Ag(i)-catalyzed cyclization of o-alkynylacetophenones facilitated through acetal formation: synthesis of C3-naphthyl indole derivatives†
Abstract
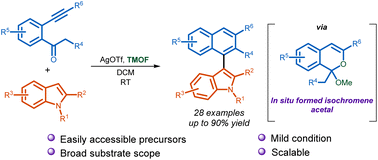
A mild method for an efficient synthesis of C3-naphthyl indoles from o-alkynylacetophenones has been developed. This Ag-catalyzed transformation is assisted by the acetal formed under the reaction condition employing trimethyl orthoformate (TMOF). The role of acetal in promoting the reaction under ambient conditions has been established with control experiments. A range of C3-naphthyl indole derivatives have been synthesized in moderate to very good yields.


 Please wait while we load your content...
Please wait while we load your content...