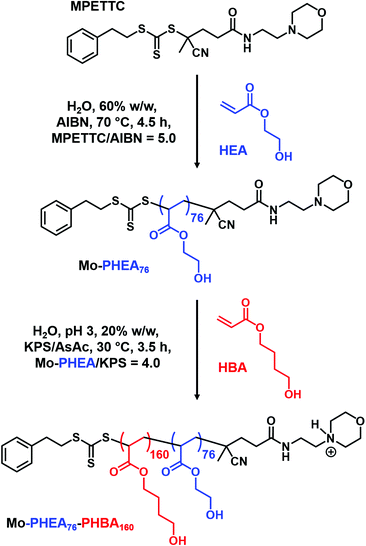
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
RAFT aqueous dispersion polymerization of 4-hydroxybutyl acrylate: effect of end-group ionization on the formation and colloidal stability of sterically-stabilized diblock copolymer nanoparticles†
Deborah L.
Beattie
,
Oliver J.
Deane
,
Oleksandr O.
Mykhaylyk
 and
Steven P.
Armes
and
Steven P.
Armes
 *
*
Dainton Building, Department of Chemistry, University of Sheffield, Brook Hill, Sheffield, South Yorkshire S3 7HF, UK. E-mail: s.p.armes@shef.ac.uk
First published on 6th January 2022
Abstract
A series of all-acrylic poly(2-hydroxyethyl acrylate)x-poly(4-hydroxybutyl acrylate)y (PHEAx–PHBAy) diblock copolymer nanoparticles were prepared via an efficient one-pot RAFT aqueous dispersion polymerization protocol using either a carboxylic acid-functionalized RAFT agent (HOOC–PHEAx–PHBAy) or a morpholine-functionalized RAFT agent (Mo–PHEAx–PHBAy). The pH-dependent colloidal stability of the resulting sterically-stabilized nanoparticles was assessed by dynamic light scattering (DLS) and aqueous electrophoresis. The HOOC–PHEA73–PHBA217 nanoparticles exhibited reversible flocculation below pH 5.1, whereas the Mo–PHEA76–PHBA160 nanoparticles flocculated above pH 5. Moreover, the HOOC–PHEA73–PHBA217 nanoparticles proved to be sensitive to added salt, with incipient flocculation occurring in the presence of 20–60 mM KCl owing to charge screening. Thus, such nanoparticles require end-group ionization to confer colloidal stability via electrosteric stabilization. However, reducing the PHBA/PHEA molar ratio and/or increasing the PHEAx stabilizer DP, leads to more efficient steric stabilization and hence enhanced colloidal stability. A series of HOOC–PHEA73–PHBA104–421 nano-objects prepared at pH 7 were characterized by visual inspection, DLS studies and shear-induced polarized light imaging. Discrepancies between these characterization techniques indicated that the worms and vesicles were unstable with respect to dilution. TEM studies were conducted after covalent stabilization of the nano-objects using glutaraldehyde (GA). More specifically, TEM studies of GA-crosslinked HOOC–PHEA73–PHBA243 and HOOC–PHEA73–PHBA421 nano-objects indicated the presence of spheres in both cases when crosslinked at 0.1% w/w and either worms or vesicles respectively when crosslinked at 10–20% w/w. Finally, HOOC–PHEA73–PHBA265 nano-objects were examined by variable temperature oscillatory rheology: thermoreversible sphere/worm and worm/vesicle transitions were observed between 2 and 50 °C.
Introduction
Polymerization-induced self-assembly (PISA) offers a robust and highly versatile synthetic route to a wide range of well-defined block copolymer nano-objects in the form of concentrated colloidal dispersions.1–8 PISA involves growing an insoluble block from one end of a soluble precursor block in a suitable solvent. Once some critical degree of polymerization is achieved for the insoluble block this causes micellar nucleation and ultimately leads to the formation of sterically-stabilized diblock copolymer nano-objects. The unreacted monomer acts as a processing aid (or co-solvent) and the high local monomer concentration within the monomer-swollen nanoparticles results in a relatively fast rate of polymerization compared to the equivalent solution polymerization.9,10 In the case of formulations based on dispersion polymerization, this approach is rather generic: PISA conducted in non-polar solvents, polar solvents or water seems to conform to essentially the same design rules.11–14 PISA syntheses involving vinyl monomers are typically conducted using reversible addition–fragmentation chain transfer (RAFT) polymerization, which is well-known to be highly tolerant of monomer functionality.8,15–19 In principle, the final copolymer morphology should depend on the relative volume fractions of the soluble and insoluble blocks.20–22 In practice, various other synthesis parameters may also play important roles.8,13,23–29 For aqueous PISA formulations, one important consideration seems to be the monomer solubility.30–35 For example, there are various RAFT aqueous emulsion polymerization formulations involving sparingly-soluble vinyl monomers for which only kinetically-trapped spheres can be obtained.36–39 In striking contrast, RAFT aqueous dispersion polymerization – for which the vinyl monomer must be water-miscible – usually provides access to the three most common copolymer morphologies (spheres, worms or vesicles) if the steric stabilizer block is not too long.14,25,40,41It has become increasingly apparent that using such water-miscible monomers leads to the production of weakly hydrophobic structure-directing blocks that often exhibit thermoresponsive behavior.42–45 Furthermore, morphological transitions can sometimes be induced simply by introducing a single charge at the end of the steric stabilizer block. This can be achieved either by ionization of a terminal carboxylic acid group28,42,46 or by protonation of a terminal morpholine group.47,48 Recently, we reported that diblock copolymer nano-objects prepared via RAFT aqueous dispersion polymerization can exhibit remarkable shape-shifting behavior.42,49,50 More specifically, a single amphiphilic diblock copolymer can form spheres, worms, vesicles or lamellae in aqueous solution simply by varying the temperature from 1 °C to 70 °C.42 In this case – which is particularly relevant to the present study – the steric stabilizer block was poly(N,N-dimethylacrylamide) (PDMAC) and diacetone acrylamide (DAAM) was statistically copolymerized with 4-hydroxybutyl acrylate (HBA) to produce a hydrophobic block that could be covalently crosslinked using adipic acid dihydrazide (ADH) to enable visualization of the copolymer morphologies using transmission electron microscopy (TEM).42 Herein we replace the PDMAC stabilizer block with poly(2-hydroxyethyl acrylate) (PHEA), which has been previously used to produce a range of PHEA-stabilized nano-objects prepared by a post-polymerization solvent switch.51–57 Recently, PHEA has been employed as a steric stabilizer for RAFT dispersion polymerization in either alcohol58 or alcohol/water mixtures.14,59,60 However, as far as we are aware PHEA has never been examined for purely aqueous PISA formulations. Moreover, we dispense with the DAAM comonomer and instead use glutaraldehyde to covalently stabilize hydroxyl-functional PHEA–PHBA diblock copolymer nano-objects for TEM studies. The sensitivity of such all-acrylic nanoparticles to changes in temperature, end-group ionization and serial dilution is explored and compared to prior PISA formulations.
