Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Robust synthesis of 2′-azido modified RNA from 2′-amino precursors by diazotransfer reaction†
Sarah
Moreno
a,
José M.
Ramos Pittol
 b,
Markus
Hartl
b,
Markus
Hartl
 b and
Ronald
Micura
b and
Ronald
Micura
 *a
*a
aInstitute of Organic Chemistry, Center for Molecular Biosciences Innsbruck, University of Innsbruck, Innrain 80-82, 6020 Innsbruck, Austria. E-mail: ronald.micura@uibk.ac.at
bInstitute of Biochemistry, Center for Chemistry and Biomedicine (CCB) Innsbruck, University of Innsbruck, Innrain 80-82, 6020 Innsbruck, Austria
First published on 29th September 2022
Abstract
Azides are versatile bioorthogonal reporter moieties that are commonly used for site-specific labeling and functionalization of RNA to probe its biology. The preparation of azido modified nucleic acids by solid-phase synthesis is problematic due to the inherent reactivity of P(III) species with azides according to the Staudinger reaction. Various strategies have been developed to bypass this limitation and are often time-consuming, low-yielding and labor-intensive. In particular, the synthesis of RNA with internal 2′-azido modifications is restricted to a single approach that employs P(V) chemistry instead of the widely used P(III) phosphoramidite chemistry. To fill this methodological gap, we present a novel convenient path toward 2′-azido RNA from readily accessible 2′-amino RNA through treatment with the diazotizing reagent fluorosulfuryl azide (FSO2N3). A diazotransfer reaction was established for oligoribonucleotides of different lengths and secondary structures. The robustness of the approach was further demonstrated for RNAs containing multiple 2′-azido moieties and for RNAs containing other sensitive modifications such as thiouridine or methylated nucleobases with a positive charge. The synthetic ease of generating 2′-azido RNA will pave the way for biotechnological applications, in particular for siRNA technologies and for referencing the growing number of RNA metabolic labeling approaches that rely on 2′-azido nucleosides.
Introduction
Azides are small and stable modifications that are rarely encountered in living organisms.1,2 They do not show any reactivity towards endogenous functional groups and are chemically engineered into the biomolecule of interest.3,4 Thus, azido modified RNA is a substantial tool for site-specific labeling and functionalization of RNA to probe its structure, dynamics, and localization, with applications in diagnostics, forensics, genetic analysis and sequencing.5,6 In all these approaches, a covalent linkage of the azido group to a reporter molecule, fluorescent dye, affinity tag or transporter unit is formed by either Staudinger ligation, Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC), strain-promoted [3 + 2] cycloaddition (SPAAC), or photo-click chemistry, with the RNA azido group being located at the ribose, the termini, the phosphate backbone or the nucleobase.4–9For chemical biology, 2′-azido modified RNA is an upcoming tool whose physicochemical properties have been reported earlier.10–12 The 2′-N3 modification favours the RNA C3′-endo sugar pucker, only causes a slight decrease in base pairing stabilities, and hardly influences the overall RNA structure.10,11 It is exceptionally well tolerated in the guide strand of siRNAs even when it is directly located at the cleavage site.10,11 This and other emerging applications in chemical biology require easy accessibility, i.e. the efficient synthesis of RNAs with site-specific 2′-N3 anchors. However, the chemical synthesis of azido modified oligonucleotides currently suffers from severe limitations since azides exhibit pronounced reactivity toward P(III) species (such as nucleoside phosphoramidite building blocks) according to Staudinger-type reactions.10–17
Various chemical and biotechnological strategies have been developed to bypass this restriction and strongly depend on the position of the azide modification. To date, the only option for the chemical synthesis of internally 2′-N3 modified RNA is provided by the integration of phospho(V)diester building blocks which are manually incorporated by a single coupling cycle of the phosphotriester method during otherwise automated standard oligonucleotide synthesis.10,11,17 Consequently, an expansion of the methodological repertoire for efficient and flexible access to 2′-N3 modified RNA is urgently needed. Therefore, we developed a convenient path starting from readily available 2′-NH2 modified RNA which is converted into 2′-N3 RNA by a diazotransfer reaction (Fig. 1).
