Scandium-catalyzed stereoselective block and alternating copolymerization of diphenylphosphinostyrenes and isoprene†
Abstract
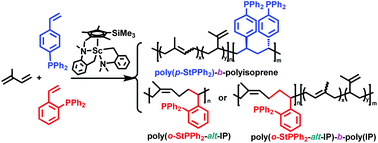
The coordination copolymerization of polar diphenylphosphinostyrenes (StPPh2) and isoprene (IP) by a half-sandwich scandium catalyst (C5Me4SiMe3)Sc(CH2C6H4NMe2-o)2 afforded a new series of phosphine-functionalized alternating and block isoprene–styrene copolymers. In the copolymerization of p-StPPh2 with IP, the incorporation of p-StPPh2 into the polymer chains started after IP was almost completely consumed, yielding the p-StPPh2–IP diblock copolymers containing a syndiotactic-rich poly(p-StPPh2) block and a poly(IP) block containing 1,4- and 3,4-structures, probably because the coordination of IP to the catalyst is favored over that of p-StPPh2. In the copolymerization of o-StPPh2 with IP, the o-StPPh2–IP alternating copolymer was exclusively formed with the coexistence of the two monomers, because the successive insertion of o-StPPh2 was difficult due to steric hindrance caused by the phosphine/styrene chelation to the catalyst metal center. When an excess amount of IP was used in the copolymerization of o-StPPh2 and IP, the formation of a poly(IP) block was achieved after o-StPPh2 was consumed in the alternating copolymerization, thus affording block copolymers containing an o-StPPh2–IP alternating block and a poly(IP) block. Moreover, the incorporation of IP in the alternating copolymerization with o-StPPh2 took place in a regio- and stereospecific cis-1,4-fashion (95%), suggesting that the coordination (chelation) of o-StPPh2 to the catalyst could influence the regio- and stereoselectivity of IP polymerization.


 Please wait while we load your content...
Please wait while we load your content...