Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Tyrosine bioconjugation – an emergent alternative
Peter A.
Szijj
,
Kristina A.
Kostadinova
,
Richard J.
Spears
and
Vijay
Chudasama
*
Department of Chemistry, University College London, London, UK. E-mail: v.chudasama@ucl.ac.uk
First published on 31st October 2022
Abstract
Correction for ‘Tyrosine bioconjugation – an emergent alternative’ by Peter A. Szijj et al., Org. Biomol. Chem., 2020, 18, 9018–9028, https://doi.org/10.1039/D0OB01912G.
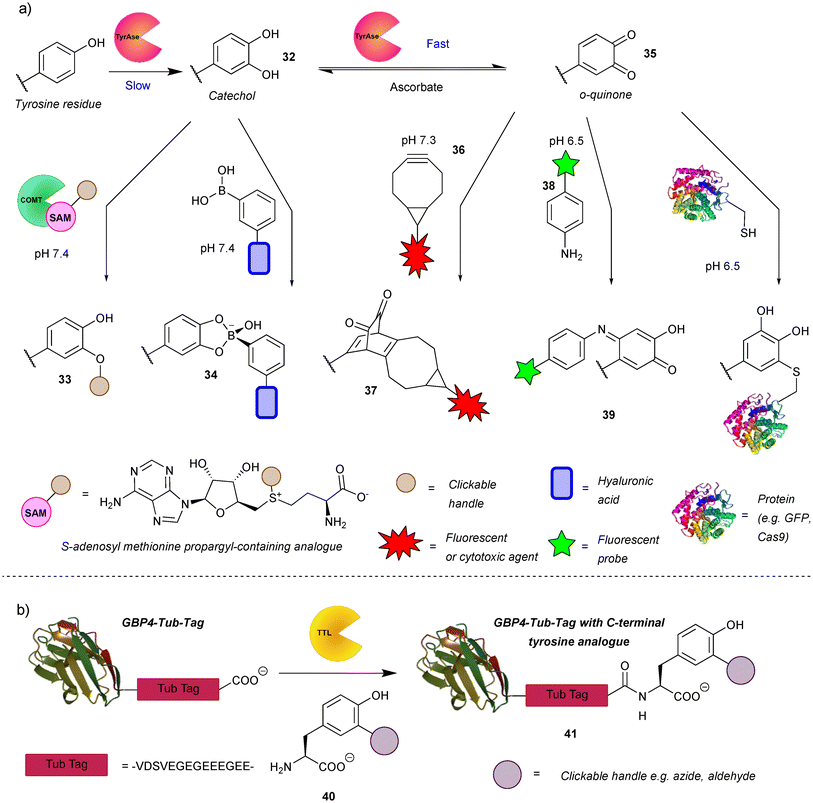
The authors regret that there were some errors in the compound numbering shown in Scheme 7. The correct scheme is shown below.
In addition, in the paragraph starting ‘The o-quinones (e.g. compound 35) produced in the second step can be attacked by nucleophiles’, the sentence ‘Recently, the suitability of anilines and cyclic amines as nucleophiles was compared, with anilines (e.g. compound 28) exhibiting higher efficiency.51’ should read ‘Recently, the suitability of anilines and cyclic amines as nucleophiles was compared, with anilines (e.g. compound 38) exhibiting higher efficiency.51’
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2022 |