophonate group of SG1 on the graft copolymers (Fig. 3). However, the presence of the nitroxide moiety was not clearly confirmed by 1H NMR probably due to its too low concentration (Fig. S13†). The glass transition temperatures (Tg) of the grafted polymers were determined by DSC (see ESI Fig. S14a–j†) and a wide range of Tg values between 63.5 to −10.5 °C, for PBA concentration of 20 and 88 wt% respectively, was achieved (Table 2). As shown in Fig. 6, the concentration of grafted PBA had a strong effect on the Tg of the modified PVC. The decrease of Tg values seemed to be linearly affected by the increase of the weight of grafted BA but a shift to lower Tg values was observed when more than 50 wt% BA were achieved (Fig. 6). The lowest Tg value was −10.5 °C despite the high concentration of grafted BA (88 wt%).
 | ||
| Fig. 6 Influence of BA concentration on Tg in PVC-g-PBA (conditions: 100 °C, BA/DMF ratio of 1/4, BA/alkoxyamine ratio of 67/1; conversions determined by 1H NMR; Tg determined by DSC). | ||
Indeed, as reported by Braslau et al., triazole groups have an anti-plasticizing impact.70
However, our PVC-g-PBA copolymers presented similar thermal properties that ones reported in the literature.
For instance, Chen et al. synthesized a PVC-g-PBA via BA ATRP initiation presenting a Tg value of 8.9 °C with 58 mol% of PBA (74 wt% of PBA).71 In contrast, Matyjaszewski et al. and Braslau et al. reported lower Tg values of −19 and −25 °C, respectively, for a PVC-g-PBA obtained by ATRP and containing 80 wt% of PBA.42,72 Additional parameters, such as the number of grafting sites or PVC chain length, would also affect the final properties of the functionalized PVC.17 Our strategy permitted to control the length of the grafted chains via NMP to provide a series of PVC-g-PBA copolymers having various Tg values.
Polyglycerol groups are efficient plasticizers of PVC.36 We extrapolated our strategy to the polymerization of polyethylene glycol acrylate (PEGA) monomer initiated by alkoxyamine grafted on PVC. A monomethylether PEG acrylate having a molar weight of 2000 g mol−1 (PEGA-2000) was reacted with PVC-Alk2 and led to three different PVC-g-PEGA copolymers containing 17, 31, and 53 wt% of PEGA (determined by 1H NMR).
The DSC analysis of the 17 wt% PVC-g-PEGA did not allow to determine indubitably the Tg of the graft polymer (Fig. S15a†).
Thus, dynamic mechanical analysis (DMA) experiments were performed, revealing a glass transition around 60 °C (Max E′′ = 54 °C, Max tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 67 °C) (Table 3, Fig. S16b†). As comparison, standard PVC exhibits a glass transition above 80 °C in the same condition (Max E′′ = 92 °C, tan
δ = 67 °C) (Table 3, Fig. S16b†). As comparison, standard PVC exhibits a glass transition above 80 °C in the same condition (Max E′′ = 92 °C, tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 98 °C, Fig. S16a†). Interestingly, DMA of the copolymer containing 31 wt% of PEGA indicated a semi-crystalline behavior, with a glass transition at a lower temperature (max E′′ = −4 °C, max tan
δ = 98 °C, Fig. S16a†). Interestingly, DMA of the copolymer containing 31 wt% of PEGA indicated a semi-crystalline behavior, with a glass transition at a lower temperature (max E′′ = −4 °C, max tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 23 °C) and a second transition around 40 °C (max tan
δ = 23 °C) and a second transition around 40 °C (max tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 43 °C Fig. S16c†). For the more PEGA enriched copolymers (53 wt% of PEGA) only a single transition around 40 °C (max tan
δ = 43 °C Fig. S16c†). For the more PEGA enriched copolymers (53 wt% of PEGA) only a single transition around 40 °C (max tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 41 °C, Fig. S16d†) is observed. The melting character of these transitions was confirmed for both products by DSC at respectively 50 °C and 52 °C (Fig. S15b and c†) and is close to the Tm value of the PEG acrylate monomer (53 °C, see ESI, Fig. S1†).