One-pot synthesis of HOOC–PHEA–PHBA diblock copolymers via RAFT aqueous dispersion polymerization
According to the literature, the homopolymerization of HEA is typically conducted at high temperatures (e.g. 70 °C) in polar organic solvents such as DMF,54–56,61 THF,58 1,4-dioxane,14,59,60 various alcohols,52,62,63 or mixtures thereof.64,65 In contrast, there are far fewer reports of the RAFT solution polymerization of HEA in aqueous media, despite such reaction conditions being highly desirable for the development of efficient one-pot PISA syntheses of diblock copolymer nanoparticles. Thus, we decided to examine the RAFT aqueous solution polymerization of HEA at 44 °C using a trithiocarbonate-based carboxylic acid-functionalized RAFT agent (PETMP), see Scheme 1. We targeted 60% w/w solids so that the HEA monomer initially acted as a co-solvent to ensure RAFT agent solubility. A low-temperature thermal initiator was used to minimize chain transfer to polymer since this side reaction is well-known for acrylic polymerizations performed at elevated temperatures.65,66 After 2.5 h, the crude HOOC–PHEA precursor was analyzed by DMF GPC and 1H NMR spectroscopy. A relatively symmetrical GPC trace suggested minimal chain transfer to polymer during the polymerization, while the relatively narrow molecular weight distribution (Mw/Mn = 1.13, see Fig. 1A) indicates good RAFT control. 1H NMR studies confirmed a final monomer conversion of 99%, which was calculated by comparing the integrated signal for the three vinyl protons assigned to the HEA monomer at 5.8–6.5 ppm to that of the five PETMP aromatic protons at 7.18–7.38 ppm (see Fig. S1B, ESI†). Moreover, a mean DP of 73 was determined for this HOOC–PHEA precursor after its isolation by precipitation (the integrated signals for the aromatic end-group at 7.18–7.38 ppm were compared to that of the oxymethylene proton signal at 4.18 ppm [Fig. S1B, ESI†]). | ||
| Fig. 1 (A) DMF GPC curves (refractive index detector; expressed relative to a series of poly[methyl methacrylate] [PMMA] calibration standards) recorded for the HOOC–PHEA73 precursor (dotted curve) and selected HOOC–PHEA73–PHBAy diblock copolymers (solid curves) prepared at pH 7. (B) The corresponding evolution in number-average molecular weight (Mn, open circles) and dispersity (Mw/Mn, crosses) with PHBA DP (raw data provided in Table S1, ESI†). Data for the HOOC–PHEA73 precursor are indicated by the red data points marked on the y axis (PHBA DP = 0) and account for the non-zero intercepts while the dashed line indicates the theoretical Mn. | ||
Subsequently, the crude HOOC–PHEA73 precursor was chain-extended via RAFT aqueous dispersion polymerization of HBA at 44 °C, targeting 20% w/w solids and a range of PHBA DPs. For this second-stage polymerization, the solution pH was adjusted to pH 7 to ensure that the carboxylic acid group located on the end of each PHEA chain was present in its anionic carboxylate form. 1H NMR studies indicated that more than 99% HBA conversion was achieved within 9 h for all target copolymer compositions, as determined by comparing the integrated HBA vinyl signals at 5.8–6.5 ppm to the integrated PHBA signal at 3.63 ppm (Fig. S1C, ESI†). Similarly, final diblock copolymer compositions (Table S1, ESI†) were determined by comparing the PHEA signal at 3.78 ppm with the PHBA signal at 3.63 ppm (Fig. S1C, ESI†). DMF GPC studies indicated reasonably high blocking efficiencies (see Fig. 1A) and a relatively linear evolution of Mn with PHBA DP (see Fig. 1B).
Notably each of the diblock copolymer GPC traces shown in Fig. 1A contains a high molecular weight shoulder, which becomes more prominent when targeting higher PHBA DPs. There is a corresponding broadening of the molecular weight distribution (see Fig. 1B and Table S1, ESI†), with dispersities increasing gradually up to a PHBA DP of 316, before rising more rapidly for higher DPs. This is most likely the result of chain transfer to polymer65,66 and/or light branching owing to trace amounts of diacrylate impurities in the HBA monomer.67,68 It is perhaps worth emphasizing that appropriate steps were taken to minimize such problems: the HBA monomer was extensively purified via exhaustive solvent extraction with n-hexane to remove diacrylate impurities and its subsequent polymerization was conducted at a relatively low temperature (44 °C).
In developing this PISA protocol, it became apparent that the resulting HOOC–PHEA73–PHBAy diblock copolymer nano-objects exhibited unusual colloidal stability behavior. In initial experiments, a one-pot synthesis protocol similar to that reported by Byard et al.42 for the preparation of PDMAC-P(HBA-stat-DAAM) diblock copolymer nano-objects was explored (see Fig. S2A, ESI†). This involved synthesis of a new HOOC–PHEA70 precursor at 60% w/w solids using a redox initiator at 30 °C. Within 1.75 h, >99% HEA conversion was achieved and the subsequent RAFT aqueous dispersion polymerization of HBA was conducted at pH 2.5 targeting 20% w/w solids. However, macroscopic phase separation unexpectedly occurred forming two distinct layers (see Fig. S2B, ESI†). DMF GPC studies conducted on the precursor, the viscous yellow lower layer and the free-flowing transparent upper layer confirmed that chain extension had occurred but the upper layer contained some unreacted HOOC–PHEA70 precursor (see Fig. S2B, ESI†). One important difference between this initial synthesis route and that shown in Scheme 1 is the solution pH. The carboxylic acid end-group located on each PHEA steric stabilizer chain is ionized at pH 7.0, whereas it is in its neutral form at pH 2.5. According to our prior studies, this subtle difference can significantly affect the aqueous solubility of a weakly hydrophilic water-soluble polymer.69 Accordingly, we undertook a series of experiments as a function of solution pH to examine the reason for the unexpected poor colloidal stability observed at pH 2.5.
Effect of varying the solution pH on colloidal stability
Dynamic light scattering (DLS) and aqueous electrophoresis studies were conducted by adjusting the pH of a 0.1% w/w aqueous dispersion of HOOC–PHEA73–PHBA217 nanoparticles prepared according to Scheme 1 (Fig. 2A). Between pH 10 and pH 5.5, the z-average diameter remained constant at approximately 35 nm. Within this pH range, the zeta potential only varied between −16 and −20 mV, with the slightly less negative zeta potentials observed between pH 6.5 and 5.5 coinciding with a modest increase in the particle size. However, lowering the pH to 5.1 led to significant flocculation: the apparent z-average diameter more than doubled to 108 nm, although the zeta potential was only marginally lower at −14.6 mV. Interestingly, this onset of aggregation coincides with the pKa determined for the HOOC–PHEA73 precursor (see Fig. S3, ESI†). This suggests that the HOOC–PHEA73–PHBA217 nanoparticles require more than 50% of their carboxylic acid end-groups to be ionized to retain colloidal stability, as schematically illustrated in Fig. 2B. Lowering the pH further results in a correspondingly higher degree of flocculation. Macroscopic precipitation was observed at around pH 4.5, for which the zeta potential was approximately −8.5 mV. These data are consistent with the formation of colloidally unstable nano-objects when performing the RAFT aqueous dispersion polymerization of HBA when using a HOOC–PHEA70 precursor at pH 2.5. | ||
| Fig. 2 (A) Variation in apparent z-average diameter (red circles) and zeta potential (blue squares) with solution pH for a 0.1% w/w aqueous dispersion of HOOC–PHEA73–PHBA217 nanoparticles at 25 °C in the presence of 1 mM KCl. The vertical black dotted line indicates the pKa of 5.1 as determined by acid titration of the HOOC–PHEA73 precursor (see Fig. S3, ESI†). (B) Schematic representation of the protonation of carboxylic acid end-groups on the PHEA steric stabilizer chains that causes nanoparticle flocculation below pH 5.5. | ||
In related work, Gibson et al.69 found that colloidal dispersions of poly(N-2-(methacryloyloxy)ethyl pyrrolidone)-poly(2-hydroxypropyl methacrylate) (PNMEP–PHPMA) nanoparticles could be prepared using a similar carboxylic acid-functionalized RAFT agent at pH 7, but not at pH 3. In this prior study, ionization of such end-groups is critical for colloidal stability since this raises the cloud point for the HOOC–PNMEPx stabilizer block by up to 25 °C. For PISA syntheses conducted at 44 °C, this represents the difference between effective steric stabilization and colloidal instability.69
However, PHEA should not exhibit such inverse temperature solubility: according to the literature, it should exhibit a cloud point above 100 °C.70 Indeed, turbidimetry studies conducted between 25 and 90 °C on 1.0% w/w dispersions of three HOOC–PHEAx precursors (x = 49, 72 or 100) indicated no significant change in transmittance at either pH 2.5 or pH 7.0. Clearly, regardless of whether their carboxylic acid end-groups are ionized or not, these three HOOC–PHEAx precursors possess cloud points above 90 °C (see Fig. S4, ESI†). Thus, although ionization of carboxylic acid end-groups is certainly essential for the successful aqueous PISA synthesis of HOOC–PHEA73–PHBAy nano-objects, this appears to involve a subtly different mechanism to that reported by Gibson et al.69 for HOOC–PNMEPx–PHPMAy nano-objects.