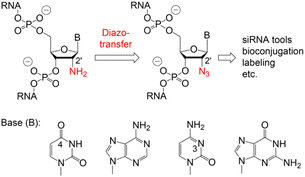 | ||
| Fig. 1 Proposed conversion of 2′-amino into 2′-azido RNA using mild reagent mediated diazotransfer reactions. | ||
Results and discussion
Diazotransfer on oligonucleotides
The potential of diazotransfer reactions on aminoalkylated DNA was first reported by Defrancq and coworkers18 in 2011 by the use of imidazole-1-sulfonylazide hydrochloride (ISAHC) in the presence of divalent metal ions. In 2020, Meng et al.19 demonstrated the rapid and quantitative conversion of a variety of small, organic amines into their corresponding azides in a biphasic solvent system containing potassium bicarbonate. Remarkably, the reaction requires only one equivalent of fluorosulfuryl azide (FSO2N3) and was deemed complete within 5 min at ambient temperature for most of the substrates. Shortly after, Krasheninina et al.20 demonstrated the applicability of FSO2N3 on native RNA and RNA–peptide conjugates containing an aliphatic primary amino group. The reaction conditions were found to be rather similar with the exception that full conversion required two equivalents of FSO2N3 and longer reaction times. To further expand the scope of substrates and to enhance the methodological repertoire specifically for the synthesis of 2′-N3 modified RNAs, the conversion of 2′-NH2-RNAs into their corresponding 2′-N3 analogues has been envisioned here. Because the 2′-position is much more sterically hindered than that in all the previously reported substrates, FSO2N3 may however not be able to approach the reaction centre sufficiently.Conversion of 2′-NH2 RNA into 2′-N3 RNA
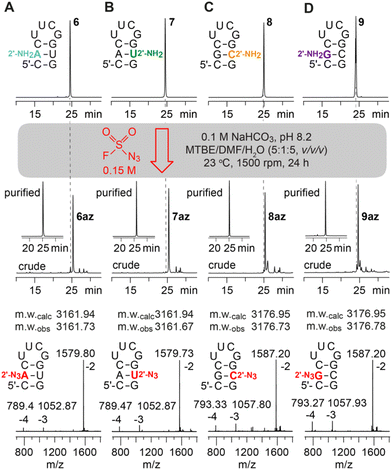
The canonical 2′-NH2 nucleoside phosphoramidites were obtained from commercial sources or were synthesized in-house;21–23 the corresponding 2′-NH2 modified RNA was prepared using standard solid-phase synthesis and deprotection protocols.Incubation of the 2′-NH2 RNA precursor 1 with FSO2N3 following the conditions described by Krasheninina et al.,20 however, did not lead to any product formation. Only when significantly longer reaction times and increased concentration of the diazotizing reagent were used, we achieved complete conversion (ESI Fig. 1–5†). Thereby, the 5 nt long RNA (2–5) without any secondary structure (single strand) showed much faster reaction progress than the 10 nt hairpin (6–9) or the 8 nt palindrome (1, 10–12) that exhibits defined secondary structures (double helices). A summary of the diazotransfer on the 10 nt hairpin for all canonical 2′-NH2 modified RNAs (6–9) is shown in Fig. 2. In short, 100 μl of 150 mM FSO2N3 in methyl tert-butyl ether (MTBE) and 24 h reaction time were found to be suitable to achieve full conversion.
To further analyse the structure dependency and to apply the diazotransfer reaction to longer sequences, the 27 nt sarcin-ricin loop24 was synthesized and a single 2′-NH2-A was placed in the middle of the stem (13), in the loop region (14) or at the 5′-terminus (15) (ESI Fig. 6†). As expected, the loop modified sarcin-ricin structure showed the fastest reaction progress followed by the terminally modified RNA and the RNA where the modification resides in the Watson–Crick paired region. This is consistent with the initial observations on 2′ accessibility.
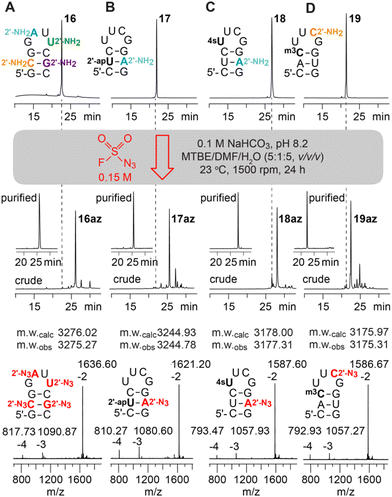
Next, RNAs containing more than one aliphatic amino group were synthesized and their amenability for multiple diazotransfer was tested. Hence, the 10 nt hairpin (16) was equipped with all four standard 2′-NH2 nucleotides (A, C, G, U) at once, and a second hairpin (17) was prepared forming a base pair between 2′-NH2-A and 2′-O-(3-aminopropyl)uridine.12,13,25 The modified RNAs were treated with FSO2N3 for 24 h and clean conversions for the sequences were observed (Fig. 3A and B).