δ = 41 °C, Fig. S16d†) is observed. The melting character of these transitions was confirmed for both products by DSC at respectively 50 °C and 52 °C (Fig. S15b and c†) and is close to the Tm value of the PEG acrylate monomer (53 °C, see ESI, Fig. S1†).
A low concentration of PEGA-2000 monomer allowed to decrease the glass transition temperature of the copolymer. Unfortunately, the crystallinity of the PEG chains dramatically limits this strategy, affording a crystalline behaviour to the resulting copolymer.
3. Experimental
3.1. Materials
Chemicals and solvents were purchased from commercial sources (Aldrich, Acros, ABCR, TCI) and used without purification. PVC was provided by Aldrich (Mn = 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and Mw = 22
000 g mol−1 and Mw = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 according to Aldrich: Mn = 30
000 g mol−1 according to Aldrich: Mn = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 g mol−1, Đ = 2.1 from THF-SEC analyses using PS standard). MAMA SG1-NHS was synthesized according to the literature (see ESI†).73
400 g mol−1, Đ = 2.1 from THF-SEC analyses using PS standard). MAMA SG1-NHS was synthesized according to the literature (see ESI†).73
PEG-2000 acrylate (PEGA) was prepared according to the literature (see ESI†) using monoether-PEG and 2-propenoyl chloride.741H NMR (400 MHz, CDCl3, Fig. S1†) δ 6.36 (d, J = 17.3 Hz, 1H), 6.09 (dd, J = 17.3, 10.4 Hz, 1H), 5.77 (d, J = 10.4 Hz, 1H), 4.25 (t, J = 4.8 Hz, 2H), 3.58 (m, J = 5.5 Hz, 178H), 3.31 (s, 3H); THF-SEC using PS standard: Mn = 2700 g mol−1, Đ = 1.05.
NMR spectra were recorded on Bruker AVL 300 or 400 (1H-NMR 300.13 or 400.13, 13C-NMR 75.46 or 100.60 and 31P-NMR 121.4 and 161.9 MHz respectively) using CDCl3 (reference: 7.26 ppm or 77.16 ppm) or DMSO-D6 (2.50 ppm or 39.52 ppm) as solvent.
Splitting patterns are indicated as follows: s, singlet; d, doublet; t, triplet; m, multiplet; br m, broad multiplet. 13C-NMR-ATP (attached proton test) sequence was applied to characterize the compounds. High resolution mass spectrometry (electrospray ESI-MS) was recorded at Spectropole-AMU (Marseille, France, see ESI†). FTIR spectra were recorded on an IR-ATR (attenuated total reflectance IR) PerkinElmer spectrometer. Elemental analyses were purchased from ICSN CNRS laboratory (Gif-sur-Yvette, France) or Spectropole-AMU (Marseille, France, see ESI†). Temperature analysis (Tg, Tm) of the polymers were studied on a DSC Q20 (sequence: 5 min isotherm at −50 °C, then 10 °C min−1 temperature rate to 150 °C, sample mass: 8–15 mg). Values of Tg or Tm were determined from DSC thermograms using the universal V4.5A TA Instruments software. Dynamic mechanical analysis (DMA) experiments were performed at Westlake Catalyse on a PerkinElmer DMA 8000 apparatus, in torsion mode (1 Hz), between −100 °C and 150 °C, at 3 °C min−1. Polymer molecular weights were determined by THF-SEC using PSS EcoSEC equipped with RI detector: THF rate of 0.3 mL min−1 for sample (0.15 mL min−1 pump ref), SEC columns: PL Resipore (50 × 4.6 mm) and two columns PL Resipore (250 × 4.6 mm) at 40 °C, sample concentrations of 0.25% in THF containing 0.25% of toluene as reference, injection volume of 20 μL. Mn (number average molecular weight), Mw (weight average molecular weight) and Đ (dispersity) were calculated with PS standard using PS-M Easivial (Agilent, USA).