The acid-induced flocculation of the HOOC–PHEA73–PHBA217 nanoparticles shown in Fig. 2 was found to be reasonably reversible: after a pH 10 – pH 2 – pH 10 cycle, DLS studies indicated that, above pH 6.5, the apparent nanoparticle diameter was only slightly larger than the initial value (see Fig. S5, ESI†). This modest increase in size is likely to be the result of the build-up of background salt, which would also account for the slightly less negative zeta potentials. Such nanoparticle redispersion is in good agreement with the reversible pH-responsive behavior reported for similar sterically-stabilized diblock copolymer nanoparticles by Lovett and co-workers,28 Penfold et al.47 and Byard et al.42 However, it is in contrast to the irreversible aggregation behavior reported by Gibson and co-workers.69
Next, the effect of added salt on colloid stability was examined. Thus, a series of 0.1% w/w aqueous dispersions of HOOC–PHEA73–PHBA217 nanoparticles were prepared in the presence of KCl and their apparent z-average particle diameters were assessed by DLS at pH 7 (see Fig. 3). As expected, the colloidal stability was very sensitive to the salt concentration, with the apparent particle size doubling in the presence of only 20 mM KCl (see Fig. 3 and Fig. S6A, ESI†). Moreover, multimodal particle size distributions were obtained as the salt concentration was raised to 50–60 mM KCl, indicating extensive flocculation under such conditions (see Fig. S6B, ESI†). This suggests that the nanoparticle aggregation observed in the presence of added salt is the result of charge screening of the anionic carboxylate end-groups. As noted above, the HOOC–PHEA73 block is not sufficiently long to confer efficient steric stabilization in its neutral form. Given that the HOOC–PHEA73–PHBA217 nanoparticles exhibit both pH- and salt-dependent colloidal stability, this is consistent with an electrosteric stabilization mechanism.69,71,72
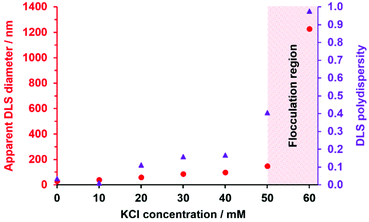 | ||
| Fig. 3 Variation in apparent z-average diameter (red circles) and polydispersity (purple triangles) with KCl concentration indicated by DLS studies of a 0.1% w/w aqueous dispersion of HOOC–PHEA73–PHBA217 nanoparticles at pH 7 (see also Fig. S6, ESI†). | ||
Once the pH-dependent colloidal stability of HOOC–PHEA73–PHBA217 nanoparticles was established, the effect of adjusting the HOOC–PHEA stabilizer DP while maintaining a similar PHBA DP (i.e. varying the HBA/HEA molar ratio) was investigated. More specifically, HOOC–PHEA105–PHBA194 and HOOC–PHEA140–PHBA205 dispersions (HBA/HEA molar ratios = 1.8 and 1.5, respectively) were prepared at pH 7 and then DLS and aqueous electrophoresis studies were conducted as a function of pH (see Fig. 4). Fig. 4 indicates that the critical pH for flocculation can be suppressed by increasing the DP of the stabilizer block, with aggregation being observed at pH 5.1 and pH 4.5 for the HOOC–PHEA73–PHBA217 and HOOC-PHEA105–PHBA194 nanoparticles, respectively. Thus, increasing the DP of the HOOC–PHEAx block reduces the fraction of stabilizer chains bearing anionic carboxylate end-groups required to maintain colloidal stability. Moreover, the HOOC–PHEA140–PHBA205 dispersion remains colloidally stable across the entire pH range. This suggests that there is a critical DP above which (and/or a critical HBA/HEA molar ratio below which) end-group ionization is no longer required to confer colloidal stability to such nanoparticles, i.e. purely steric stabilization is sufficient to prevent aggregation.
To investigate whether the enhanced colloidal stability observed for the HOOC–PHEA140–PHBA205 dispersion in Fig. 4 is the result of a longer PHEA DP or a lower HBA/HEA molar ratio, the colloidal stability of a HOOC–PHEA140–PHBA408 dispersion was studied over the same pH range. This diblock copolymer has a PHBA/PHEA molar ratio of 2.9, which is very similar to that for the HOOC–PHEA73–PHBA217 diblock copolymer (PHBA/PHEA molar ratio = 3.0), thus enabling a meaningful comparison of the pH-dependence of their colloidal stability. Accordingly, DLS and aqueous electrophoresis studies were performed on a 0.1% w/w aqueous dispersion of HOOC–PHEA140–PHBA408 nanoparticles. In Fig. 5A and B, these data are compared with that obtained for the HOOC–PHEA73–PHBA217 nanoparticles shown in Fig. 2. Similar behavior is observed above pH 5.1, which corresponds to the common pKa value obtained for the two corresponding stabilizer chains: the particle size remains around 50 nm on lowering the pH from 7.7 to 5.5 (see Fig. 5A) and there is a concomitant modest reduction in zeta potential (see Fig. 5B). However, the two types of nanoparticles exhibit divergent behavior below pH 5.1. As previously noted, the apparent z-average diameter of the HOOC–PHEA73–PHBA217 nanoparticles increases dramatically, indicating the onset of flocculation. In contrast, the apparent z-average diameter for the HOOC–PHEA140–PHBA408 nanoparticles gradually increases on lowering the pH from 5.0 to 4.3 before attaining a constant value of approximately 103 nm below pH 4.3 (see Fig. 5A). Meanwhile, the corresponding zeta potential is reduced from −8.2 mV at pH 5.0 to approximately −0.5 mV at pH 2.0 (see Fig. 5B). This indicates that these nanoparticles retain a reasonably high degree of dispersion in the form of relatively small flocs at low pH, despite the absence of any anionic end-groups under such conditions. Furthermore, these data suggest that longer HOOC–PHEA stabilizer chains confer more effective steric stabilization and do not require end-group ionization to prevent macroscopic precipitation.