Subsequently, the compatibility of the diazotransfer reaction with other sensitive and reactive RNA modifications like 4-thiouridine (4sU) and 3-methylcytidine (m3C) was tested (18, 19) (Fig. 3C and D). The corresponding phosphoramidite building blocks were synthesized according to published procedures26,27 and the modified RNAs were prepared as described in the ESI.† 4sU is one of the most widely applied labels on RNA due to its broad and selective reactivity scope (disulfide formation, oxidative alkylations, photo-crosslinking, etc.) and used e.g. for biotinylation and RNA pull-down, covalent attachment to fluorophores, or photoactivatable-ribonucleoside-enhanced crosslinking and immunoprecipitation (PAR-CLIP).28–31 Moreover, metabolic labeling-based RNA sequencing methods (e.g. Thiouridine-to-Cytidine Conversion Sequencing, TUC-seq) have emerged which became indispensable tools for analysing cellular RNA dynamics.26 The 4sU modification, however, can also undergo undesired side reactions like oxidation, dimerization, hydrolysis and degradation. Selective derivatization of 4sU modified RNA that leaves the sensitive 4-thio moiety intact is therefore rare. We show that the treatment of 4sU containing RNA (18) with FSO2N3 indeed results in the selective formation of the desired 2′-N3 modified RNA (18az) preserving the parent 4sU modification. The obtained 4sU and 2′-N3 modified RNA offers two orthogonal conjugation sites to combine the novel RNA sequencing approaches with efficient bioconjugation reactions.
Next, we focused on testing the diazotransfer reaction for RNA (19) containing 3-methylcytidine (m3C) which is another fragile modification due to its positively charged nucleobase. In tRNA, m3C has long been known,32,33 but recently this modification gained increasing attention due to its evidential occurrence in the mRNA of mice and humans.34 Furthermore, the self-methylation activity of a naturally occurring riboswitch scaffold35 and various approaches for detection and genome-wide m3C sequencing36–38 have been reported. Although m3C is prone to nucleobase loss (depyrimidization) and/or hydrolysis to 3-methyluridine (see ref. 35), the diazotransfer reaction proceeded smoothly in our hands for the m3C and 2′-NH2-C modified RNA, furnishing the m3C containing 2′-N3 analog (19az) in good yields.
Taken together, we have demonstrated that 2′-amino RNAs can be converted into their 2′-azido counterparts by the diazotransfer reaction (see ESI Table 1† for a complete list) in nearly quantitative yields for short RNAs and yields of more than 80 to 90% for longer RNAs. Although extended reaction times (>12 h) are needed, RNA degradation appears minimal under the biphasic reaction conditions, reflected in HPLC traces with no or only a few faster migrating RNA side products. Interestingly, slower migrating side products are observed (less than 10 to 20%) that may indicate covalent RNA-reagent intermediates or even crosslinked RNA dimers.
