Controlled copolymerization of α-NCAs and α-NNTAs for preparing peptide/peptoid hybrid polymers with adjustable proteolysis†
Abstract
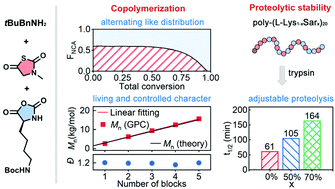
Peptides and polypeptides have excellent biocompatibility and numerous biological functions; however, their application is restricted by their proneness to proteolytic degradation. Although the incorporation of peptoid residues into peptides can improve their stability against proteases, the synthesis of peptide/peptoid hybrid oligomers suffers from the tedious and time consuming solid-phase synthesis process. Hereby, we report a living and controlled copolymerization of α-amino acid N-carboxyanhydrides (α-NCAs) and N-substituted α-amino acid N-thiocarboxyanhydrides (α-NNTAs) for the facile synthesis of peptide/peptoid hybrid polymers with an alternating-like distribution of residues, controllable molecular weight and narrow dispersity. Moreover, this polymerization strategy enables quick synthesis of peptide mimicking peptide/peptoid hybrid polymers with adjustable proteolysis by varying the proportion of peptoid residues. Our studies shed some light on the facile synthesis of peptide mimicking peptide/peptoid hybrid polymers with tunable proteolysis and open new avenues for preparing novel peptide mimics with tunable proteolysis and large structural diversity.


 Please wait while we load your content...
Please wait while we load your content...