3.2. Alkoxyamine synthesis
1H NMR (400 MHz, CDCl3, Fig. S2†) δ: 8.43 (s, 1H, NH), 4.14 (m, 5H, CH2), 3.82 (ddd, J = 17.3, 4.3, 2.4, 1H, CH2), 3.34 (d, J = 26.3 Hz, 1H, CH–P), 2.12 (s, 1H, CH), 1.70 (s, 3H, CH3), 1.57 (s, 3H, CH3), 1.33 (dt, J = 16.3, 7.1, 6H, CH3–CH2), 1.21 (s, 9 H, CH3), 1.10 (s, 9H, CH3).
13C NMR (300 MHz, CDCl3, Fig. S3†) δ: 176.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 85.8 (C), 71.1–69.7 (d, J = 133.3 Hz CH), 71.0 (CH), 61.7 (CH2), 60.0 (CH2), 29.6 (CH3), 29.3 (CH3), 28.7 (CH2), 27.0 (CH3), 24.3 (CH3), 16.5 (CH3), 16.5 (CH3). Additional NMR spectra: correlation spectroscopy COSY NMR (Fig. S4†), Heteronuclear Multiple-Quantum Correlation HMQC NMR (Fig. S5†), Heteronuclear Multiple Bond Correlation HMBC NMR (Fig. S6†). ATR-IR spectrum (Fig. S7,† cm−1): 2980, 1667, 1515, 1368, 1222, 1142, 1053, 1022, 954, 743, 650, 596, 559. MS (ESI) calcd for [C20H40N2O5P]+m/z = 419.267; found 419.274 for [M + H]+.
O), 85.8 (C), 71.1–69.7 (d, J = 133.3 Hz CH), 71.0 (CH), 61.7 (CH2), 60.0 (CH2), 29.6 (CH3), 29.3 (CH3), 28.7 (CH2), 27.0 (CH3), 24.3 (CH3), 16.5 (CH3), 16.5 (CH3). Additional NMR spectra: correlation spectroscopy COSY NMR (Fig. S4†), Heteronuclear Multiple-Quantum Correlation HMQC NMR (Fig. S5†), Heteronuclear Multiple Bond Correlation HMBC NMR (Fig. S6†). ATR-IR spectrum (Fig. S7,† cm−1): 2980, 1667, 1515, 1368, 1222, 1142, 1053, 1022, 954, 743, 650, 596, 559. MS (ESI) calcd for [C20H40N2O5P]+m/z = 419.267; found 419.274 for [M + H]+.
1H NMR: (400 MHz, CDCl3, Fig. S8†) δ: 4.56–4.73 (m, 5H), 3.95 (m, 5H), 3.69 (m, 1H), 3.26 (d, J = 21.59, 8.26 Hz, 1H), 2.45–2.56 (m, 2H), 1.16 (s, 9H), 1.10 (s, 6H), 1.03 (s, 9H).
13C NMR (CDCl3, 75.47 MHz, Fig. S9†): 16.6 (dd, CH3, 1C); 23.5 (CH3, 1C); 28.0 (CH3, 1C); 28.2 (CH3, 3C); 30.0 (d, CH3, 3C); 30.0 (Cq, 1C); 40.4 (Cq, 1C); 41.2 (CH2, 1C); 52.0 (CH2, 1C); 41.2 (CH2, 1C); 59.4 (d, CH2, 1C); 62.0 (Cq, 1C); 62.4 (d, CH2, 1C); 68.9 (CH, 1C); 75.3 (CH, 1C); 77.2 (Cq, 1C); 83.2 (CH, 1C); 172.1 (Cq, 1C); 181.4 (Cq, 1C).
31P NMR (CDCl3): 24.4 ppm. HRMS (ESI) calcd for [C23H42NO8P]+m/z = 491.2648; found 491.2648 for [M]+. IR spectrum (see below).