Reverse transcription of 2′-NH2 and 2′-N3 RNA
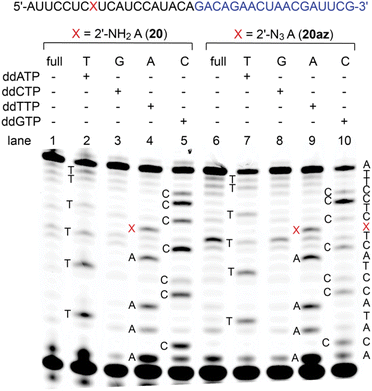
2′-Amino and 2′-azido nucleotide triphosphates (NTPs) are only modestly accepted substrates for native RNA polymerases; however, polymerase mutants have been described that make such NTPs effective for enzymatic RNA synthesis.39–41 Here, to shed light on the performance of 2′-NH2 and 2′-N3 modified RNA templates in reverse transcription, we performed primer extension using SuperScript IV reverse transcriptase on synthetic 37 nt long RNAs (20, 20az) containing either a single 2′-NH2- or a single 2′-N3 adenosine at the same internal position, flanked by 5′-C and 3′-U (Fig. 4 and ESI Fig. 7, 8†). Both modifications, 2′-NH2-A and 2′-N3-A, were well recognized by thymidine, and hence, they preserved canonical base recognition. However, slightly enhanced termination of reverse transcription at the 2′-N3 modification (Fig. 4, lane 6 and 9) was observed while this was not then case for the 2′-NH2 modification (Fig. 4, lane 1 and 4). This observation may be attributed to different preferences of the modified ribose pucker (C3′- versus C2′ endo conformation) and/or to the larger steric hindrance of 2′-N3 compared to that of the 2′-NH2 group.Potential of 2′-NH2 and 2′-N3 siRNAs
Earlier on, we have pointed out the promising potential of 2′-azido modified RNA for RNA interference,10,11 being equivalent or even superior to 2′-F and 2′-OCH3 modified siRNAs that have found biomedical and therapeutic applications.42,43 As shown previously, single 2′-N3 moieties were excellently tolerated in the guide strand of the siRNA duplex and caused efficient gene silencing even when the modification was located in the seed region or at the cleavage site.10,11With the new method for efficient 2′-N3 RNA synthesis in our hand, we further aimed at demonstrating their siRNA potential by directly comparing with the corresponding 2′-NH2 counterparts and by explicitly placing multiple azides into the modification-sensitive seed region of the siRNA antisense strand. Knockdown of the brain acid soluble protein 1 (BASP1)44 from the DF-1 chicken cell line by transient siRNA transfection was selected as an established model system (Fig. 5A and ESI Table 2†).10–12
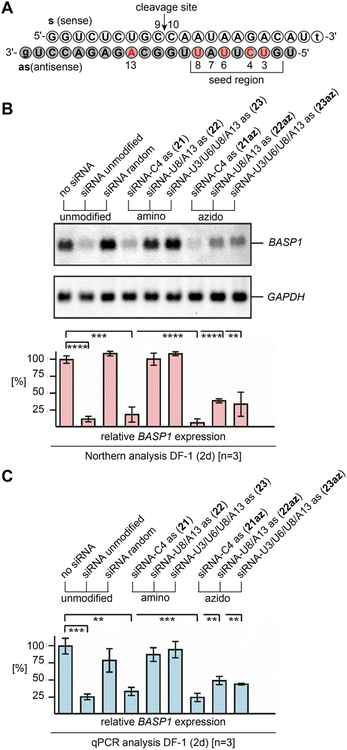 | ||
| Fig. 5 Gene silencing by 2′-NH2 and 2′-N3 modified siRNAs. (A) Sequence of the brain acid soluble protein 1 (BASP1)44 targeting siRNA duplex used in this study; nucleosides in red indicate positions for the modification tested. (B) Biological activities of 2′-NH2 and 2′-N3 modified siRNAs, directed against BASP1 mRNA. Chicken DF-1 cells grown on 60 mm dishes were transiently nucleofected with 0.24 nmol (∼3.0 μg) aliquots of the individual siRNAs. An equal aliquot of siRNA with a shuffled (random) nucleotide sequence was used as a control. Total RNAs were isolated 2 days after siRNA delivery, and 5 μg of aliquots were analyzed by Northern hybridization using a digoxigenin-labeled DNA probe specific for the chicken BASP1 gene, and subsequently with a digoxigenin-labeled probe specific for the housekeeping chicken GAPDH gene. Sizes for the mRNAs are: BASP1, 2.0 kb; GAPDH, 1.4 kb. The levels (%) of BASP1 expression were determined using the program ImageQuant TL and are depicted as bars in relation to mock transfections (no siRNA, 100%). Vertical bars show standard deviations (SD) from independent experiments (n = 3). Statistical significance was assessed by using a paired Student t-test (**P < 0.01, ***P < 0.001, ****P < 0.0001). (C) The same as (B) but analyzed by quantitative polymerase chain reaction (qPCR) using each 2.5 ng cDNA template reverse transcribed from total RNA, and primers specific for chicken BASP1 or GAPDH. All siRNAs depicted contain overhangs of 2′-deoxynucleosides (lower case letters). | ||
The Northern blot and the corresponding graph depicted in Fig. 5B show that the unmodified siRNA efficiently silences the BASP1 gene, while a control siRNA duplex with two compensatory base pair mutations in the seed region (U7as–A13s and A5as–U15s; siRNA random) did not downregulate.