Tailoring colloidal stability via choice of RAFT agent
Bearing in mind the above end-group effect, we decided to evaluate a morpholine-functionalized RAFT agent (MPETTC) for the aqueous PISA synthesis of Mo–PHEAx–PHBAy nanoparticles, where Mo denotes an 2-(N-morpholino)ethyl end-group. In principle, such dispersions should exhibit complementary pH-dependent colloidal stability. Accordingly, Mo–PHEA76–PHBA160 nanoparticles were prepared using MPETTC at pH 3 targeting 20% w/w solids according to the one-pot protocol outlined in Scheme 2. Analysis of the Mo–PHEA76 precursor and final diblock copolymer by 1H NMR spectroscopy confirmed that 99% conversion was achieved for each step, while DMF GPC analysis indicated relatively narrow molecular weight distributions in each case (Mw/Mn = 1.15 and 1.35, respectively) and a reasonably high blocking efficiency (see Fig. S7A, ESI†). Importantly, the resulting aqueous dispersion showed no signs of flocculation at pH 3 (see Fig. S7A, ESI†), suggesting good colloidal stability under such conditions.DLS and electrophoresis studies were conducted on a 0.1% w/w aqueous dispersion of the Mo–PHEA76–PHBA160 nanoparticles (see Fig. 6A). As expected, the apparent z-average diameter remained constant at approximately 42 nm below pH 5, while zeta potentials ranged from +5.9 to +8.5 mV. However, weak flocculation was evident at pH 5.6, for which the zeta potential was +3.3 mV. Above pH 5.7, the apparent DLS size exceeded the 6 μm upper limit for the DLS instrument, indicating macroscopic precipitation (see Fig. 6B). Thus, complete loss of colloidal stability occurs well below the pKa of 6.4 determined for the conjugate acid form of the morpholine end-groups on the PHEA stabilizer chains (see Fig. S7B, ESI;† this pKa is in good agreement with that reported by Penfold et al. for a PGMA50 homopolymer prepared using MPETTC).47 This suggests that a relatively high proportion of morpholine end-groups must be protonated to maintain colloidal stability.
Effect of dilution on the morphology of HOOC–PHEA73–PHBAy nano-objects
Fig. 7 indicates the visual appearance at 25 °C of a series of 20% w/w aqueous dispersions of HOOC–PHEA73–PHBAy nanoparticles prepared at pH 7. These dispersions remain transparent up to a PHBA DP of 265, above which they become increasingly turbid. The four central inverted vials indicate that the dispersion viscosity increases monotonically up to a maximum value at a PHBA DP of 265, with free-flowing milky-white dispersions being obtained at higher PHBA DPs. Based on our previous experience of various PISA formulations,42,73,74 this strongly suggests the formation of transparent dispersions of free-flowing spheres (y = 109–189), relatively transparent worm gels (y = 217–289), and relatively turbid vesicles (y = 316–421). | ||
| Fig. 7 Digital photograph recorded at 25 °C indicating the physical appearance of a series of as-synthesized 20% w/w aqueous dispersions of HOOC–PHEA73–PHBAy nano-objects (where y varies from 109 to 421, see tube labels) prepared at pH 7 according to the protocol outlined in Scheme 1. | ||
Accordingly, DLS studies were conducted on 0.1% w/w aqueous dispersions of HOOC–PHEA73–PHBAy nanoparticles at pH 7 after sequential dilution (see Fig. 8 and Table S1, ESI†). As shown in Fig. 8A, the apparent z-average diameter at 25 °C increased linearly from 14 nm to 52 nm with PHBA DP. Moreover, particle size distributions were relatively narrow, with most dispersions exhibiting DLS polydispersities below 0.10. These observations are precisely what would be expected for a series of kinetically-trapped spheres.75–77 However, this is clearly not consistent with the visual appearance of the series of 20% w/w aqueous dispersions shown in Fig. 7. For example, nanoparticles with an apparent z-average diameter of 33 nm should not form a transient free-standing gel, as observed for the 20% w/w HOOC–PHEA73–PHBA265 dispersion. Similarly, aqueous dispersions with z-average diameters ranging from 35 to 52 nm should not exhibit strong turbidity (as shown in Fig. 7 for 20% w/w HOOC–PHEA73–PHBAy nanoparticles when y ≥ 289). Furthermore, the DLS data do not suggest that such turbidity results from colloidal instability. Instead, these observations suggest that (some of) the nano-objects are unstable with respect to dilution, with worms or vesicles undergoing in situ dissociation to form relatively large spheres. As far as we are aware, such dilution instability is seldom reported for PISA-synthesized nano-objects.45 Nevertheless, this explanation is consistent with the relatively narrow DLS particle size distributions, small z-average diameters and the linear increase in apparent nanoparticle diameter with PHBA DP.75 Moreover, we have recently reported that the structure-directing PHBA block is only weakly hydrophobic.42,50
 | ||
| Fig. 8 (A) Variation of the apparent DLS diameter, Dh (circles) and DLS polydispersity (PDI, crosses) with PHBA DP (y) for a series of 0.1% w/w aqueous dispersions of HOOC–PHEA73–PHBAy nano-objects at pH 7. (B) DLS size distributions recorded for selected nano-objects (see also Table S1, ESI†). | ||
Even without the additional complication of dilution-induced instability, assigning morphologies for acrylic diblock copolymer nano-objects is generally rather more problematic than for the corresponding methacrylic nano-objects.14,42,51,73,78,79 This is because the former nano-objects typically exhibit a sub-ambient glass transition temperature (Tg), which prevents conventional TEM analysis owing to nanoparticle deformation and/or film formation during grid preparation. In principle, cryo-TEM can be used to image such low Tg nanoparticles.29,73 However, in situ dilution is required for cryo-TEM sample preparation. Alternatively, small-angle X-ray scattering (SAXS) can readily distinguish between spheres, worms and vesicles and this technique can be conducted at relatively high copolymer concentration.80–82 However, a structure factor is typically observed under such conditions; this significantly complicates the data analysis, particularly for worms (see Fig. S8, ESI†). Indeed, SAXS patterns recorded at 1.0% w/w for the charged nano-objects described herein exhibit a pronounced structure factor arising from strong inter-particle electrostatic repulsion (see Fig. S8B, ESI†). Such interactions also have consequences for DLS measurements, whereby the incurred systematic error leads to an apparent particle diameter (see Fig. 2A, 3, 4, 5A, 6A and 8A).
An alternative approach is nanoparticle crosslinking: we have recently reported that such covalent stabilization can facilitate nano-object imaging by conventional TEM.42,50 For example, Deane et al. utilized the pendent hydroxyl group present in each PHBA residue as a reactive site for crosslinking using a well-known biological fixative, glutaraldehyde (GA).50,83 Importantly, such covalent stabilization should allow TEM studies of the original HOOC–PHEA73–PHBAy spheres, worms and vesicles and also any new nano-objects that may be formed on dilution.