3.3. Preparation of azide functional PVC (PVC-N3)
Typical procedure for PVC-N3 preparation: in a 250 mL round bottom flask, PVC (4 g, 64.0 mmol of -CH2CHCl-units) and NaN3 (4 g, 61.5 mmol) were mixed in 70 mL of DMF. The mixture was heated at 50 or 60 °C for 0.33 to 16.5 h (Table 1). The mixture was filtered and then precipitated via a dropwise addition of the solution onto 400 mL of ethanol under high magnetic stirring. After filtration, the solid was dissolved in 100 mL of THF and precipitated in 400 mL of ethanol. The solid was dried under reduced pressure. The concentrations of grafted azide were determined by CHN elemental analysis (see Table S1 and Fig. S11†).1H NMR (400 MHz, CDCl3) δ: 4.6–4.15 (br m, 0.6H, –CHCl), 4.1–3.6 (br m, 0.4H, –CHN3), 2.4–1.85 (br m, 1.2H, –CH2CHCl), 1.85–1.6 (br m, 0.8H, –CH2CHN3) (the PVC-N3 described contains 43% of N3 units, see ESI, Fig. S10†).
IR (cm−1): 2910, 2108, 1426, 1331, 1254, 1098, 962, 655, 611 (see spectrum below).
3.4. Preparation of alkoxyamine functional PVC-alkoxyamine 2 (PVC-Alk2, Fig. 2)
In a 250 mL round bottom flask, PVC-N3 (0.16 mmol, 1 equiv., 1.0 g), and alkoxyamine 2 (0.8 mmol, 4 equiv., 400 mg), copper bromide (0.52 mmol, 3 equiv., 75 mg) and 2,2′-bipyridine (1.15 mmol, 7 equiv., 180 mg) were mixed in 100 mL of DMF and degassed under argon. The mixture let to react under argon for 24 h at RT. The solution was then precipitated in a mixture of 100 mL of ethanol and 300 mL of water in the presence of 150 mg of EDTA. The mixture was placed at −10 °C and the resulting solid was filtered, washed with acetone, and dried under reduced pressure. (m = 1.129 g, r = 80%, Tg = 75 °C, MW = 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 g mol−1, Đ = 3.9). 1H NMR: (400 MHz, CDCl3, Fig. 4) δ: 4.46 (m, 1H, CHCl), 2.51 (m, 2H, CH2), 1.20 (m, 1H, CH3). PVC-Alk2 was analyzed by elemental analysis (Table S2†).
100 g mol−1, Đ = 3.9). 1H NMR: (400 MHz, CDCl3, Fig. 4) δ: 4.46 (m, 1H, CHCl), 2.51 (m, 2H, CH2), 1.20 (m, 1H, CH3). PVC-Alk2 was analyzed by elemental analysis (Table S2†).
3.5. Preparation of PVC-g-PBA
In a Schlenk flask under argon, PVC-Alk2 (1 equiv.) and butyl acrylate (67 equiv.) were mixed in DMF (volume ratio of DMF/BA of 4/1). The mixture was heated at 100 °C for 6 to 12 h (see ESI†). After reaction an aliquot of the reaction medium was analyzed by 1H NMR to determine the BA conversion and the copolymer was precipitated via addition of 200 mL of ethanol or cold pentane (for high BA conversion polymerization). The resulting solid was filtered, washed with ethanol or pentane and dry under reduced pressure. The BA concentration in the copolymer was determined by 1H NMR. Before DSC analysis, the polymer sample was dry for 4–8 h under vacuum to ensure the elimination of traces of DMF. 1H NMR of the copolymers are presented in ESI.†1H NMR (400 MHz, CDCl3, Fig. S14a–j†) δ: 4.8–3.5 (br m, overlapped signals of –CHCl–, –CHN– and –OCH2– of PBA), 2.6–1.75 (br m, overlapped signals of –CHC(O) and –OCH2–CH2–, 3H), 1.75–1.1 (br m, overlapped signals of –CH2–CH2–, 4H), 1.0–0.7 (br m, –CH3 of PBA, 3H).No SEC analysis of PVC-g-PBA could be provided due to the very broad dispersity of the polymers.