As expected, a single 2′-NH2 or 2′-N3 installed at C4 (21, 21az) in the seed region led to efficient silencing. The picture changed when more modifications were present. For the 2′-amino modified siRNAs U8/A13as (22) and U3/U6/U8/A13as (23), the silencing capabilities were abolished. Interestingly, the two corresponding 2′-azido modified siRNAs U8/A13as (22az) and U3/U6/U8/A13 (23az) still exhibited significant silencing activity, which strongly underlines their virtue for siRNA design and performance. The results were independently confirmed by qPCR analysis (Fig. 5C and ESI†).
Conclusions
2′-Azido RNA has attracted much interest recently because of novel opportunities for the cell and tissue-specific metabolic labeling of nascent RNA. Spitale and co-workers demonstrated the efficient metabolic incorporation of 2′-azidoadenosine into cellular RNA.45 2′-Azidoadenosine is incorporated transcriptionally by RNA polymerase enzymes and post-transcriptionally by poly(A)polymerases. Additionally, through the manipulation of enzymes in the nucleotide salvage pathway, further azido-modified nucleosides have been applied for RNA metabolic labeling. It has been shown that the overexpression of nucleoside kinase UCK2 or deoxynucleoside kinase dCK enables RNA labeling with 2′-azidouridine46 and 2′-azidocytidine,47 respectively. Moreover, 2′-azidoguanosine has been utilized for AIR-seq (azidonucleoside-incorporated RNA sequencing) which enabled the genome-wide analysis of transcription upon heat stress in Escherichia coli, and hence, represents a new benchmark for metabolic RNA labeling to probe RNA dynamics in bacteria.48 All these novel developments have significantly increased the demand for site-specifically labeled, chemically synthesized 2′-azido RNAs which are needed to reference the approaches. Additionally, 2′-azido RNA is required for siRNA technologies.Thus, to satisfy the growing demand, a novel synthetic methodology was conceptualized and diazotransfer reactions on readily accessible 2′-amino RNA with fluorosulfuryl azide (FSO2N3) were investigated. This reagent was previously introduced for NH2-to-N3 conversions on small organic compounds,19,49 and also used on native RNAs and synthetic RNA–peptide conjugates with structurally well accessible primary amino groups.20 For the here demonstrated conversion of the sterically hindered 2′-NH2 groups in RNA into their 2′-N3 modified counterparts, the diazotransfer reaction had to be carefully optimized, and finally was successful for longer RNA containing multiple 2′-NH2 moieties and other sensitive nucleoside modifications, such as 4-thiouridine and 3-methylcytosine. The synthetic ease of generating 2′-azido RNA will pave the way for novel applications in RNA biology, and strengthen existing ones in siRNA technologies and RNA metabolic labeling.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Anna Rázková (University of Innsbruck) for synthetic contributions, and Daniel Fellner (University of Innsbruck) and Ulrike Schober (University of Innsbruck) for the technical support. This work was supported by the Austrian Science Fund FWF (P31691, F8011-B to R.M.; P33662 to M.H.), the Austrian Research Promotion Agency FFG [West Austrian BioNMR 858017], and the Wiener Wissenschafts-, Forschungs- und Technologiefonds (WWTF LS17-003).References
- N. J. Agard, J. M. Baskin, J. A. Prescher, A. Lo and C. R. Bertozzi, ACS Chem. Biol., 2006, 1, 644 CrossRef CAS PubMed
.
- S. L. Scinto, D. A. Bilodeau, R. Hincapie, W. Lee, S. S. Nguyen, M. Xu, C. W. am Ende, M. G. Finn, K. Lang, Q. Lin and J. P. Pezacki, Nat. Rev. Methods Primers, 2021, 1, 1 CrossRef
.
- N. Klöcker, F. P. Weissenboeck and A. Rentmeister, Chem. Soc. Rev., 2020, 49, 8749 RSC
.
- N. Z. Fantoni, A. H. El-Sagheer and T. Brown, Chem. Rev., 2021, 121, 7122 CrossRef CAS
.
- M. L. Winz, A. Samanta, D. Benzinger and A. Jäschke, Nucleic Acids Res., 2012, 40, e78 CrossRef CAS PubMed
.
- J. T. George and S. G. Srivatsan, Chem. Commun., 2020, 56, 12307 RSC
.
