Thiol–ene ionogels based on polymerizable imidazolium ionic liquids
Abstract
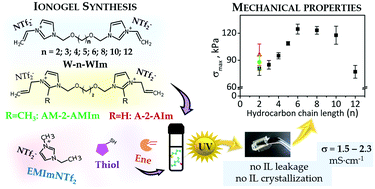
In this work, we report the synthesis of polymerizable ionic liquids (PILs) and the synthesis of ionogels by thiol–ene photopolymerization. A series of gemini imidazolium-based bis(trifluoromethylsulfonyl)imide polymerizable ionic liquids with ene functional groups, reactive in thiol–ene polymerization, were synthesized. The obtained polymerizable ionic liquids differ by (i) type of reactive group: vinyl, allyl, (ii) length of hydrocarbon chain spacer (from C2 to C12) between two imidazole rings in cations in the vinyl series of PILs (divinyl-PIL), and (iii) type of substituent (–H or –CH3) at C(2) position at the imidazole ring in diallyl-PILs. Then ionogel systems on the basis of the prepared PILs used as ene, 1,3,5-trimethylolpropane trimethacrylate (TMPTM), difunctional aliphatic polyester urethane acrylate (DAPU), and tris(3-mercaptobutyloxethyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione (TMTT) were synthesized in 70 wt% of ionic liquid 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMImNTf2). The mechanical properties (tensile strength), ionic conductivity (electrochemical impedance spectroscopy), and thermal properties (differential scanning calorimetry, thermogravimetric analysis) of the prepared ionogels as well as the course of photopolymerization by the photo-DSC method were studied. The resulting ionogels are fully transparent, highly flexible, with no leakage (also at an elevated temperature 80 °C and during mechanical testing), all thanks to the presence of PILs in the polymer matrix of ionogels. Interestingly, DSC studies have shown that the ionic liquid EMImNTf2 does not crystallize in the synthesized ionogels in a broad range of temperatures (−170 °C to 30 °C). The PIL structure influences the polymerization kinetics as well as properties of ionogels. All synthesized ionogels are stable in the temperature range 304 °C–325 °C, and ionogels with allyl-PILs have higher thermal stability than ionogels with vinyl-PILs. The strength (for the ionogels with PILs C2–C6) and Young's modulus of the obtained ionogels increase with the length of the hydrocarbon chain of the spacer in vinyl-PILs but elongation and ionic conductivity decrease. However, the conductivity of ionogels is greater than 1 mS cm−1, which allows the use of the obtained materials, e.g., in electrochemical capacitors.
- This article is part of the themed collection: Photopolymer science


 Please wait while we load your content...
Please wait while we load your content...