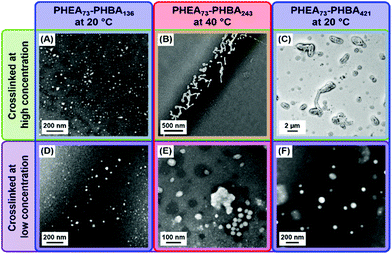
Accordingly, a transparent, free-flowing dispersion of HOOC–PHEA73–PHBA136 nano-objects (tentatively assigned as spheres) and a turbid, free-flowing dispersion of HOOC–PHEA73–PHBA421 nano-objects (tentatively assigned as vesicles) were each crosslinked in turn at 20 °C at either 0.1 or 10% w/w (see Scheme S1, ESI†). Similarly, HOOC–PHEA73–PHBA243 nano-objects (tentatively assigned as worms) were crosslinked in the form of a 20% w/w transparent gel at 40 °C, which corresponds to the critical gelation temperature (CGT, see Fig. S9B and Scheme S1, ESI†) and also under the same conditions after dilution to 0.1% w/w. TEM studies (see Fig. 9) revealed the presence of small HOOC–PHEA73–PHBA136 spheres at 20 °C regardless of whether GA crosslinking is conducted at 10 or 0.1% w/w. Furthermore, there was no evidence for interparticle crosslinking when conducting such experiments at 10% w/w, despite the presence of reactive hydroxyl groups within the PHEA stabilizer chains (see Table S2, ESI†). Conversely, GA crosslinking the HOOC–PHEA73–PHBA243 nano-objects at 20% w/w reveals the presence of anisotropic worm-like nanoparticles, whereas only isotropic spherical nanoparticles are obtained after covalent stabilization at 0.1% w/w. Similarly, TEM studies of PHEA73–PHBA421 nano-objects crosslinked at 10% w/w indicate the presence of large vesicles, as expected given the turbid appearance of such dispersions. In contrast, GA crosslinking at 0.1% w/w afforded much smaller spheres. These observations are consistent with the corresponding DLS data obtained for the linear nano-objects at 0.1% w/w. In summary, these DLS and TEM studies provide strong evidence for the instability of HOOC–PHEA73–PHBAy worms and vesicles with respect to spheres on serial dilution.
Characterization of concentrated dispersions
In order to further examine the morphology of the as-synthesized aqueous dispersions of HOOC–PHEA73–PHBAy nanoparticles, alternative analytical techniques applicable to concentrated dispersions were required. Thus, the series of 20% w/w HOOC–PHEA73–PHBAy nanoparticles was studied using shear-induced polarized light imaging (SIPLI) at 25 °C. SIPLI is an opto-rheological technique that enables the presence of anisotropic nanoparticles to be identified via the appearance of a characteristic Maltese cross motif.45,84,85 This feature is the result of the birefringence generated by in situ alignment of the anisotropic particles under shear. Furthermore, the plate-plate geometry utilized for SIPLI experiments results in a continuous gradient of shear rates radially distributed from zero to a maximum value at the disk edge. This allows the critical shear rate required for the alignment of anisotropic particles to be identified, where the central dark area of the Maltese cross transitions to the light area, indicating alignment. If no Maltese cross is observed, this indicates the presence of isotropic nanoparticles. Therefore, this technique should enable highly anisotropic worms to be identified if such species are present in the as-synthesized concentrated aqueous dispersions of HOOC–PHEA73–PHBAy nanoparticles.Angular speed sweeps were conducted between 0.0008 and 8 rad s−1 (corresponding to maximum shear rates at the disk edge of 0.01 and 100 s−1) with polarized light images being captured periodically as the maximum shear rate was gradually increased. A representative polarized light image recorded at 25 °C for each dispersion of HOOC–PHEA73–PHBAy nanoparticles is shown in Fig. 10. These images were recorded at various shear rates depending on whether a Maltese cross was observed or not; if this feature appeared, then the shear rate was adjusted to achieve the optimal image in each case. Morphologies were assigned based on these SIPLI images taking into account the visual appearance of each 20% w/w aqueous copolymer dispersion at 25 °C, see Fig. 7.
 | ||
| Fig. 10 Digital polarized light images recorded under shear at various maximum shear rates (0.2–100 s−1) when conducting SIPLI experiments at 25 °C on a series of 20% w/w aqueous dispersions of HOOC–PHEA73–PHBAy nano-objects (y = 109–421; see red crosses marked on the horizontal PHBA DP axis). The optimized maximum (sample edge) shear rate at which each dispersion was assessed is stated for each polarized light image. Shaded regions indicate the assigned morphology based on the presence or absence of a Maltese cross, the corresponding shear rate and the visual appearance of each copolymer dispersion at 25 °C (see Fig. 8). The white arrows indicate the relative orientation of the polarizer (P) with respect to the analyzer (A) planes, respectively. | ||
For 20% w/w HOOC–PHEA73–PHBAy dispersions with y = 109 or 136, no Maltese cross was observed up to a maximum shear rate of 100 s−1 (see Fig. 10). This is consistent with the presence of isotropic spherical nanoparticles, with mean apparent diameters indicated by DLS studies of the corresponding diluted dispersions (Fig. 8). As y is increased from 163 to 217, a Maltese cross is observed at a progressively lower maximum shear rate (ranging from 100 s−1 to 8 s−1), with this feature gradually becoming more intense. Since the dispersion viscosity clearly increases with PHBA DP (see Fig. 7), this suggests the presence of a mixed phase comprising isotropic spheres and (increasingly) anisotropic worms for such copolymer compositions. This is commonly observed for PISA formulations that lie intermediate between pure spheres and pure worms.25,58,86 As y is increased further to 243 or 265, intense Maltese crosses are obtained at lower maximum shear rates (2 and 0.8 s−1 respectively, see Fig. 10); this is not unexpected given that such dispersions form transparent viscous fluids (Fig. 7), which suggests the presence of highly anisotropic linear worms. The dispersions become more turbid and less viscous for y = 289–421. Nevertheless, Maltese crosses were still observed over this range at relatively low maximum shear rates between 4 and 0.2 s−1. This suggests that a mixed phase comprising worms and vesicles persists over this compositional range, with a gradual increase in the proportion of the latter species accounting for the increase in turbidity (see Fig. 7).
It is perhaps surprising that a worm population appears to persist over such a wide range of copolymer compositions.25 However, it is likely that the anionic surface charge required to ensure electrosteric stabilization of these nano-objects impedes the worm-to-vesicle transition as the PHBA DP is increased, thereby delaying the formation of pure vesicles. Indeed, it is well documented in the PISA literature that introducing charge into the steric stabilizer chains often prevents (or delays) the formation of worms or vesicles.27,87 Furthermore, Lovett et al. reported that, although pH-insensitive methyl ester-capped PGMA59–PHPMA160 worm gels exhibited thermoresponsive behavior at both neutral and low pH, the corresponding pH-sensitive HOOC–PGMA56–HPMA155 worm gel only exhibited thermoresponsive behavior when the majority of its carboxylic acid end-groups were protonated (i.e. below its pKa of 4.7).28 This is important because it provides direct evidence that anionic end-groups can prevent (or hinder) access to higher order morphologies. Alternatively, TEM studies of HOOC–PHEA73–PHBA421 vesicles crosslinked at 10% w/w provided evidence for the coexistence of populations of both spherical and tubular vesicles (see Fig. 9C). In principle, the latter weakly anisotropic nano-objects might account for the persistence of the Maltese cross at high PHBA DPs. Furthermore, the highly hydrated nature of the PHBA membranes may lead to some degree of elongation under applied shear (see Table S2, ESI†).
In a further set of experiments, the thermoresponsive behavior of a 20% w/w aqueous dispersion of HOOC–PHEA73–PHBA265 nano-objects was assessed by visual inspection at 2, 23 and 53 °C, allowing 10 min equilibration at each temperature (see Fig. 11). On cooling from 23 to 2 °C, the initial transient free-standing gel became a free-flowing fluid, see Fig. 11A(i) and 11A(ii). For other PISA systems,41,74,88 including the closely related thermoresponsive HBA-rich nanoparticles reported by Byard et al.,42 such degelation is associated with a worm-to-sphere transition (see Fig. 11B). Reheating the flowing dispersion to 23 °C resulted in reconstitution of a transient free-standing gel [Fig. 11A(iii)], suggesting that this worm-to-sphere transition is reversible. Moreover, heating to 53 °C yielded a turbid free-flowing dispersion [Fig. 11A(iv)], which is consistent with a worm-to-vesicle transition (see Fig. 11B).42,49 This second thermal transition also appeared to be reversible on returning to 23 °C [Fig. 11A(v)].