- M. Fonvielle, D. Mellal, D. Patin, M. Lecerf, D. Blanot, A. Bouhss, M. Santarem, D. Mengin-Lecreulx, M. Sollogoub, M. Arthur and M. Ethève-Quelquejeu, Chem. – Eur. J., 2012, 19, 1357 CrossRef PubMed
.
- F. Müggenburg and S. Müller, Chem. Rec., 2022, 22, e202100322 CrossRef
.
- K. Krell and H.-A. Wagenknecht, Biomolecules, 2020, 10, 480 CrossRef CAS
.
- M. Aigner, M. Hartl, K. Fauster, J. Steger, K. Bister and R. Micura, ChemBioChem, 2011, 12, 47 CrossRef CAS PubMed
.
- K. Fauster, M. Hartl, T. Santner, M. Aigner, C. Kreutz, K. Bister, E. Ennifar and R. Micura, ACS Chem. Biol., 2012, 7, 581 CrossRef CAS PubMed
.
- T. Santner, M. Hartl, K. Bister and R. Micura, Bioconjugate Chem., 2014, 25, 188 CrossRef CAS
.
- A. M. Jawalekar, N. Meeuwenoord, J. S. G. Cremers, H. S. Overkleeft, G. A. van der Marel, F. P. Rutjes and F. L. van Delft, J. Org. Chem., 2008, 73, 287 CrossRef CAS PubMed
.
- T. Wada, A. Mochizuki, S. Higashiya, H. Tsuruoka, S. I. Kawahara, M. Ishikawa and M. Sekine, Tetrahedron Lett., 2001, 42, 9215 CrossRef CAS
.
- M. Warminski, J. Kowalska and J. Jemielity, Org. Lett., 2017, 19, 3624 CrossRef CAS
.
- J. Lietard, A. Meyer, J. J. Vasseur and F. Morvan, Tetrahedron Lett., 2007, 48, 8795 CrossRef CAS
.
- N. N. Polushin, I. P. Smirnov, A. N. Verentchikov and J. M. Coull, Tetrahedron Lett., 1996, 37, 3227 CrossRef CAS
.
- R. Lartia, P. Murat, P. Dumy and E. Defrancq, Org. Lett., 2011, 13, 5672 CrossRef CAS PubMed
.
- G. Meng, T. Guo, T. Ma, J. Zhang, Y. Shen, B. Sharpless and J. Dong, Nature, 2019, 574, 86 CrossRef CAS PubMed
.
- O. A. Krasheninina, J. Thaler, M. D. Erlacher and R. Micura, Angew. Chem., Int. Ed., 2021, 60, 6970 CrossRef CAS
.
- C. Falschlunger and R. Micura, Monatsh. Chem., 2019, 150, 795 CrossRef CAS
.
- H. Aurup, T. Tuschl, F. Benseler, J. Ludwig and F. Eckstein, Nucleic Acids Res., 1994, 22, 20–24 CrossRef CAS PubMed
.
- D. M. Williams, W. A. Pieken and F. Eckstein, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 918 CrossRef CAS PubMed
.
- V. Olieric, U. Rieder, K. Lang, A. Serganov, C. Schulze-Briese, R. Micura, P. Dumas and E. Ennifar, RNA, 2009, 15, 707 CrossRef CAS PubMed
.
- M. Egger, R. Bereiter, S. Mair and R. Micura, Angew. Chem., Int. Ed., 2022, 61, e202207590 CrossRef CAS PubMed
.
- C. Riml, T. Amort, D. Rieder, C. Gasser, A. Lusser and R. Micura, Angew. Chem., Int. Ed., 2017, 56, 13479 CrossRef CAS PubMed
.
- S. Moreno, L. Flemmich and R. Micura, Monatsh. Chem., 2022, 153, 285 CrossRef CAS PubMed
.
- S. Moreno, M. Brunner, I. Delazer, D. Rieder, A. Lusser and R. Micura, RSC Chem. Biol., 2022, 3, 447 RSC
.
- L. Dölken, Z. Ruzsics, B. Rädle, C. C. Friedel, R. Zimmer, J. Mages, R. Hoffmann, P. Dickinson, T. Forster, P. Ghazal and U. H. Koszinowski, RNA, 2008, 14, 1959 CrossRef PubMed
.