3.6. Preparation of PVC-g-PEGA
In a Schlenk under argon, PVC-Alk2 (1.2 to 1.85 g, see ESI†) and PEG acrylate (0.71 to 1.60 g, see ESI†) were mixed in 15 mL of DMF. The mixture was heated at 100 °C for 18 h. After reaction an aliquot of the reaction medium was analyzed by 1H NMR to determine the PEGA conversion and the copolymer was precipitated via addition of 300 mL of ethanol. The resulting solid was filtered, washed with ethanol and diethylether, dried under reduced pressure. The PEGA concentration in the copolymer was determined by 1H NMR. Before DSC analysis, the polymer sample was dry for 4–8 h under vacuum to ensure the elimination of traces of DMF.1H NMR of the copolymers are presented in ESI.†1H NMR (300 MHz, DMSO, Fig. S15a–c†) δ 4.65–4.2 (br m, overlapped signals of –CHCl– and –CHN–), 3.65–3.45 (br s, OCH2– signals of PEG), 3.25 (s, –CH3), 2.6–2.0 (br m, overlapped signals of –CH2–CHC(O)– and –CH2–CHCl–).
4. Conclusions
A series of PVC-g-PBA and PVC-g-PEGA were synthesized via NMP initiated by alkoxyamines grafted on PVC chains. An alkyne function was introduced on MAMA-SG1 alkoxyamines via the modification of the terminal acid group into an alkyne amide group (alkoxyamine 1) or via the 1,2 intermolecular radical addition of an alkyne acrylate (alkoxyamine 2). The control of time and temperature of the reaction permitted to adjust the substitution degree of Cl atoms on PVC with azide groups, leading to PVC containing 1.4 to 44% azide units. Alkoxyamines 1 and 2 were grafted on the azide modified PVC via CuAAC reaction. The reaction with alkoxyamines 1 led to a very poorly soluble material, probably due to radical cross-linking reactions. The radical polymerization of BA initiated by PVC-Alk2 permitted to synthesize PVC-g-PBA copolymers having between 20 to 88 wt% of PBA and presenting a wide range of Tg values between 63.5 to −10.5 °C. PEG-2000 acrylate was grafted to PVC (between 17 and 53 wt% of PEGA) using the same strategy and the DMA analysis revealed a semi-crystalline behavior and a low Tg of −4 °C and a Tm of 43 °C (31 wt% PEGA).Radical polymerization through grafted alkoxyamine on PVC is a strong strategy to covalently link a plasticizer on polymer chains and to finely tune its thermal properties without using toxic plasticizers, opening the way to safe soft materials with high permanence and durability. Eventually, this NMP “polymerization from” strategy could be extended to numerous different monomers to introduce various properties to PVC chains.
Author contributions
AV, MD, and LA: methodology, formal analysis, and investigation. CD and KL: conceptualization, supervision, funding acquisition and writing – original draft. DG: conceptualization and validation. AK: supervision, conceptualization, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Westlake Global Compounds, CNRS and Aix-Marseille Université are acknowledged for supports. We also acknowledge Dr M. Rollet for polymer analysis and M. Arnautu for dynamic mechanical analysis.Notes and references
- P. W. Skelly, L. Li and R. Braslau, Polym. Rev., 2021 DOI:10.1080/15583724.2021.1986066.
- M. Rahman and C. S. Brazel, Prog. Polym. Sci., 2004, 29, 1223–1248 CrossRef CAS.
- S. Jobling, T. Reynolds, R. White, M. G. Parker and J. P. Sumpter, Environ. Health Perspect., 1995, 103, 582–587 CrossRef CAS PubMed.