Finally, the same 20% w/w aqueous dispersion of HOOC–PHEA73–PHBA265 nano-objects was studied by variable temperature oscillatory rheology at pH 7 (see Fig. 11C and D). At sub-ambient temperatures, the loss modulus (G′′) exceeds the storage modulus (G′), indicating a fluid rather than a gel. However, as the complex viscosity, |η*|, remains at about 3 Pa s, the free-flowing dispersion is still relatively viscous, see Fig. 11A(ii). As the temperature is raised, G′, G′′ and |η*| each pass through a maximum value at around 22–28 °C before tending towards 1 Pa and 2 Pa s, respectively, above 40 °C. Unusually, gelation occurs after these maxima, with CGT of 32 °C and 34 °C being observed on heating and cooling, respectively, as |η*| rapidly decreases. This rheology profile is similar to that reported by Byard et al. for a 20% w/w aqueous dispersion of HBA-rich nano-objects.42 In this prior study, TEM studies of covalently-stabilized nano-objects that had been crosslinked at various temperatures provided confirmation of reversible sphere-to-worm and worm-to-vesicle morphological transitions between 1 and 50 °C. Furthermore, almost perfect thermoreversibility with minimal hysteresis is indicated by the rheological data shown in Fig. 11C and D, which is consistent with visual inspection [see Fig. 11A(i–v)].
Rheological studies conducted on three other HOOC–PHEA73–PHBAy nano-objects (y = 217, 243 or 289) indicated that lower PHBA DPs lead to higher CGTs (see Fig. S9, ESI†). Similar observations were reported by Verber et al. for aqueous dispersions of other thermoresponsive diblock copolymer worms.89 Notably, all gels formed by these HOOC–PHEA73–PHBAy nano-objects were relatively weak, with maximum |η*| values increasing from approximately 14 to 40 Pa s with increasing PHBA DP (see Fig. 11D and Fig. S9, ESI†). Presumably, this is the result of repulsive inter-worm interactions arising from the anionic end-groups, which reduces the number of inter-worm contacts that are believed to be responsible for macroscopic gelation.90 Similar observations were reported by Penfold and co-workers.47,48 One reviewer of this manuscript has suggested that it would be interesting to perform further rheology studies to examine the effect of varying the solution pH on the thermoresponsive behaviour of such nano-objects. This is an interesting suggestion that warrants further consideration but unfortunately such experiments are beyond the scope of the current study.
Conclusions
The synthesis of new all-acrylic PHEAx–PHBAy diblock copolymer nano-objects has been achieved via an efficient one-pot aqueous PISA protocol. Such nano-objects proved to be unusually sensitive to changes in pH, salt concentration, temperature and also copolymer concentration. When using a suitable carboxylic acid-functionalized RAFT agent, DLS and aqueous electrophoresis studies indicated that HOOC–PHEA73–PHBA217 spheres underwent reversible flocculation below pH 5.1, which corresponds to the pKa for the carboxylic acid end-groups located on the PHEA stabilizer chains. Moreover, these nanoparticles also exhibited high salt sensitivity, with incipient flocculation occurring in the presence of 20–60 mM KCl. However, unlike the pH-dependent colloidal stability reported by Gibson et al. for the analogous HOOC–PNMEPx–PHPMAy nanoparticles,69 such aggregation appears to be unrelated to LCST-type behavior. Instead, the PHEA73 stabilizer is not sufficiently long to confer effective steric stabilization so ionization of the carboxylic acid end-groups is required to confer additional colloidal stability via electrosteric stabilization. Furthermore, when HOOC–PHEAx–PHBAy spheres were prepared using longer PHEA stabilizers (x = 105 and 140), relatively effective steric stabilization was maintained even when targeting longer PHBA DPs, as indicated by the elimination of the requirement for end-group ionization. Conversely, by switching the RAFT agent functionality from carboxylic acid to morpholine, colloidally stable Mo–PHEA76–PHBA160 nanoparticles could be prepared that exhibited complementary pH-dependent colloidal stability behavior: such dispersions remained stable at low pH but became highly flocculated above pH 5.Discrepancies between the physical appearance of a series of HOOC–PHEA73–PHBAy nanoparticles (where y = 104–421) prepared at 20% w/w and their corresponding DLS data obtained at 0.1% w/w suggested that such nano-objects were unstable to dilution. This unexpected instability was supported by TEM studies of HOOC–PHEA73–PHBA243 and HOOC–PHEA73–PHBA421 nanoparticles crosslinked using glutaraldehyde at either 0.1% w/w or 10–20% w/w. At 0.1% w/w, such covalent stabilization simply indicated a spherical morphology for both diblock copolymers. In contrast, worms (y = 243) or vesicles (y = 421) were observed by TEM when these formulations were crosslinked at 20% w/w or 10% w/w, respectively. Similarly, the strong Maltese cross observed in SIPLI studies of the as-synthesized 20% w/w HOOC–PHEA73–PHBA243 nano-objects also suggested a highly anisotropic worm morphology. Therefore, morphological assignments of spheres, a sphere/worm mixed phase, pure worms and a worm/vesicle mixed phase were made based on (i) the physical appearance of the 20% w/w dispersions and (ii) SIPLI studies conducted at the same concentration. However, TEM studies of GA-crosslinked HOOC–PHEA73–PHBA421 nano-objects provided evidence for a population of tubular vesicles rather than worms.
Finally, the thermoresponsive behavior of a 20% w/w aqueous dispersion of HOOC–PHEA73–PHBA265 nano-objects was examined at pH 7 using variable temperature oscillatory rheology. The rheological profile and corresponding visual appearance observed between 2 and 50 °C was similar to that reported by Byard et al. for similar thermoresponsive HBA-rich nanoparticles42 and almost perfect thermoreversibility was obtained. This suggests that HOOC–PHEA73–PHBA265 forms spheres at sub-ambient temperatures, worms at around ambient temperature, and vesicles at elevated temperatures.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the EPSRC for a DTA PhD studentship to support D.L.B. S.P.A. acknowledges an EPSRC Particle Technology Fellowship grant (EP/R003009).Notes and references
- B. Charleux, G. Delaittre, J. Rieger and F. D'Agosto, Macromolecules, 2012, 45, 6753–6765 CrossRef CAS.
- J. T. Sun, C. Y. Hong and C. Y. Pan, Polym. Chem., 2013, 4, 873–881 RSC.
- N. J. Warren and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 10174–10185 CrossRef CAS PubMed.
- J. Rieger, Macromol. Rapid Commun., 2015, 36, 1458–1471 CrossRef CAS PubMed.
- Y. Pei, A. B. Lowe and P. J. Roth, Macromol. Rapid Commun., 2017, 38, 1600528 CrossRef PubMed.
- N. J. W. Penfold, J. Yeow, C. Boyer and S. P. Armes, ACS Macro Lett., 2019, 8, 1029–1054 CrossRef CAS.
- X. Wang and Z. An, Macromol. Rapid Commun., 2019, 40, 1800325 CrossRef PubMed.
- F. D'Agosto, J. Rieger and M. Lansalot, Angew. Chem., Int. Ed., 2020, 59, 8368–8392 CrossRef PubMed.
- I. Chaduc, W. Zhang, J. Rieger, M. Lansalot, F. D'Agosto and B. Charleux, Macromol. Rapid Commun., 2011, 32, 1270–1276 CrossRef CAS PubMed.