- M. Hafner, M. Landthaler, L. Burger, M. Khorshid, J. Hausser, P. Berninger, A. Rothballer, M. Ascano, A.-C. Jungkamp, M. Munschauer, A. Ulrich, G. S. Wardle, S. Dewell, M. Zavolan and T. Tuschl, Cell, 2010, 141, 129 CrossRef CAS PubMed
.
- C. Danan, S. Manickavel and M. Hafner, Methods Mol. Biol., 2022, 2404, 167 CrossRef PubMed
.
- R. H. Hall, Biochem. Biophys. Res. Commun., 1963, 12, 361 CrossRef CAS
.
- J. P. Garel, D. Hentzen, M. Schlegel and G. Dirheimer, Biochimie, 1976, 58, 1089 CrossRef CAS
.
- L. Xu, X. Liu, N. Sheng, K. S. Oo, J. Liang, Y. H. Chionh, J. Xu, F. Ye, Y.-G. Gao, P. C. Dedon and X.-Y. Fu, J. Biol. Chem., 2017, 292, 14695 CrossRef CAS PubMed
.
- L. Flemmich, S. Heel, S. Moreno, K. Breuker and R. Micura, Nat. Commun., 2021, 12, 3877 CrossRef CAS
.
- A. Liaqat, M. V. Sednev, C. Stiller and C. Höbartner, Angew. Chem., Int. Ed., 2021, 60, 19058 CrossRef CAS PubMed
.
- V. Marchand, L. Ayadi, F. G. Ernst, J. Hertler, V. Bourguignon-Igel, A. Galvanin, A. Kotter, M. Helm, D. L. Lafontaine and Y. Motorin, Angew. Chem., Int. Ed., 2018, 57, 16785 CrossRef CAS PubMed
.
- J. Cui, Q. Liu, E. Sendinc, Y. Shi and R. I. Gregory, Nucleic Acids Res., 2021, 49, e27 CrossRef CAS PubMed
.
- M. Flamme, L. K. McKenzie, I. Sarac and M. Hollenstein, Methods, 2019, 161, 64 CrossRef CAS PubMed
.
- H. Aurup, A. Siebert, F. Benseler, D. Williams and F. Eckstein, Nucleic Acids Res., 1994, 22, 4963 CrossRef CAS PubMed
.
- M.-L. Winz, A. Samanta, D. Benzinger and A. Jäschke, Nucleic Acids Res., 2012, 40, e78 CrossRef CAS PubMed
.
- E. L. Chernolovskaya and M. A. Zenkova, Curr. Opin. Mol. Ther., 2010, 12, 158 CAS
.
- M. R. Hassler, A. A. Turanov, J. F. Alterman, R. A. Haraszti, A. H. Coles, M. F. Osborn, D. Echeverria, M. Nikan, W. E. Salomon, L. Roux, B. M. D. C. Godinho, S. M. Davis, D. V. Morrissey, P. D. Zamore, S. A. Karumanchi, M. J. Moore, N. Aronin and A. Khvorova, Nucleic Acids Res., 2018, 46, 2185 CrossRef CAS PubMed
.
- M. Hartl, A. Nist, M. I. Khan, T. Valovka and K. Bister, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 5604 CrossRef CAS PubMed
.
- S. Nainar, S. Beasley, M. Fazio, M. Kubota, N. Dai, I. R. Corrêa and R. C. Spitale, ChemBioChem, 2016, 17, 2149 CrossRef CAS PubMed
.
- S. Nainar, B. J. Cuthbert, N. M. Lim, W. E. England, K. Ke, K. Sophal, R. Quechol, D. L. Mobley, C. W. Goulding and R. C. Spitale, Nat. Methods, 2020, 17, 311 CrossRef CAS PubMed
.
- D. Wang, Y. Zhang and R. E. Kleiner, J. Am. Chem. Soc., 2020, 142, 14417 CrossRef CAS PubMed
.
- L. Meng, Y. Guo, Q. Tang, R. Huang, Y. Xie and X. Chen, Nucleic Acids Res., 2020, 48, 12566 CrossRef CAS PubMed
.
- J. M. Kofsky, G. C. Daskhan, M. S. Macauley and C. J. Capicciotti, Eur. J. Org. Chem., 2022, e202200108 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Procedures for RNA solid-phase synthesis, purification, and characterization; procedures for RNA conversion; procedures for siRNA applications; HPLC spectra; ESI mass spectra; primer extension assays (gels). See DOI: https://doi.org/10.1039/d2ob01560a |
| This journal is © The Royal Society of Chemistry 2022 |