- U. Heudorf, V. Mersch-Sundermann and J. Angerer, Int. J. Hyg. Environ. Health, 2007, 210, 623–634 CrossRef CAS PubMed.
- L. Trasande, B. Liu and W. Bao, Environ. Pollut., 2022, 292, 118021 CrossRef CAS PubMed.
- R. A. Rudel, D. E. Camann, J. D. Spengler, L. R. Korn and J. G. Brody, Environ. Sci. Technol., 2003, 37, 4543–4553 CrossRef CAS PubMed.
- S. Benjamin, E. Masai, N. Kamimura, K. Takahashi, R. C. Anderson and P. A. Faisal, J. Hazard. Mater., 2017, 340, 360–383 CrossRef CAS PubMed.
- H. C. Erythropel, M. Maric, J. A. Nicell, R. L. Leask and V. Yargeau, Appl. Microbiol. Biotechnol., 2014, 98, 9967–9981 CrossRef CAS PubMed.
- J. A. Tickner, T. Schettler, T. Guidotti, M. McCally and M. Rossi, Am. J. Ind. Med., 2001, 39, 100–111 CrossRef CAS PubMed.
- G. Latini, C. De Felice and A. Verrotti, Reprod. Toxicol., 2004, 19, 27–33 CrossRef CAS PubMed.
- F. Chiellini, M. Ferri, A. Morelli, L. Dipaola and G. Latini, Prog. Polym. Sci., 2013, 38, 1067–1088 CrossRef CAS.
- S. Kumar, Ind. Eng. Chem. Res., 2019, 58, 11659–11672 CrossRef CAS.
- Z. Zhang, P. Jiang, D. Liu, S. Feng, P. Zhang, Y. Wang, J. Fu and H. Agus, J. Mater. Sci., 2021, 56, 10155–10182 CrossRef CAS.
- M. G. A. Vieira, M. Altenhofen da Silva, L. Oliveira dos Santos and M. M. Beppu, Eur. Polym. J., 2011, 47, 254–263 CrossRef CAS.
- M. Bocque, C. Voirin, V. Lapinte, S. Caillol and J.-J. Robin, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 11–33 CrossRef CAS.
- Addit. Polym., 2017, 2017, 10–11 Search PubMed.
- S. Moulay, Prog. Polym. Sci., 2010, 35, 303–331 CrossRef CAS.
- P. Jia, L. Hu, G. Feng, C. Bo, M. Zhang and Y. Zhou, Mater. Chem. Phys., 2017, 190, 25–30 CrossRef CAS.
- Y. Ma, S. Liao, Q. Li, Q. Guan, P. Jia and Y. Zhou, React. Funct. Polym., 2020, 147, 104458 CrossRef CAS.
- M. Arslan, G. Acik and M. A. Tasdelen, Polym. Chem., 2019, 10, 3806–3821 RSC.
- Z. M. Zhou, M. Zhu, X. D. Wu and D. Z. Qian, Polymer, 1994, 35, 2888–2892 CrossRef CAS.
- T. Kameda, Y. Fukuda, G. Grause and T. Yoshioka, J. Appl. Polym. Sci., 2010, 116, 36–44 CrossRef CAS.
- R. Navarro, M. Pérez Perrino, C. García, C. Elvira, A. Gallardo and H. Reinecke, Macromolecules, 2016, 49, 2224–2227 CrossRef CAS.
- R. Navarro, M. Pérez Perrino, M. Gómez Tardajos and H. Reinecke, Macromolecules, 2010, 43, 2377–2381 CrossRef CAS.
- C. Mijangos, A. Martinez and A. Michel, Eur. Polym. J., 1986, 22, 417–421 CrossRef CAS.
- G. Martìnez, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2476–2486 CrossRef.
- M. Wang, X. Kong, X. Song, Y. Chen and Q. Bu, New J. Chem., 2021, 45, 3441–3447 RSC.