- A. Blanazs, J. Madsen, G. Battaglia, A. J. Ryan and S. P. Armes, J. Am. Chem. Soc., 2011, 133, 16581–16587 CrossRef CAS PubMed.
- L. A. Fielding, M. J. Derry, V. Ladmiral, J. Rosselgong, A. M. Rodrigues, L. P. D. Ratcliffe, S. Sugihara and S. P. Armes, Chem. Sci., 2013, 4, 2081–2087 RSC.
- A. B. Lowe, Polymer, 2016, 106, 161–181 CrossRef CAS.
- S. Y. Khor, N. P. Truong, J. F. Quinn, M. R. Whittaker and T. P. Davis, ACS Macro Lett., 2017, 6, 1013–1019 CrossRef CAS.
- J. Tan, J. He, X. Li, Q. Xu, C. Huang, D. Liu and L. Zhang, Polym. Chem., 2017, 8, 6853–6864 RSC.
- J. Chiefari, Y. K. B. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo, S. H. Thang and C. South, Macromolecules, 1998, 31, 5559–5562 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379–410 CrossRef CAS.
- M. R. Hill, R. N. Carmean and B. S. Sumerlin, Macromolecules, 2015, 48, 5459–5469 CrossRef CAS.
- M. Benaglia, J. Chiefari, Y. K. Chong, G. Moad, E. Rizzardo and S. H. Thang, J. Am. Chem. Soc., 2009, 131, 6914–6915 CrossRef CAS PubMed.
- G. Moad and E. Rizzardo, Polym. Int., 2020, 69, 658–661 CrossRef CAS.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, J. Chem. Soc., Faraday Trans. 2, 1976, 72, 1525–1568 RSC.
- J. N. Israelachvili, Intermolecular and Surface Forces, Academic Press, London, 3rd edn, 2011 Search PubMed.
- A. Blanazs, S. P. Armes and A. J. Ryan, Macromol. Rapid Commun., 2009, 30, 267–277 CrossRef CAS PubMed.
- Z. An, Q. Shi, W. Tang, C. K. Tsung, C. J. Hawker and G. D. Stucky, J. Am. Chem. Soc., 2007, 129, 14493–14499 CrossRef CAS PubMed.
- S. Boissé, J. Rieger, G. Pembouong, P. Beaunier and B. Charleux, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3346–3354 CrossRef.
- A. Blanazs, A. J. Ryan and S. P. Armes, Macromolecules, 2012, 45, 5099–5107 CrossRef CAS.
- W. Zhang, F. D'Agosto, O. Boyron, J. Rieger and B. Charleux, Macromolecules, 2012, 45, 4075–4084 CrossRef CAS.
- M. Semsarilar, V. Ladmiral, A. Blanazs and S. P. Armes, Langmuir, 2012, 28, 914–922 CrossRef CAS PubMed.
- J. R. Lovett, N. J. Warren, L. P. D. Ratcliffe, M. K. Kocik and S. P. Armes, Angew. Chem., Int. Ed., 2015, 54, 1279–1283 CrossRef CAS PubMed.
- G. Mellot, J. M. Guigner, L. Bouteiller, F. Stoffelbach and J. Rieger, Angew. Chem., Int. Ed., 2019, 58, 3173–3177 CrossRef CAS PubMed.
- W. Zhang, F. D'Agosto, P. Y. Dugas, J. Rieger and B. Charleux, Polymer, 2013, 54, 2011–2019 CrossRef CAS.
- A. A. Cockram, T. J. Neal, M. J. Derry, O. O. Mykhaylyk, N. S. J. Williams, M. W. Murray, S. N. Emmett and S. P. Armes, Macromolecules, 2017, 50, 796–802 CrossRef CAS PubMed.
- F. L. Hatton, A. M. Park, Y. Zhang, G. D. Fuchs, C. K. Ober and S. P. Armes, Polym. Chem., 2019, 10, 194–200 RSC.
- E. E. Brotherton, F. L. Hatton, A. A. Cockram, M. J. Derry, A. Czajka, E. J. Cornel, P. D. Topham, O. O. Mykhaylyk and S. P. Armes, J. Am. Chem. Soc., 2019, 141, 13664–13675 CrossRef CAS PubMed.
- X. Dai, L. Yu, Y. Zhang, L. Zhang and J. Tan, Macromolecules, 2019, 52, 7468–7476 CrossRef CAS.
- P. Galanopoulo, P. Y. Dugas, M. Lansalot and F. D'Agosto, Polym. Chem., 2020, 11, 3922–3930 RSC.
- C. J. Ferguson, R. J. Hughes, D. Nguyen, B. T. T. Pham, R. G. Gilbert, A. K. Serelis, C. H. Such and B. S. Hawkett, Macromolecules, 2005, 38, 2191–2204 CrossRef CAS.
- J. Rieger, W. Zhang, F. Stoffelbach and B. Charleux, Macromolecules, 2010, 43, 6302–6310 CrossRef CAS.
- N. P. Truong, M. V. Dussert, M. R. Whittaker, J. F. Quinn and T. P. Davis, Polym. Chem., 2015, 6, 3865–3874 RSC.
- S. L. Canning, V. J. Cunningham, L. P. D. Ratcliffe and S. P. Armes, Polym. Chem., 2017, 8, 4811–4821 RSC.
- F. H. Sobotta, M. T. Kuchenbrod, C. Grune, D. Fischer, S. Hoeppener and J. C. Brendel, Polym. Chem., 2021, 12, 1668–1680 RSC.
- J. Tan, Y. Bai, X. Zhang and L. Zhang, Polym. Chem., 2016, 7, 2372–2380 RSC.
- S. J. Byard, C. T. O'Brien, M. J. Derry, M. Williams, O. O. Mykhaylyk, A. Blanazs and S. P. Armes, Chem. Sci., 2020, 11, 396–402 RSC.
- F. H. Sobotta, M. Kuchenbrod, S. Hoeppener and J. C. Brendel, Nanoscale, 2020, 12, 20171–20176 RSC.
- X. Wang, J. Zhou, X. Lv, B. Zhang and Z. An, Macromolecules, 2017, 50, 7222–7232 CrossRef CAS.
- N. J. Warren, M. J. Derry, O. O. Mykhaylyk, J. R. Lovett, L. P. D. Ratcliffe, V. Ladmiral, A. Blanazs, L. A. Fielding and S. P. Armes, Macromolecules, 2018, 51, 8357–8371 CrossRef CAS PubMed.
- J. R. Lovett, N. J. Warren, S. P. Armes, M. J. Smallridge and R. B. Cracknell, Macromolecules, 2016, 49, 1016–1025 CrossRef CAS PubMed.
- N. J. W. Penfold, J. R. Lovett, N. J. Warren, P. Verstraete, J. Smets and S. P. Armes, Polym. Chem., 2016, 7, 79–88 RSC.
- N. J. W. Penfold, J. R. Lovett, P. Verstraete, J. Smets and S. P. Armes, Polym. Chem., 2017, 8, 272–282 RSC.
- L. P. D. Ratcliffe, M. J. Derry, A. Ianiro, R. Tuinier and S. P. Armes, Angew. Chem., Int. Ed., 2019, 58, 18964–18970 CrossRef CAS PubMed.
- O. J. Deane, J. Jennings and S. P. Armes, Chem. Sci., 2021, 12, 13719–13729 RSC.
- L. Zhang, K. Katapodi, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2177–2194 CrossRef CAS.
- Y. Chan, T. Wong, F. Byrne, M. Kavallaris and V. Bulmus, Biomacromolecules, 2008, 9, 1826–1836 CrossRef CAS PubMed.