- P. Jia, L. Hu, Q. Shang, R. Wang, M. Zhang and Y. Zhou, ACS Sustainable Chem. Eng., 2017, 5, 6665–6673 CrossRef CAS.
- P. Jia, Y. Ma, F. Song, Y. Hu, C. Zhang and Y. Zhou, React. Funct. Polym., 2019, 144, 104363 CrossRef CAS.
- P. Jia, Y. Ma, Q. Kong, L. Xu, Y. Hu, L. Hu and Y. Zhou, Mater. Today Chem., 2019, 13, 49–58 CrossRef CAS.
- J. Lafarge, N. Kébir, D. Schapman and F. Burel, React. Funct. Polym., 2013, 73, 1464–1472 CrossRef CAS.
- C. Altinkok, H. R. F. Karabulut, M. A. Tasdelen and G. Acik, Mater. Today Commun., 2020, 25, 101425 CrossRef CAS.
- A. Earla and R. Braslau, Macromol. Rapid Commun., 2014, 35, 666–671 CrossRef CAS PubMed.
- A. Earla, L. Li, P. Costanzo and R. Braslau, Polymer, 2017, 109, 1–12 CrossRef CAS.
- P. Yang, J. Yan, H. Sun, H. Fan, Y. Chen, F. Wang and B. Shi, RSC Adv., 2015, 5, 16980–16985 RSC.
- K. W. Lee, J. W. Chung and S.-Y. Kwak, Macromol. Rapid Commun., 2016, 37, 2045–2051 CrossRef CAS PubMed.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS PubMed.
- L. Li, Y. Schneider, A. B. Hoeglund and R. Braslau, Synlett, 2021, 32, 497–501 CAS.
- Y. Xia, Y. Huo, Q. Yang, H. Zhou, X. Lin and G. Li, J. Macromol. Sci., Part A: Pure Appl. Chem., 2021, 58, 636–641 CrossRef CAS.
- V. Percec and F. Asgarzadeh, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 1120–1135 CrossRef CAS.
- V. Percec, A. Cappotto and B. Barboiu, Macromol. Chem. Phys., 2002, 203, 1674–1683 CrossRef CAS.
- H. J. Paik, S. G. Gaynor and K. Matyjaszewski, Macromol. Rapid Commun., 1998, 19, 47–52 CrossRef CAS.
- S. Brown, Y. Yue, L.-J. Kuo, N. Mehio, M. Li, G. Gill, C. Tsouris, R. T. Mayes, T. Saito and S. Dai, Ind. Eng. Chem. Res., 2016, 55, 4139–4148 CrossRef CAS.
- N. Bicak, B. Karagoz and D. Emre, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1900–1907 CrossRef CAS.
- D. K. Roh, J. T. Park, S. H. Ahn, H. Ahn, D. Y. Ryu and J. H. Kim, Electrochim. Acta, 2010, 55, 4976–4981 CrossRef CAS.
- X.-S. Shao, J.-H. Li, Q. Zhou, J. Miao and Q.-Q. Zhang, J. Appl. Polym. Sci., 2013, 129, 2472–2478 CrossRef CAS.
- L.-F. Fang, H. Matsuyama, B.-K. Zhu and S. Zhao, J. Appl. Polym. Sci., 2018, 135, 45832 CrossRef.
- N. Bicak and M. Ozlem, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 3457–3462 CrossRef CAS.
- E. Rusen, S. Raluca, C. Busuioc and A. Diacon, RSC Adv., 2020, 10, 35692–35700 RSC.
- H. Wu, T. Li, B. Liu, C. Chen, S. Wang and J. C. Crittenden, Appl. Surf. Sci., 2018, 455, 987–996 CrossRef CAS.
- M.-Y. Zhou, L.-F. Fang, C.-C. Sun, C.-E. Lin, B.-K. Zhu and J.-H. Chen, J. Membr. Sci., 2019, 572, 401–409 CrossRef CAS.
- W. Liu, Y. Dong, S. Zhang, Z. Wu and H. Chen, Chem. Commun., 2019, 55, 858–861 RSC.
- J. Nicolas, Y. Guillaneuf, C. Lefay, D. Bertin, D. Gigmes and B. Charleux, Prog. Polym. Sci., 2013, 38, 63–235 CrossRef CAS.
- A. Kermagoret and D. Gigmes, Tetrahedron, 2016, 72, 7672–7685 CrossRef CAS.
- S. Coiai, E. Passaglia and F. Cicogna, Polym. Int., 2019, 68, 27–63 CrossRef CAS.
- M. Abbasian and A. A. Entezami, Polym. Adv. Technol., 2007, 18, 306–312 CrossRef CAS.
- L. Couvreur, C. Lefay, J. Belleney, B. Charleux, O. Guerret and S. Magnet, Macromolecules, 2003, 36, 8260–8267 CrossRef CAS.
- J. Nicolas, B. Charleux, O. Guerret and S. Magnet, Angew. Chem., Int. Ed., 2004, 43, 6186–6189 CrossRef CAS PubMed.
- S. Banerjee, I. Domenichelli and B. Ameduri, ACS Macro Lett., 2016, 5, 1232–1236 CrossRef CAS.
- F. Chauvin, P.-E. Dufils, D. Gigmes, Y. Guillaneuf, S. R. A. Marque, P. Tordo and D. Bertin, Macromolecules, 2006, 39, 5238–5250 CrossRef CAS.
- B. Clément, T. Trimaille, O. Alluin, D. Gigmes, K. Mabrouk, F. Féron, P. Decherchi, T. Marqueste and D. Bertin, Biomacromolecules, 2009, 10, 1436–1445 CrossRef PubMed.
- S. Marque, C. Le Mercier, P. Tordo and H. Fischer, Macromolecules, 2000, 33, 4403–4410 CrossRef CAS.
- L. Coltro, J. B. Pitta and E. Madaleno, Polym. Test., 2013, 32, 272–278 CrossRef CAS.
- J. L. Millàn, G. Martinez, C. Mijangos and M. Gómez-Daza, Makromol. Chem., 1989, 190, 223–230 CrossRef.
- S. Marque, H. Fischer, E. Baier and A. Studer, J. Org. Chem., 2001, 66, 1146–1156 CrossRef CAS PubMed.
- D. Bertin, D. Gigmes, C. Le Mercier, S. R. A. Marque and P. Tordo, J. Org. Chem., 2004, 69, 4925–4930 CrossRef CAS PubMed.
- D. Bertin, P.-E. Dufils, I. Durand, D. Gigmes, B. Giovanetti, Y. Guillaneuf, S. R. A. Marque, T. Phan and P. Tordo, Macromol. Chem. Phys., 2008, 209, 220–224 CrossRef CAS.
- D. Bertin, D. Gigmes, S. R. A. Marque and P. Tordo, Macromolecules, 2005, 38, 2638–2650 CrossRef CAS.
- H. Akat and M. Ozkan, eXPRESS Polym. Lett., 2011, 5, 318–326 CrossRef CAS.
- C. M. Higa, A. T. Tek, R. J. Wojtecki and R. Braslau, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 2397–2411 CrossRef CAS.
- G. Chen, X. Zhu, Z. Cheng and J. Lu, J. Appl. Polym. Sci., 2005, 96, 183–189 CrossRef CAS.
- L. Li, Y. Schneider, A. B. Hoeglund and R. Braslau, J. Appl. Polym. Sci., 2021, 138, 50747 CrossRef CAS.
- J. Vinas, N. Chagneux, D. Gigmes, T. Trimaille, A. Favier and D. Bertin, Polymer, 2008, 49, 3639–3647 CrossRef CAS.
- V. Pertici, C. Pin-Barre, C. Rivera, C. Pellegrino, J. Laurin, D. Gigmes and T. Trimaille, Biomacromolecules, 2019, 20, 149–163 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py00308b |
| This journal is © The Royal Society of Chemistry 2022 |