- C. D. Vo, J. Rosselgong, S. P. Armes and N. Tirelli, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 2032–2043 CrossRef CAS.
- H. Mori and H. Tanaka, Macromol. Chem. Phys., 2011, 212, 2349–2359 CrossRef CAS.
- W. Steinhauer, R. Hoogenboom, H. Keul and M. Moeller, Macromolecules, 2013, 46, 1447–1460 CrossRef CAS.
- B. Louage, Q. Zhang, N. Vanparijs, L. Voorhaar, S. Vande Casteele, Y. Shi, W. E. Hennink, J. Van Bocxlaer, R. Hoogenboom and B. G. De Geest, Biomacromolecules, 2015, 16, 336–350 CrossRef CAS PubMed.
- M. Lu, Y. Y. Khine, F. Chen, C. Cao, C. J. Garvey, H. Lu and M. H. Stenzel, Biomacromolecules, 2019, 20, 273–284 CrossRef CAS PubMed.
- J. Zhou, W. Zhang, C. Hong and C. Y. Pan, Polym. Chem., 2016, 7, 3259–3267 RSC.
- V. Tkachenko, C. Matei Ghimbeu, C. Vaulot, L. Vidal, J. Poly and A. Chemtob, Polym. Chem., 2019, 10, 2316–2326 RSC.
- J. Tan, Y. Peng, D. Liu, C. Huang, M. Yu, D. Jiang and L. Zhang, Macromol. Chem. Phys., 2016, 217, 1723–1728 CrossRef CAS.
- W. Steinhauer, R. Hoogenboom, H. Keul and M. Moeller, Macromolecules, 2010, 43, 7041–7047 CrossRef CAS.
- J. T. Lai, D. Filla and R. Shea, Macromolecules, 2002, 35, 6754–6756 CrossRef CAS.
- R. Chapman, G. G. Warr, S. Perrier and K. A. Jolliffe, Chem. – Eur. J., 2013, 19, 1955–1961 CrossRef CAS PubMed.
- P. E. Millard, L. Barner, J. Reinhardt, M. R. Buchmeiser, C. Barner-Kowollik and A. H. E. Müller, Polymer, 2010, 51, 4319–4328 CrossRef CAS.
- L. Martin, G. Gody and S. Perrier, Polym. Chem., 2015, 6, 4875–4886 RSC.
- T. Junkers and C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7585–7605 CrossRef CAS.
- Y. Li and S. P. Armes, Angew. Chem., Int. Ed., 2010, 49, 4042–4046 CrossRef CAS PubMed.
- I. Bannister, N. C. Billingham, S. P. Armes, S. P. Rannard and P. Findlay, Macromolecules, 2006, 39, 7483–7492 CrossRef CAS.
- R. R. Gibson, S. P. Armes, O. M. Musa and A. Fernyhough, Polym. Chem., 2019, 10, 1312–1323 RSC.
- G. Vancoillie, D. Frank and R. Hoogenboom, Prog. Polym. Sci., 2014, 39, 1074–1095 CrossRef CAS.
- M. B. Einarson and J. C. Berg, J. Colloid Interface Sci., 1993, 155, 165–172 CrossRef CAS.
- M. Save, M. Manguian, C. Chassenieux and B. Charleux, Macromolecules, 2005, 38, 280–289 CrossRef CAS.
- L. P. D. Ratcliffe, B. E. McKenzie, G. M. D. Le Bouëdec, C. N. Williams, S. L. Brown and S. P. Armes, Macromolecules, 2015, 48, 8594–8607 CrossRef CAS.
- A. Blanazs, R. Verber, O. O. Mykhaylyk, A. J. Ryan, J. Z. Heath, C. W. I. Douglas and S. P. Armes, J. Am. Chem. Soc., 2012, 134, 9741–9748 CrossRef CAS PubMed.
- B. Akpinar, L. A. Fielding, V. J. Cunningham, Y. Ning, O. O. Mykhaylyk, P. W. Fowler and S. P. Armes, Macromolecules, 2016, 49, 5160–5171 CrossRef CAS PubMed.
- V. J. Cunningham, A. M. Alswieleh, K. L. Thompson, M. Williams, G. J. Leggett, S. P. Armes and O. M. Musa, Macromolecules, 2014, 47, 5613–5623 CrossRef CAS.
- M. Williams, N. J. W. Penfold and S. P. Armes, Polym. Chem., 2016, 7, 384–393 RSC.
- M. Chenal, L. Bouteiller and J. Rieger, Polym. Chem., 2013, 4, 752–762 RSC.
- G. Liu, Q. Qiu and Z. An, Polym. Chem., 2012, 3, 504–513 RSC.
- J. Bang, S. Jain, Z. Li, T. P. Lodge, J. S. Pedersen, E. Kesselman and Y. Talmon, Macromolecules, 2006, 39, 1199–1208 CrossRef CAS.
- A. Czajka and S. P. Armes, Chem. Sci., 2020, 11, 11443–11454 RSC.
- C. Cao, F. Chen, C. J. Garvey and M. H. Stenzel, ACS Appl. Mater. Interfaces, 2020, 12, 30221–30233 CrossRef CAS PubMed.
- O. J. Deane, J. Jennings, T. J. Neal, O. M. Musa, A. Fernyhough and S. P. Armes, Chem. Mater., 2021, 33, 7767–7779 CrossRef CAS.
- O. O. Mykhaylyk, N. J. Warren, A. J. Parnell, G. Pfeifer and J. Laeuger, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 2151–2170 CrossRef CAS.
- O. O. Mykhaylyk, Soft Matter, 2010, 6, 4430–4440 RSC.
- J. Tan, H. Sun, M. Yu, B. S. Sumerlin and L. Zhang, ACS Macro Lett., 2015, 4, 1249–1253 CrossRef CAS.
- M. Semsarilar, V. Ladmiral, A. Blanazs and S. P. Armes, Langmuir, 2013, 29, 7416–7424 CrossRef CAS PubMed.
- Y. Pei, N. C. Dharsana, J. A. Van Hensbergen, R. P. Burford, P. J. Roth and A. B. Lowe, Soft Matter, 2014, 10, 5787–5796 RSC.
- R. Verber, A. Blanazs and S. P. Armes, Soft Matter, 2012, 8, 9915–9922 RSC.
- J. R. Lovett, M. J. Derry, P. Yang, F. L. Hatton, N. J. Warren, P. W. Fowler and S. P. Armes, Chem. Sci., 2018, 9, 7138–7144 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and characterization details; supporting figures, schemes and tables: assigned 1H NMR spectra; summary table of DMF GPC and DLS data for a series of HOOC–PHEA73–PHBAy dispersions; reaction scheme and DMF GPC curves for a one-pot synthesis of HOOC–PHEA70–PHBA100 at pH 2.5; acid titration curve for the HOOC–PHEA73 precursor; normalized transmittance at 600 nm vs. temperature plot for a range of HOOC–PHEAx precursors; variation of apparent DLS diameter and zeta potential with pH; DLS particle size distributions at various salt concentrations; DMF GPC curves for a Mo–PHEA76 precursor and a Mo–PHEA76–PHBA160 diblock copolymer; acid titration curve for the Mo–PHEA76 precursor; SAXS patterns for HOOC–PHEA70–PHBA300 nano-objects; glutaraldehyde crosslinking scheme; summary table of TEM and variable temperature oscillatory rheology data for a range of HOOC-PHEA73-PHBAy nano-objects. See DOI: 10.1039/d1py01562a |
| This journal is © The Royal Society of Chemistry 2022 |